A Novel POSS-Based Copolymer Functionalized Graphene: An Effective Flame Retardant for Reducing the Flammability of Epoxy Resin
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of OH-bisDOPO
2.3. Synthesis of bisDOPOMA Monomer
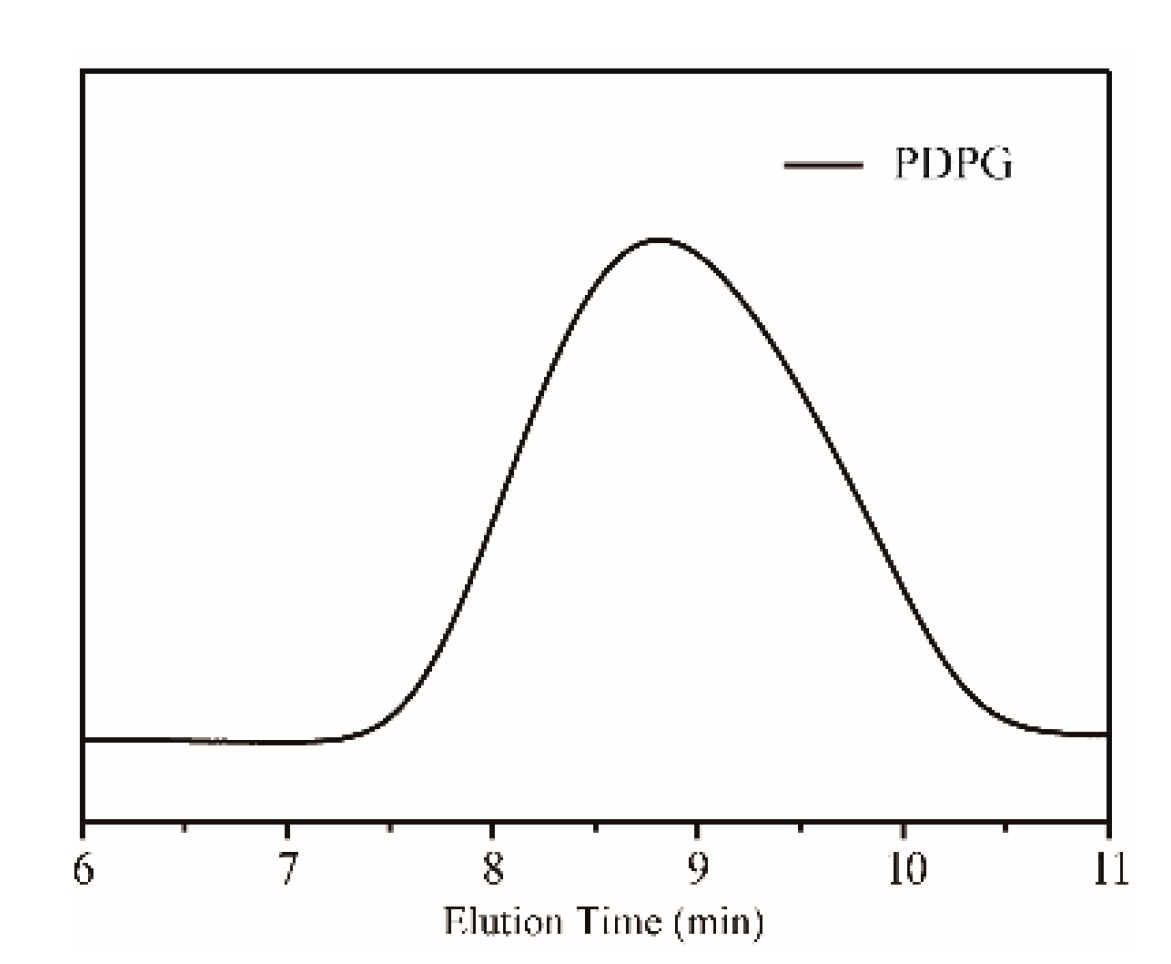
2.4. Synthesis of PDPG
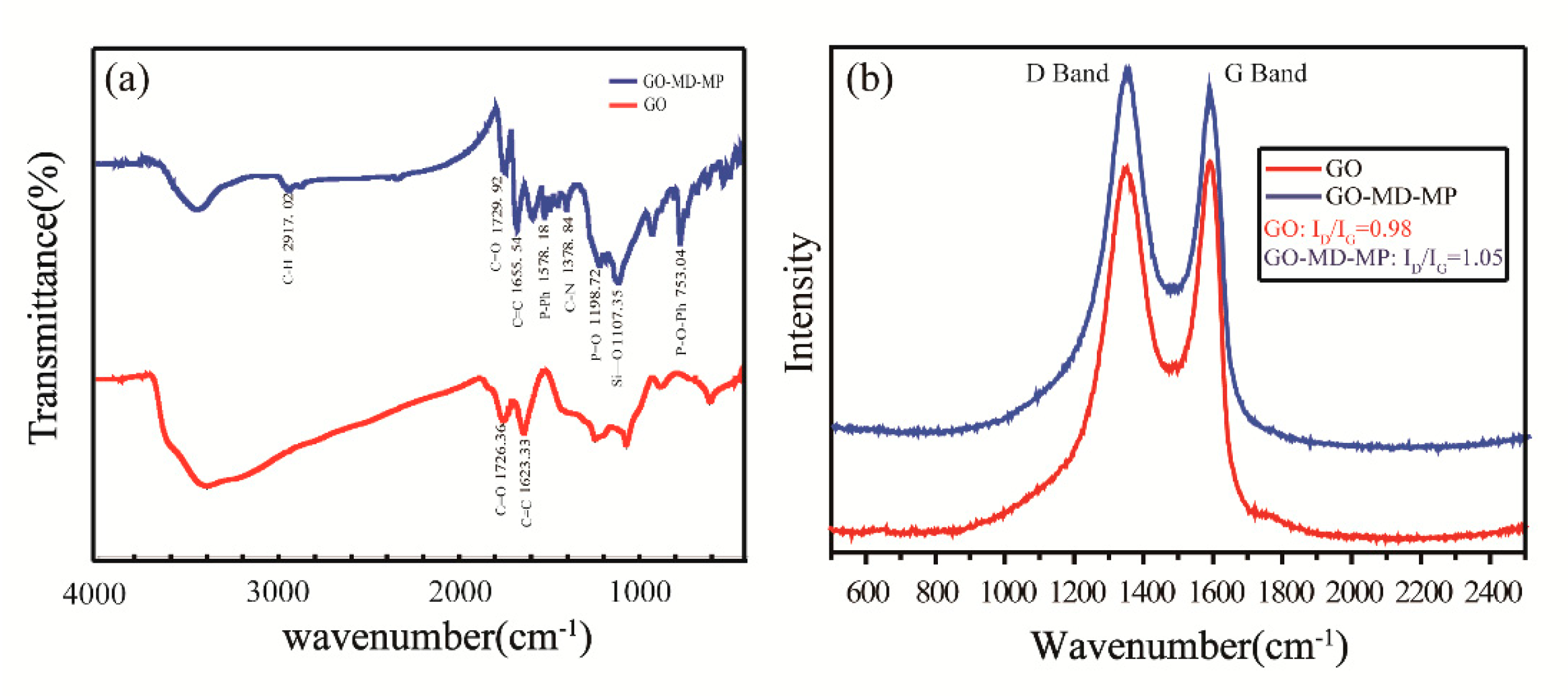
2.5. Synthesis of GO-MD-MP
2.6. Preparation of the Flame-Retardant EP Composites
2.7. Characterizations
3. Results and Discussion
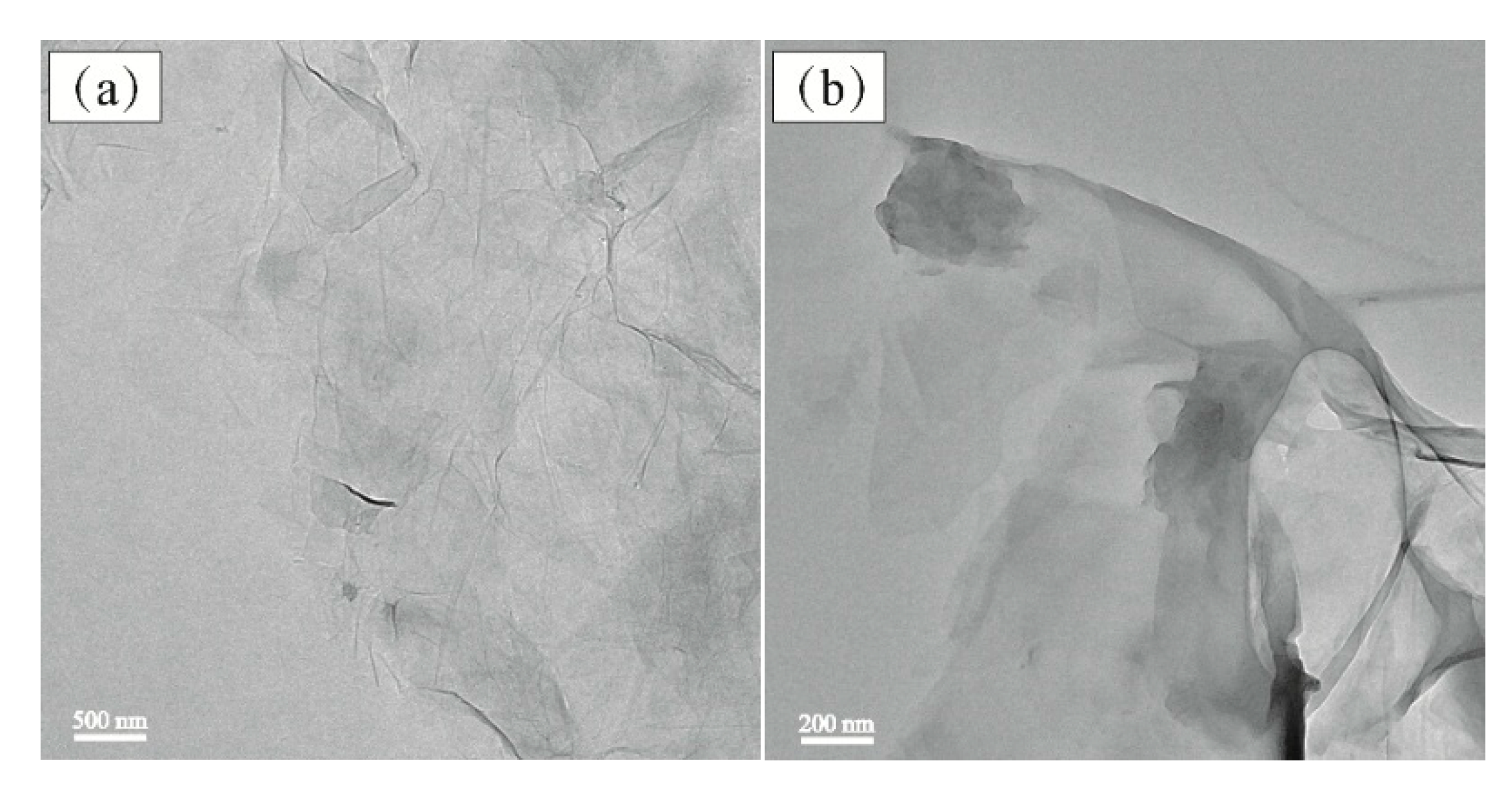
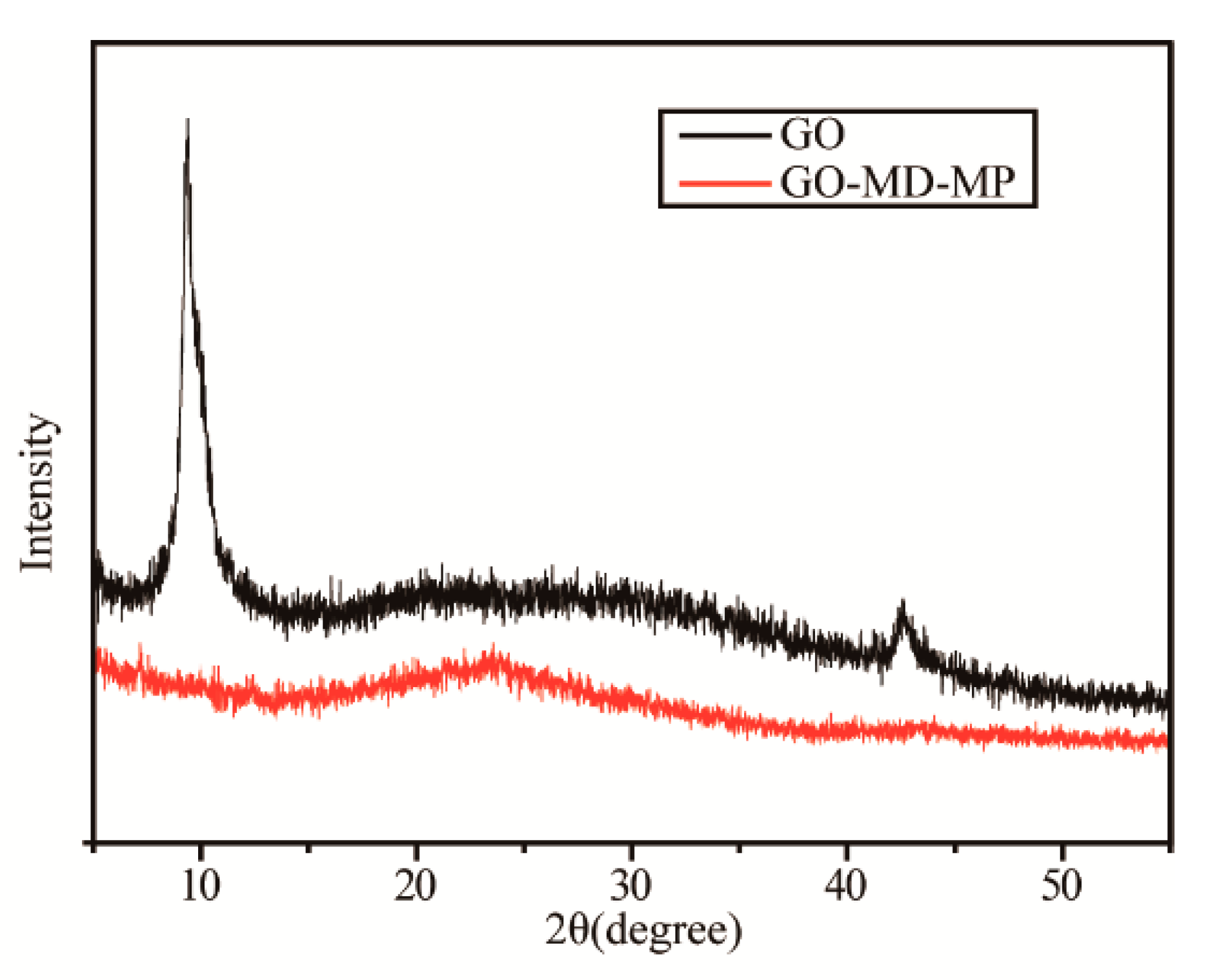
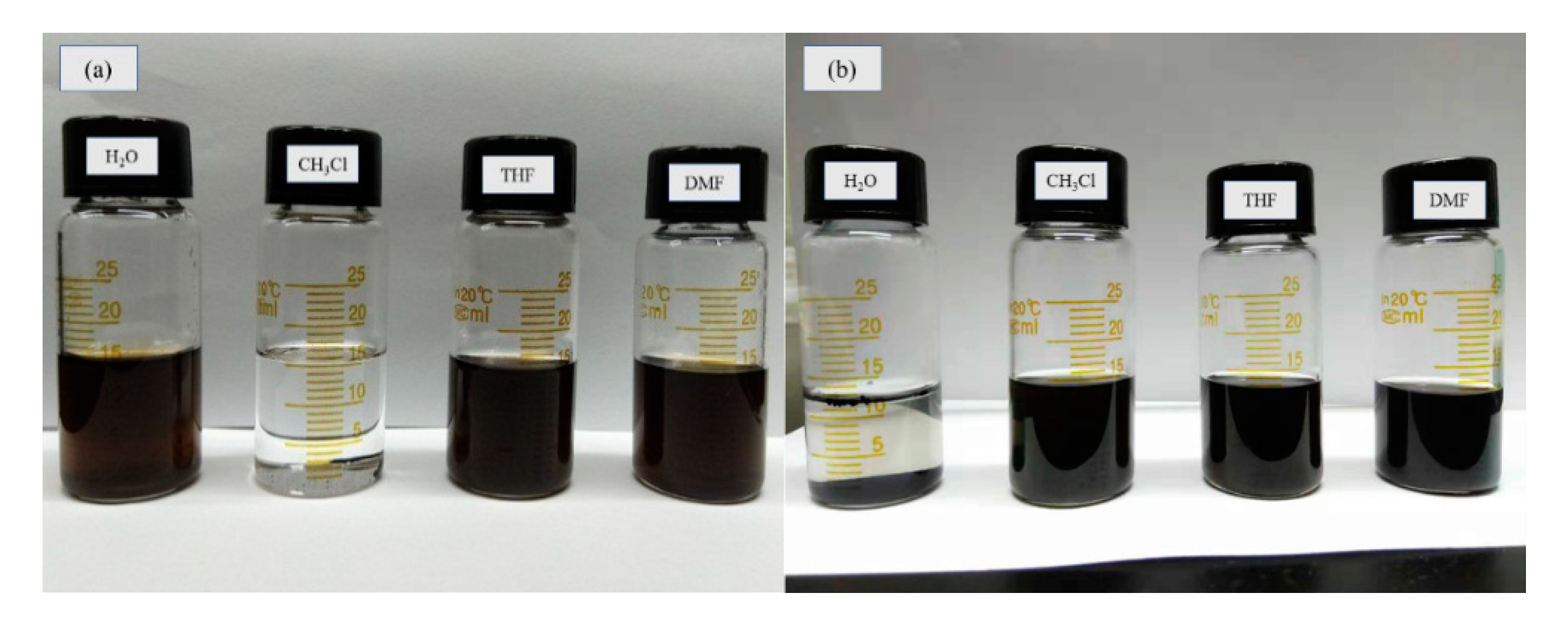
3.1. Structural and Morphological Characterization of GO-MD-MP and Cured EP Composites
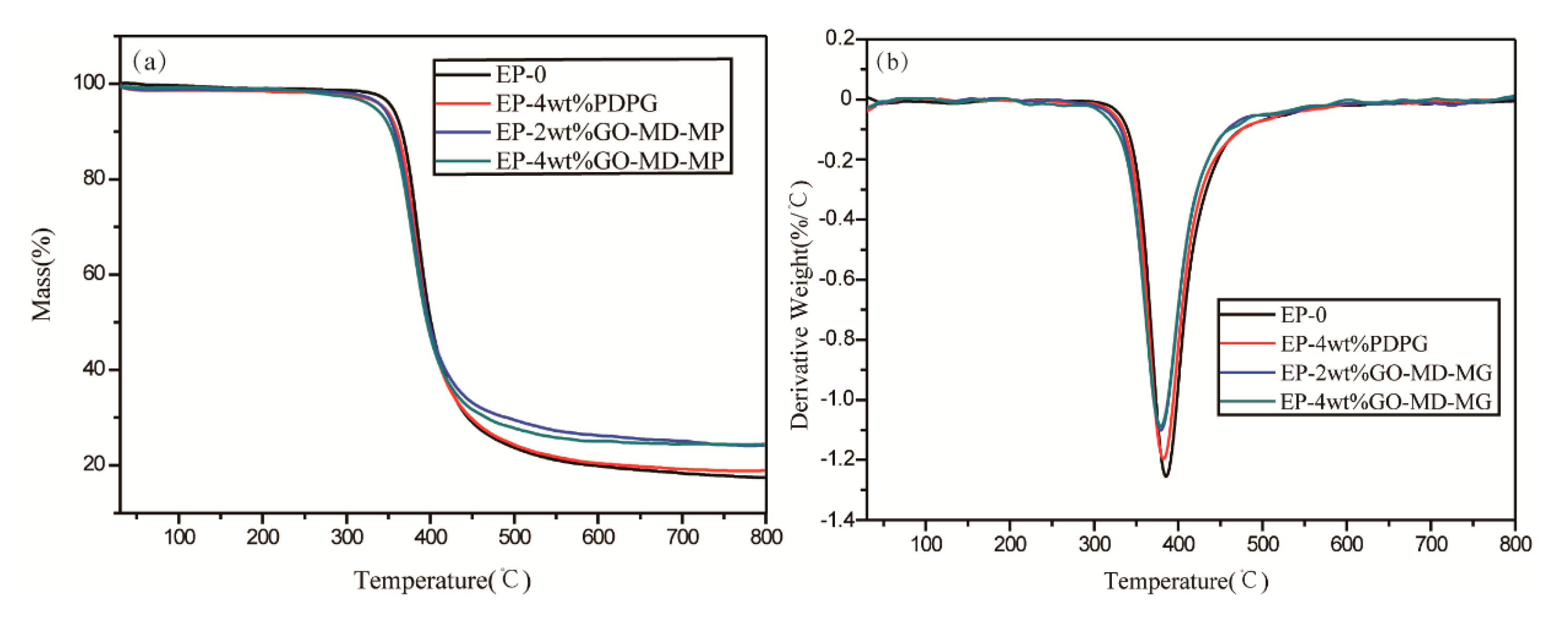
3.2. Thermal Properties and Flame Retardance of GO-MD-MP/EP Composites
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.W. Simultaneous improvement in the flame resistance and thermal conductivity of epoxy/Al2O3 composites by incorporating polymeric flame retardant-functionalized graphene. J. Mater. Chem. A 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Zhang, X.; Alloul, O.; Zhu, J.; He, Q.; Luo, Z.; Colorado, H.A.; Haldolaarachchige, N.; Young, D.P.; Shen, T.D.; Wei, S.; et al. Iron-core carbon-shell nanoparticles reinforced electrically conductive magnetic epoxy resin nanocomposites with reduced flammability. RSC Adv. 2013, 3, 9453–9464. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Cai, H.; Zhang, B.; Chen, X.; Wu, Q.; Yang, L. Synergistic effect between a novel triazine-based flame retardant and DOPO/HPCP on epoxy resin. Polym. Adv. Technol. 2018, 29, 2774–2783. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Gui, H.; Xu, P.; Hu, Y.; Wang, J.; Yang, X.; Bahader, A.; Ding, Y. Synergistic effect of graphene and an ionic liquid containing phosphonium on the thermal stability and flame retardancy of polylactide. RSC Adv. 2015, 5, 27814–27822. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zhu, J.; Yuan, C.; Zeng, B.; Chen, G.; Xu, Y.; Dai, L. Synthesis of a novel azaphosphorine flame retardant and its application in epoxy resins. J. Appl. Polym. Sci. 2018, 135, 45721. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Yu, B.; Wang, B.; Yuan, B.; Shi, Y.; Hu, Y.; Yuen, R.K. Novel organic-inorganic flame retardants containing exfoliated graphene: Preparation and their performance on the flame retardancy of epoxy resins. J. Mater. Chem. A 2013, 1, 6822–6830. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S.; et al. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–306. [Google Scholar] [CrossRef]
- Ciesielski, M.; Schafer, A.; Doring, M. Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym. Adv. Technol. 2008, 19, 507–515. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Fu, T.; Xu, Y.J.; Li, D.F.; Wang, X.L.; Wang, Y.Z. Novel phosphorus-containing halogen-free ionic liquid toward fire safety epoxy resin with well-balanced comprehensive performance. Chem. Eng. J. 2018, 354, 208–219. [Google Scholar] [CrossRef]
- Duan, L.; Yang, H.; Shi, Y.; Hou, Y.; Zhu, Y.; Gui, Z.; Hu, Y. A Novel Branched Phosphorus-Containing Flame Retardant: Synthesis and Its Application into Poly(Butylene Terephthalate). Ind. Eng. Chem. Res. 2016, 55, 10218–10225. [Google Scholar] [CrossRef]
- Hoang, D.; Kim, J.; Jang, B.N. Synthesis and performance of cyclic phosphorus-containing flame retardants. Polym. Degrad. Stab. 2008, 93, 2042–2047. [Google Scholar] [CrossRef]
- Wang, C.S.; Shieh, J.Y. Phosphorus-containing epoxy resin for an electronic application. J. Appl. Polym. Sci. 1999, 73, 353–361. [Google Scholar] [CrossRef]
- Haddad, T.S.; Choe, E.; Lichtenhan, J.D. Hybrid styryl-based polyhedral oligomeric silsesquioxane (POSS) polymers. Mater. Res. Soc. Symp. Proc. 1996, 435, 25–32. [Google Scholar] [CrossRef]
- Schwab, J.J.; Lichtenhan, J.D. Polyhedral oligomeric silsesquioxane (POSS)-based polymers. Appl. Organomet. Chem. 1998, 12, 707–713. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Song, C.F.; Chang, Y.; Chen, G.R.; Xu, Y.T.; Dai, L.Z. Modification of epoxy resin through the self-assembly of a surfactant-like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]
- Song, G.H.; Li, X.S.; Jiang, Q.Y.; Mu, J.X.; Jiang, Z.H. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015, 5, 11980–11988. [Google Scholar] [CrossRef]
- Zhang, W.C.; He, X.D.; Song, T.L.; Jiao, Q.J.; Yang, R.J. Comparison of intumescence mechanism and blowing-out effect in flame-retarded epoxy resins. Polym. Degrad. Stab. 2015, 112, 43–51. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Li, J.F.; Zhang, X.Q.; Yue, L.N.; Chai, Z.H. The flame retardancy of epoxy resin including the modified graphene oxide and ammonium polyphosphate. Combust. Sci. Technol. 2018, 190, 1126–1140. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Zheng, Y.Y.; Yang, J.; Duan, Z.P.; Hu, Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interface Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, L.; Yang, H.Y.; Xing, W.Y.; Kandola, B.; Hua, Y. Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Pornwannchai, W.; Hu, Y.; Kandola, B. The effect of graphene presence in flame retarded epoxy resin matrix on the mechanical and flammability properties of glass fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 88–96. [Google Scholar] [CrossRef]
- Huang, G.B.; Gao, J.R.; Wang, X.; Liang, H.D.; Ge, C.H. How can graphene reduce the flammability of polymer nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, Y.S.; Liu, P.J.; Xu, C.G.; Liu, Y.; Wang, Q. The preparation and application of a graphene-based hybrid flame retardant containing a long-chain phosphaphenanthrene. Sci. Rep. 2017, 7, 8759. [Google Scholar] [CrossRef]
- Guo, W.W.; Yu, B.; Yuan, Y.; Song, L.; Hu, Y. In situ preparation of reduced graphene oxide/DOPO-based phosphonamidate hybrids towards high-performance epoxy nanocomposites. Compos. Part B Eng. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Bao, C.L.; Guo, Y.Q.; Song, L.; Kan, Y.C.; Qian, X.D.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Bao, C.L.; Song, L.; Yuan, B.H.; Hu, Y. In Situ Polymerization of Graphene, Graphite Oxide, and Functionalized Graphite Oxide into Epoxy Resin and Comparison Study of On-the-Flame Behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Liao, S.H.; Liu, P.L.; Hsiao, M.C.; Teng, C.C.; Wang, C.A.; Ger, M.D.; Chiang, C.L. One-Step Reduction and Functionalization of Graphene Oxide with Phosphorus-Based Compound to Produce Flame-Retardant Epoxy Nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Xu, Y.J.; Chen, L.; Rao, W.H.; Qi, M.; Guo, D.M.; Liao, W.; Wang, Y.Z. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018, 347, 223–232. [Google Scholar] [CrossRef]
- Cao, R.R.; Chen, S.; Liu, H.B.; Liu, H.H.; Zhang, X.X. Fabrication and characterization of thermo-responsive GO nanosheets with controllable grafting of poly(hexadecyl acrylate) chains. J. Mater. Sci. 2018, 53, 4103–4117. [Google Scholar]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.Y.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]








| Sample | Composition (mol %) | P (at%) | P/Si | ||
|---|---|---|---|---|---|
| GMA | POSSMA | bisDOPOMA | |||
| PDPG | 16 | 18 | 66 | 1.8 | 0.92 |
| UL-94 | |||||
|---|---|---|---|---|---|
| Samples | LOI (%) | Burning Grade | t1(s) | t2(s) | Dripping |
| pure EP | 23.4 | NR | >30s | - | Yes |
| PDPG/EP-4% | 27.9 | V-1 | 16.2 | 2.4 | No |
| GO-MD-MP/EP-2% | 29.4 | V-1 | 19.6 | 3.8 | No |
| GO-MD-MP/EP-4% | 31.1 | V-0 | 2.7 | 3.5 | No |
| Samples | Flexural Modulus (MPa) | Flexural Strength (N·mm−2) | Percentage of Increased Flexural Strength (%) |
|---|---|---|---|
| pure EP | 2308.89 | 142.451 | 0 |
| EP-4% PDPG | 2145.61 | 131.014 | −8.03 |
| EP-2% GO-MD-MP | 2658.62 | 154.732 | 8.62 |
| EP-4% GO-MD-MP | 2738.46 | 165.067 | 15.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, H.; Wu, W.; Li, M.; Xu, Y.; Chen, G.; Dai, L. A Novel POSS-Based Copolymer Functionalized Graphene: An Effective Flame Retardant for Reducing the Flammability of Epoxy Resin. Polymers 2019, 11, 241. https://doi.org/10.3390/polym11020241
Li M, Zhang H, Wu W, Li M, Xu Y, Chen G, Dai L. A Novel POSS-Based Copolymer Functionalized Graphene: An Effective Flame Retardant for Reducing the Flammability of Epoxy Resin. Polymers. 2019; 11(2):241. https://doi.org/10.3390/polym11020241
Chicago/Turabian StyleLi, Min, Hong Zhang, Wenqian Wu, Meng Li, Yiting Xu, Guorong Chen, and Lizong Dai. 2019. "A Novel POSS-Based Copolymer Functionalized Graphene: An Effective Flame Retardant for Reducing the Flammability of Epoxy Resin" Polymers 11, no. 2: 241. https://doi.org/10.3390/polym11020241
APA StyleLi, M., Zhang, H., Wu, W., Li, M., Xu, Y., Chen, G., & Dai, L. (2019). A Novel POSS-Based Copolymer Functionalized Graphene: An Effective Flame Retardant for Reducing the Flammability of Epoxy Resin. Polymers, 11(2), 241. https://doi.org/10.3390/polym11020241





