Abstract
Polymer cellulose triacetate membranes doped with 1-alkylimidazole as fixed carriers were applied for the investigation of the facilitated transport of Zn(II) and Mn(II) ions from an aqueous sulphate feed phase (cM = 0.001 mol/dm3). For the polymer inclusion membranes (PIMs) doped with 1-alkylimidazole (alkyl – from hexyl up to decyl), the following patterns of transport selectivity were found: Zn(II) > Mn(II). The highest initial flux of Zn(II) ions (2.65 µmol/m2·s) was found for PIMs doped with 1-decyl-imidazole, whereas the best Zn(II)/Mn(II) selectivity coefficients equal to 19.7 were found for 1-hexyl-imidazole. Permeability coefficients for Zn(II) and Mn(II) ions transported across PIMs increase with an increase in the pKa values of 1-alkylimidazole. The polymer membranes of cellulose triacetate-o-NPPE with 1-alkylimidazole were characterised by scanning electron microscopy, non-contact atomic force microscopy and thermal analysis techniques. The influence of membrane morphology on the Zn(II) and Mn(II) transport process was discussed.
1. Introduction
Nowadays, the necessity to protect the environment and the gradual depletion of metal-bearing mineral resources have caused a certain convergence of interests, which results in the necessity to remove heavy metals from effluents and solid waste; at the same time, metal-bearing waste has become a secondary raw material for the production of economically valuable metals, e.g., zinc. This causes increased interest of entrepreneurs and scientists in seeking successful and cheap methods for recovering valuable metals, including zinc, from secondary raw materials. The global production of refined zinc in 2017 amounted to 13,234 thousand tonnes [1], of which secondary zinc produced from secondary raw materials constituted approx. 30%. The zinc recovery process depends on the form and concentration of this metal in the metal-bearing material and on the degree of contamination of the raw material with other compounds.
Currently, zinc(II) and the ions of other metals can be selectively separated by means of various separation methods, such as solvent extraction [2,3,4], ion flotation [5,6,7,8,9], ionic exchange [10,11], solvent sublation [12,13], sorption [14,15], and liquid membranes [16,17,18,19,20], in which the stages of extraction and re-extraction proceed simultaneously. The separation of zinc ions can be performed by means of extracting agents/commercial carriers, i.e., organophosphorous acids or Cyphos [20,21,22,23], macrocyclic [18,23,24], as well as imidazole derivatives [25,26,27,28].
Waste alkaline zinc-manganese batteries and zinc-carbon batteries are a valuable source of secondary zinc. These waste batteries contain considerable amounts of zinc (5%–21%) and manganese (23%–33%), as well as minor amounts of other metals (0.1%–1.0% Fe, 5%–7% K, approx. 0.01% Ni) [29] and they can be processed in pyrometallurgical processes: Citron, Tera, Waelz, Batric and Sumitomo, as well as hydrometallurgical processes: Batrec (Switzerland), Batenus (Germany), Zincex MZP (Spain) and TNO (the Netherlands, Germany) [30,31,32]. However, new, more economically effective hydrometallurgical methods of recovering this metal are still being sought.
A typical hydrometallurgical metal production process consists of four major technological steps such as leaching, phase separation, the separation of metal ions from aqueous solutions and the precipitation of metals or their compounds from the aqueous phase [29,33,34]. Among the listed technological operations, particular attention should be paid to the process of separating metal ions in an aqueous solution, which decides about the degree of purity of the resulting products. This process can be carried out by means of methods such as liquid–liquid extraction, ion exchange, ion flotation or transport through liquid membranes. The selection of a proper method and conditions for conducting the process decides about its selectivity and the possibility of application on an industrial scale.
Membrane technologies have become an important alternative to conventional processes employed for wastewater treatment, separation and recovery of target metals [35,36]. The selective transport of metal ions has been widely studied with supported (SLMs) and polymer inclusion (PIMs) liquid membranes [16,17,18,19,20,21,22,23,24,25,26,27,28,35,36,37]. Their high selectivity, high diffusion rates and the ability to concentrate ions have made them particularly useful [35,38].
The aim of this work was to check the possibility of separation and recovery of Zn(II) from equimolar sulphate mixtures of Zn(II)-Mn(II) ions from aqueous solutions using PIMs doped with hydrophobic 1-alkyl-imidazoles (alkyl = 1-hexyl, 1-heptyl, 1-octyl, 1-nonyl-, 1-decyl).
The purpose of work also included investigation of the dependence of the physical properties of CTA-based membranes on their efficiency in the separation of Zn(II)-Mn(II) ions during transport across such membranes.
2. Experimental
2.1. Reagents and Apparatus
Inorganic chemicals: zinc(II), manganese(II) sulphate and tetramethylammonium hydroxide were of analytical grade and were purchased from POCh (Gliwice, Poland). Aqueous solutions were prepared with double distilled water (conductivity 5 µS/m).
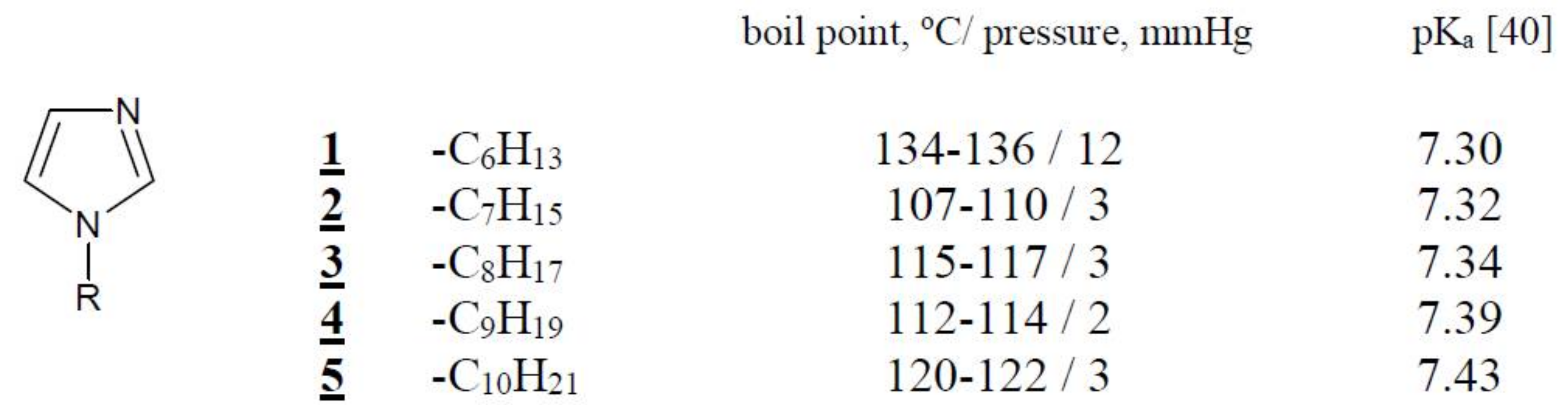
Organic reagents, i.e., cellulose triacetate (CTA), o-nitrophenyl pentyl ether (o-NPPE), and dichloromethane (all from Fluka, Switzerland) were of analytical grade and were used without further purification. 1-alkylimidazoles 1–5 (Figure 1) were synthesized according to the procedure described in paper [39]. The pKa value of all carrier is obtained from reference [40].

Figure 1.
The chemical formula 1-alkyl-imidazoles and their physicochemical properties.
2.2. Polymer Inclusion Membrane Preparation
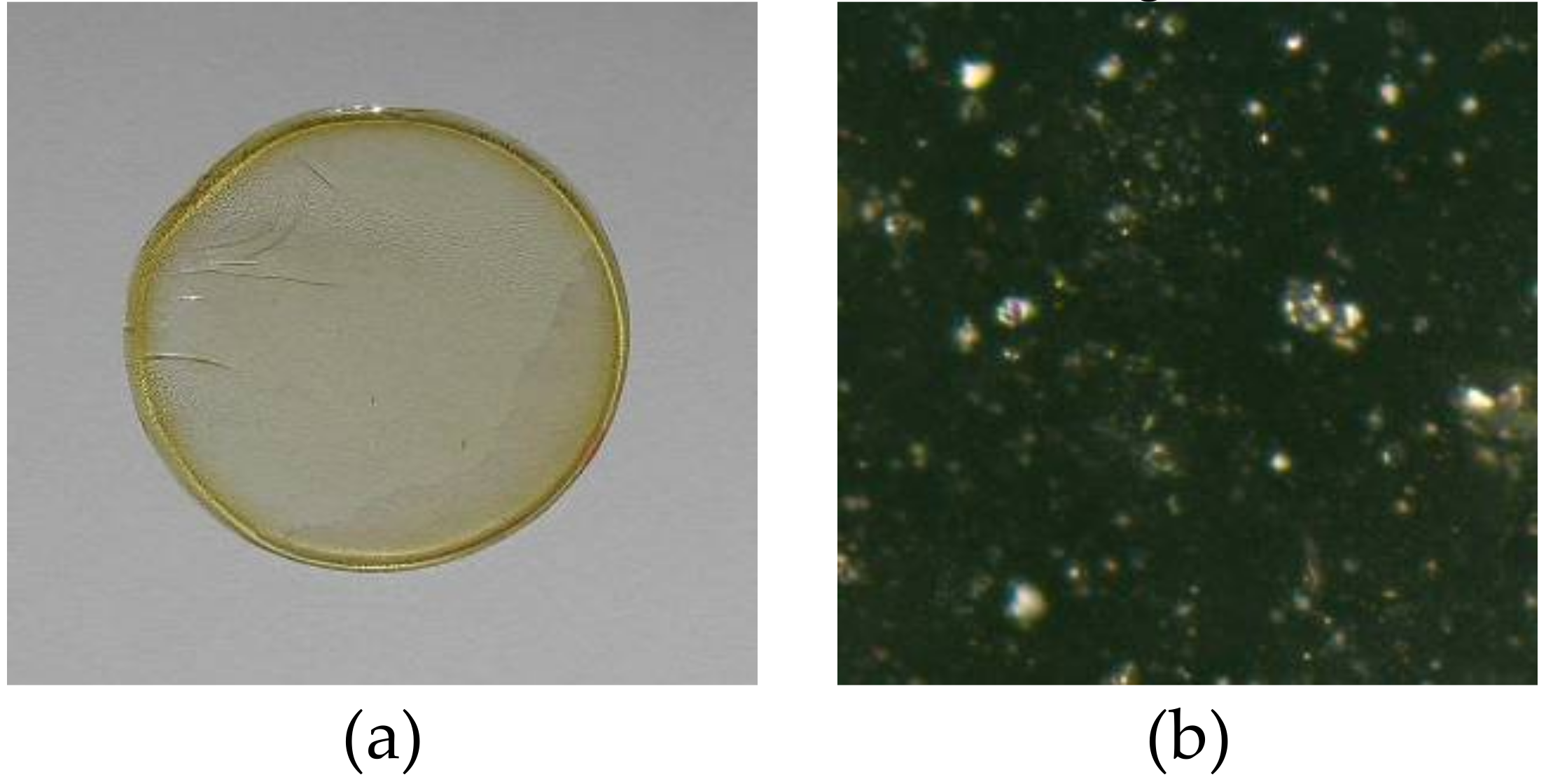
The membranes were prepared according to the procedure reported in the previous paper [25]. A solution of cellulose triacetate (CTA), plasticizer (o-nitrophenyl pentyl ether (o-NPPE)), and 1-alkylimidazoles 1–5 (Figure 1) as ion carriers in dichloromethane was prepared. 25 cm3 of the solution was poured into a membrane mould consisting of a 9.0 cm diameter glass ring fixed on a glass plate with cellulose triacetate-dichloromethane glue. After evaporation of the solvent overnight at room temperature the resulting membrane was peeled off from the glass plate by immersion in cold water. Then the membrane was soaked for 12 h in distilled water to ensure its homogeneity. Two discs were cut out from the same preparation to duplicate transport experiments. The wet membrane contained 2.67 cm3 o-NPPE /1 g CTA, and 1.0 mol/dm3 concentration of 1-alkyl-imidazoles (1–5) based on plasticizer. The example membrane and its photo taken with an optical microscope (NICON ECLIPSE E40 POL) are shown in Figure 2.
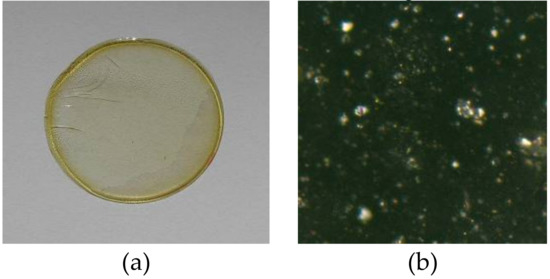
Figure 2.
PIM: CTA-o-NPPE-1-hexyl-imidazole: before process—real diameter 44 mm (a) photo from the otoscope microscope—enlargement 1000 times (b).
2.3. Characteristics of PIMs
The thickness of the PIM samples was measured using a digital micrometer (Panametrics® Magna-Mike® 8500 (San Diego, CA, USA)) with an accuracy of 0.1 µm. The thickness of a membrane was measured 10 times for each case and shown as the average value of these measurements, with the standard deviation below 1%. The thickness of membranes before and after transport was found to be the same. The average PIMs thickness varied in the range of 29–32 μm. Experimental reproducibility was high with standard deviation below 1% of the measured values.
A surface characterization study of the polymer membranes was performed using an Atomic-force MultiMode Scanning Probe Microscope IIIa (AFM) (Digital Instruments Vecco Metrology Group, Santa Barbara, CA, USA) according to the procedure described in other papers [24,25,26,27]. Pores characterization was performed using the AFM image processing program NanoScope v.5.12 (Digital Instruments Vecco Metrology Group, Santa Barbara, CA, USA), which enabled the calculation of two parameters: roughness (Rq) and porosity (ε). The Rq parameter is the standard deviation of the z values within the box cursor and is calculated as:
where: zi is the current z value, n- is the number of points within the box cursors.
Scanning electron microscope (SEM) images of the membranes were obtained with the Hitachi SU3500 SEM/EDS (energy dispersive spectroscopy) microscope operated at 10.0 kV. The membranes were visualized in 10.0 μm × 10.0 μm images (front view central and from a perpendicular cross-section along medium).
The thermal stability of the polymeric inclusion membranes was determined over the range of 20–700 °C under nitrogen, in ceramic crucibles. An empty crucible served as a reference. The apparatus used was a SDT 2960 instrument (TG209F3 Tarsus Netzsch, Selb, Germany) operated at a heating rate of 10 °C/min and a nitrogen flow rate of 100 cm3/min. The sample weight was 10 mg throughout.
2.4. Transport Studies
Transport experiments were carried out in a permeation cell described in an earlier paper by the same authors [24,25,26,27]. The membrane film (surface area 4.9 cm2) was tightly clamped between two cell compartments. Both, the feed and the receiving aqueous phases (45 cm3 each) were mechanically stirred at 600 rpm. Metal sulphate were used in the feed phase whereas the receiving phase was deionized water. The PIM transport experiments were carried out at 20 ± 0.2 °C. Small samples of the aqueous receiving phase were taken periodically from the sampling port equipped with a syringe and analysed by atomic absorption spectroscopy (AAS Spectrometer, Solar 939, Unicam, London, UK) to determine zinc(II), and manganese(II) concentrations. The pH of the feed phase (5.6) was kept constant using tetramethylammonium hydroxide.
The kinetics of the transport across the PIMs was described as a first-order process with respect to the metal-ion concentration expressed by Equation (2):
where c is the metal ions concentration in the feed phase at a given time (mol/dm3), ci is the initial metal ions concentration in the feed phase, k is the rate constant (s−1), and t is the time of transport (s). [41].
To calculate the value of k, ln(c/ci) versus time was plotted. The rate constant values for two independent transport experiments were averaged and standard deviation was calculated. The correlation between ln(c/ci) and time was linear, which was confirmed by the high correlation coefficient (R2) ranging from 0.9624 to 0.9995. The permeability coefficient (P) was calculated according to Equation (3):
where V is the volume of the aqueous feed phase [m3], k is the rate constant and A is an effective area of the membrane [m2].
The initial flux (Ji) is equal to:
The selectivity coefficient (S) was defined as the ratio of initial fluxes for M1 and M2 metal ions, respectively:
To describe the efficiency of metal removal from the feed phase, the recovery factor (RF) was calculated:
The reported values correspond to the average values of three replicates, with the standard deviation within 5%.
3. Results and Discussion
3.1. The Characteristics of Membranes
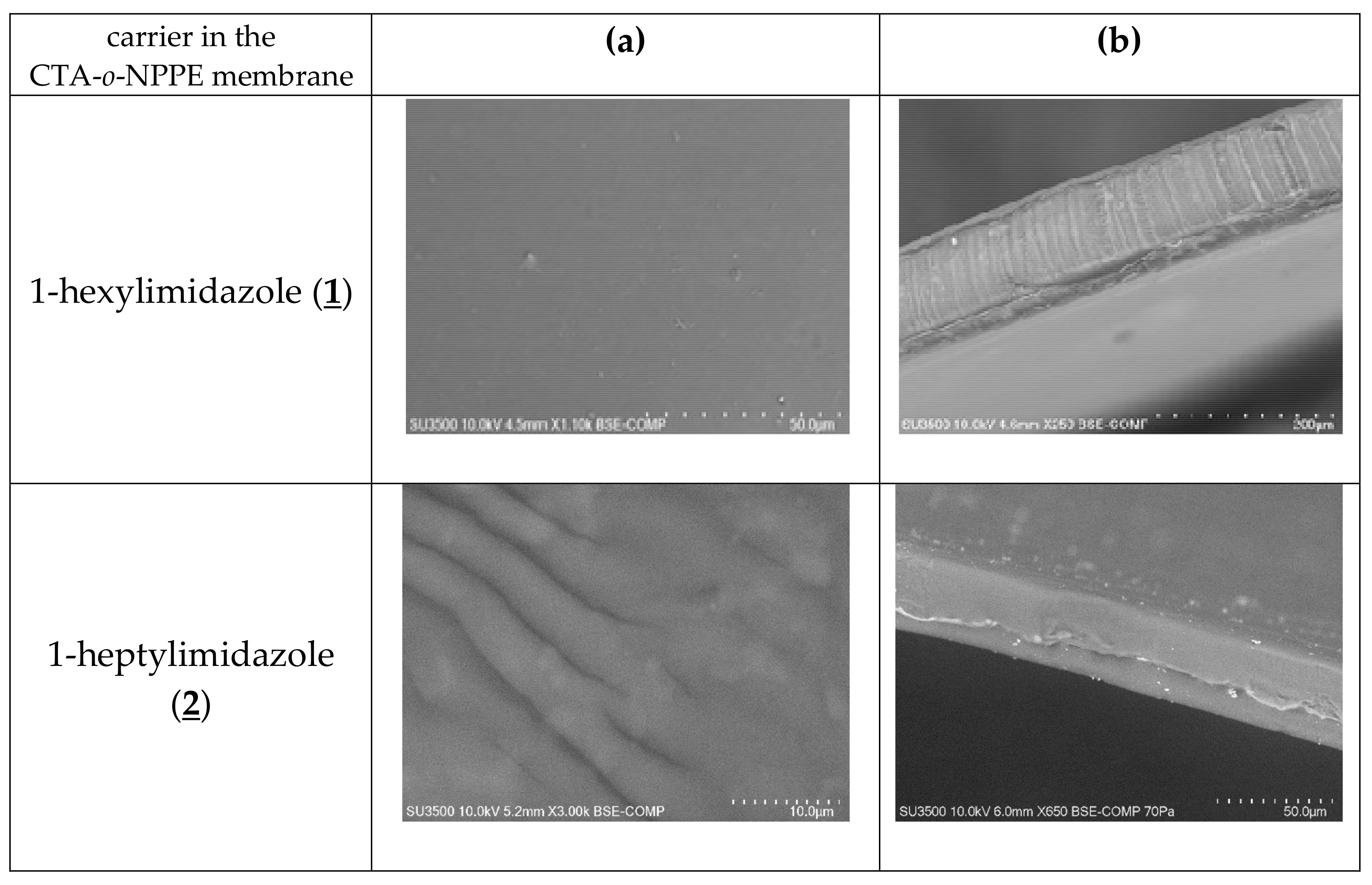
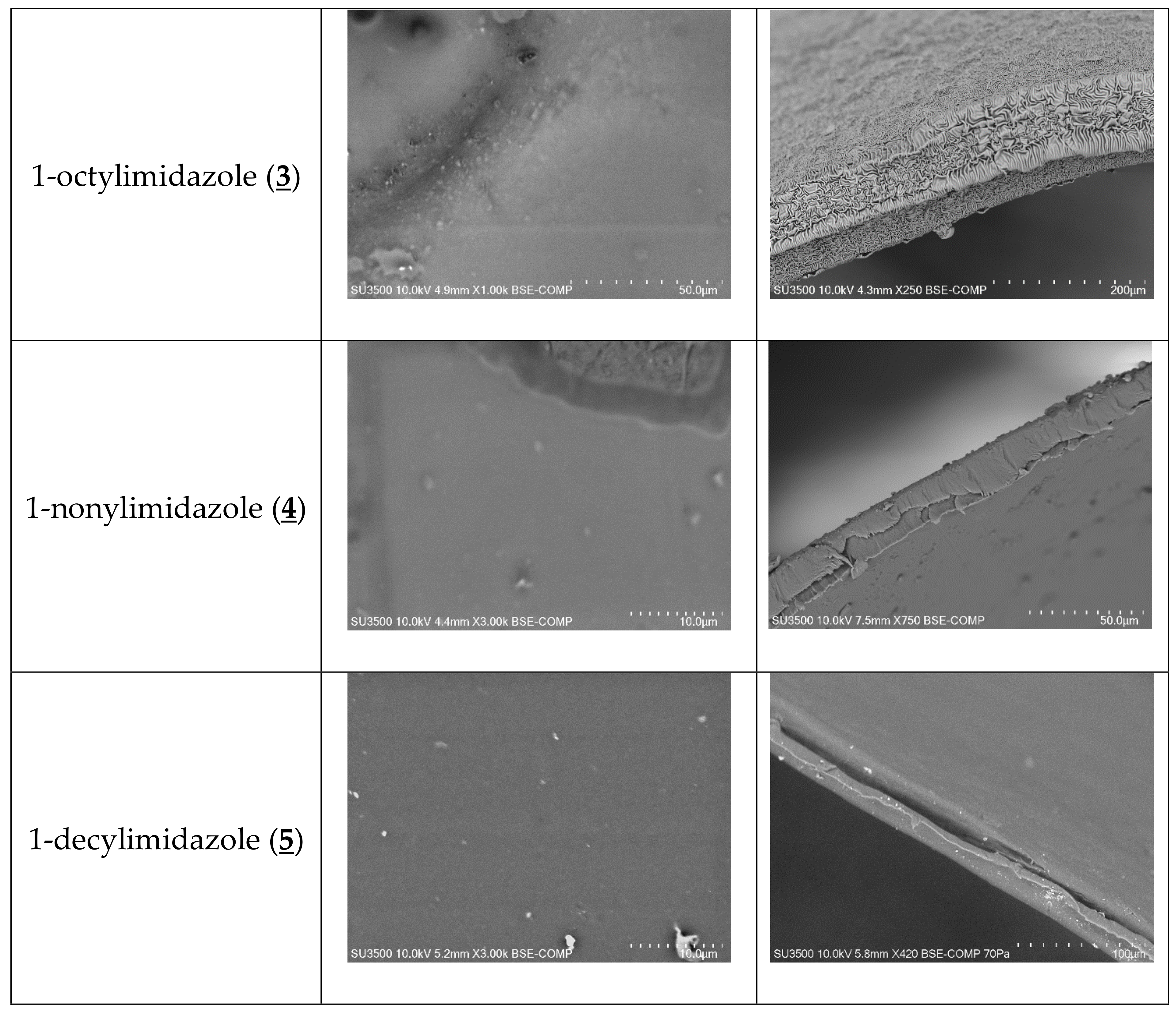
The SEM photomicrographs (Figure 3) showed that all membranes had dense and homogeneous structures. Moreover, the images showed visible roughness of film surfaces. Carriers could crystallise in the membrane and, for example, alkylimidazole molecules migrated to the membrane surface, causing its roughness and porosity.
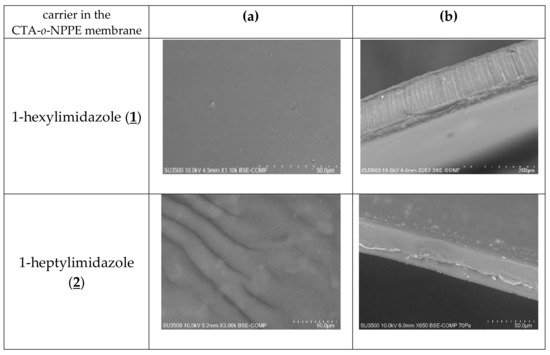
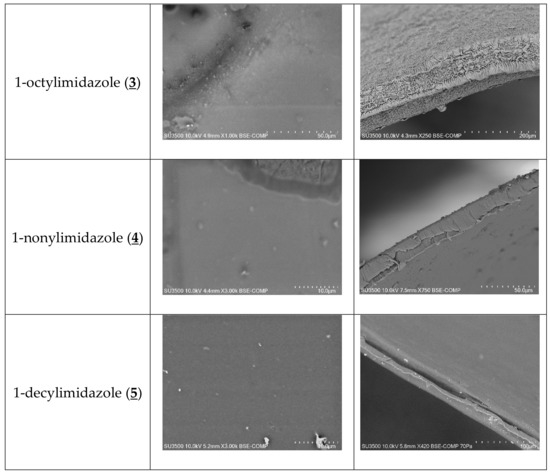
Figure 3.
SEM images of PIMs containing 1-alkyl-imidazole as a carrier: front view (a), side section (b).
The distribution of the carrier in the investigated membrane after the evaporation of dichloromethane is homogeneous on the entire surface. PIMs containing 1-alkyl-imidazole are dense and homogenous.
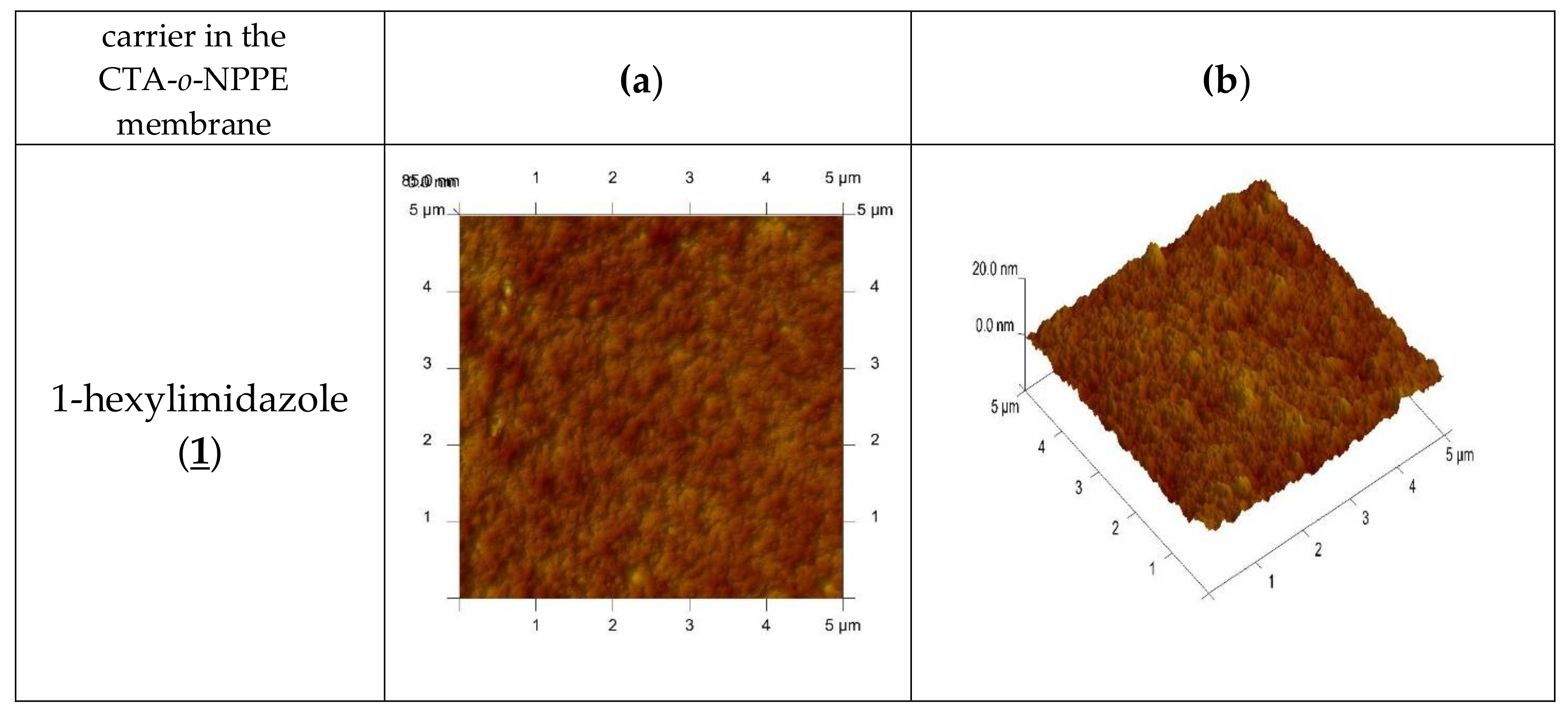
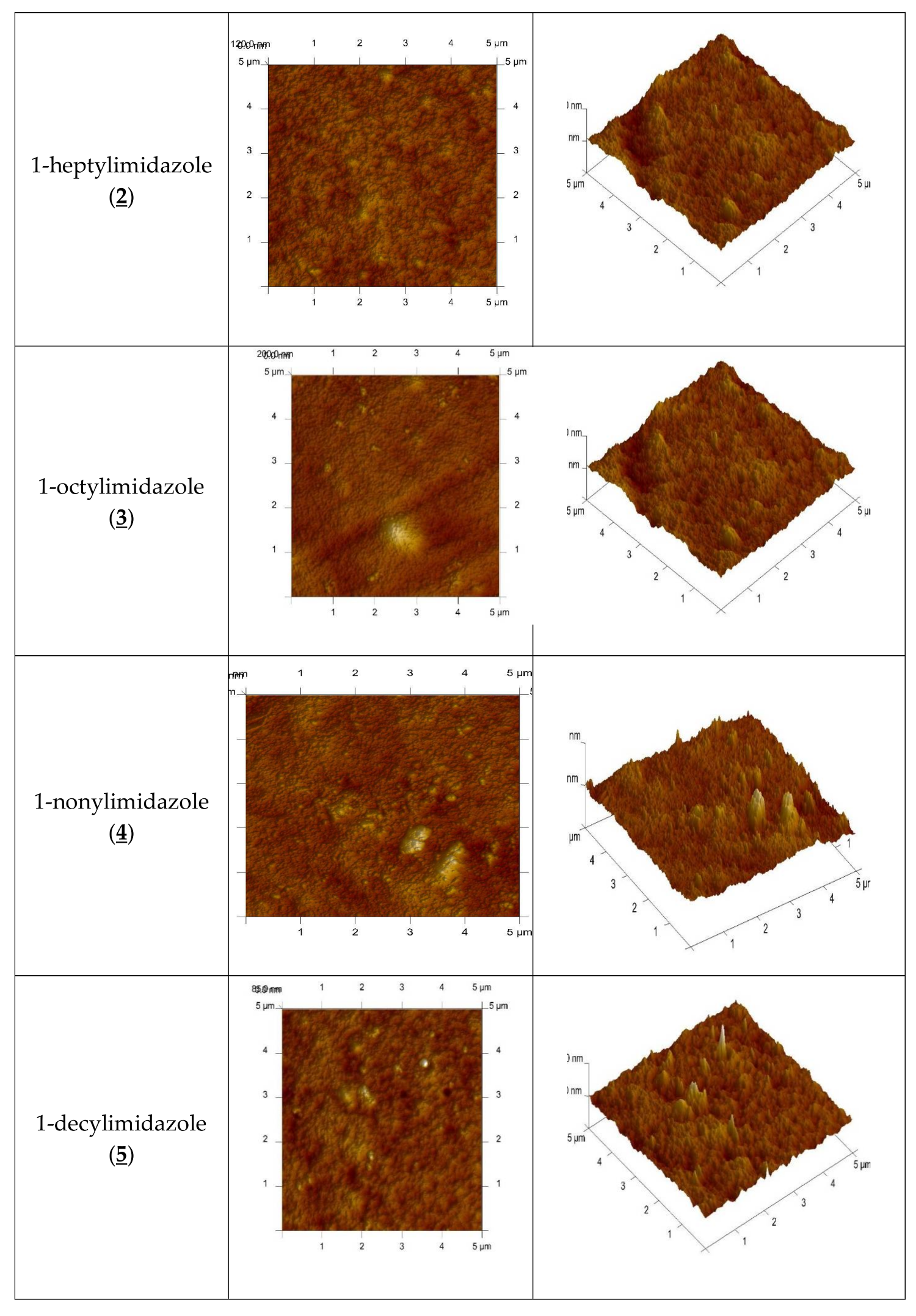
An AFM image of the PIM doped with 1-alkyl-imidazole in two- and three-dimensional forms is shown in Figure 4.
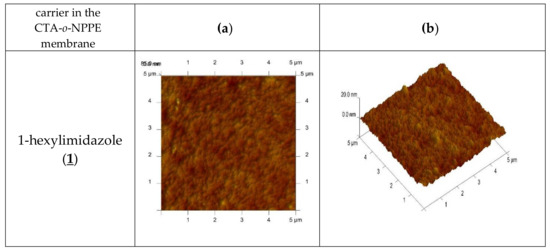
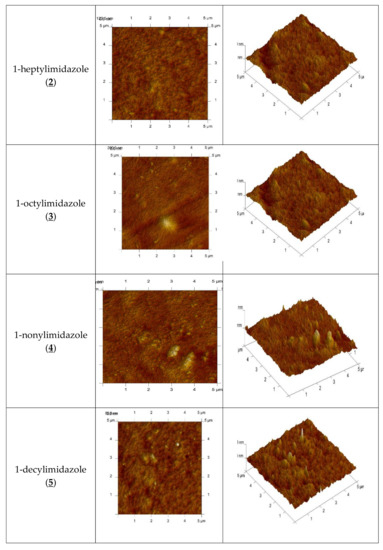
Figure 4.
2D- and 3D- view atomic force photomicrographs of PIMs with 1-alkyl-imidazole at a 1 mol/dm3 carrier concentration. 2D-view (a) and 3D- view (b).
In the image of PIM samples (Figure 4) there are clearly visible elongated pores, also called “cavity channels” (darker regions). Such morphology of the membrane surface can be related to the crystallinity of the CTA.
A wide network of pores 5–25 nm in size is likely to be responsible for the improved transport performance of the membranes.
The roughness (Rq) and effective pore sizes of the membrane were calculated using atomic force microscopy (AFM) and they are shown in Table 1 together with the tortuosity of the membrane, determined from the dependence developed by Wolf and Strieder [42]:
τ = 1 − lnε

Table 1.
AFM characterization parameters for PIM doped with 1-alkyl-imidazole.
In the image of PIM samples (Figure 4), there are clearly visible elongated pores, also called “cavity channels” (darker regions). Such morphology of the membrane surface can be related to the crystallinity of the CTA.
A wide network of pores 5–25 nm in size is likely to be responsible for the improved transport performance of the membranes.
The roughness (Rq) and effective pore sizes of the membrane were calculated using atomic force microscopy (AFM) and they are shown in Table 1, together with the tortuosity of the membrane, determined from the dependence developed by Wolf and Strieder [42]:
The roughness, the effective size of pores and tortuosity of the investigated membranes increase along with an increase in the hydrophobicity of the carrier molecules.
As demonstrated in a number of papers [24,43,44,45,46], the microstructure of the membrane has an impact on the transport process. CTA membranes have porous structures, and the distribution of pores is nearly uniform (porosity of 50%) [41]. The pores in a CTA matrix were filled with a plasticiser (o-NPPE) and the carrier. The carrier crystallises inside the membrane, with the texture of the surface being relatively homogeneous, with varying porosity and roughness. The roughness of a CTA membrane obtained by Tor et al. [46] equalled 14 nm.
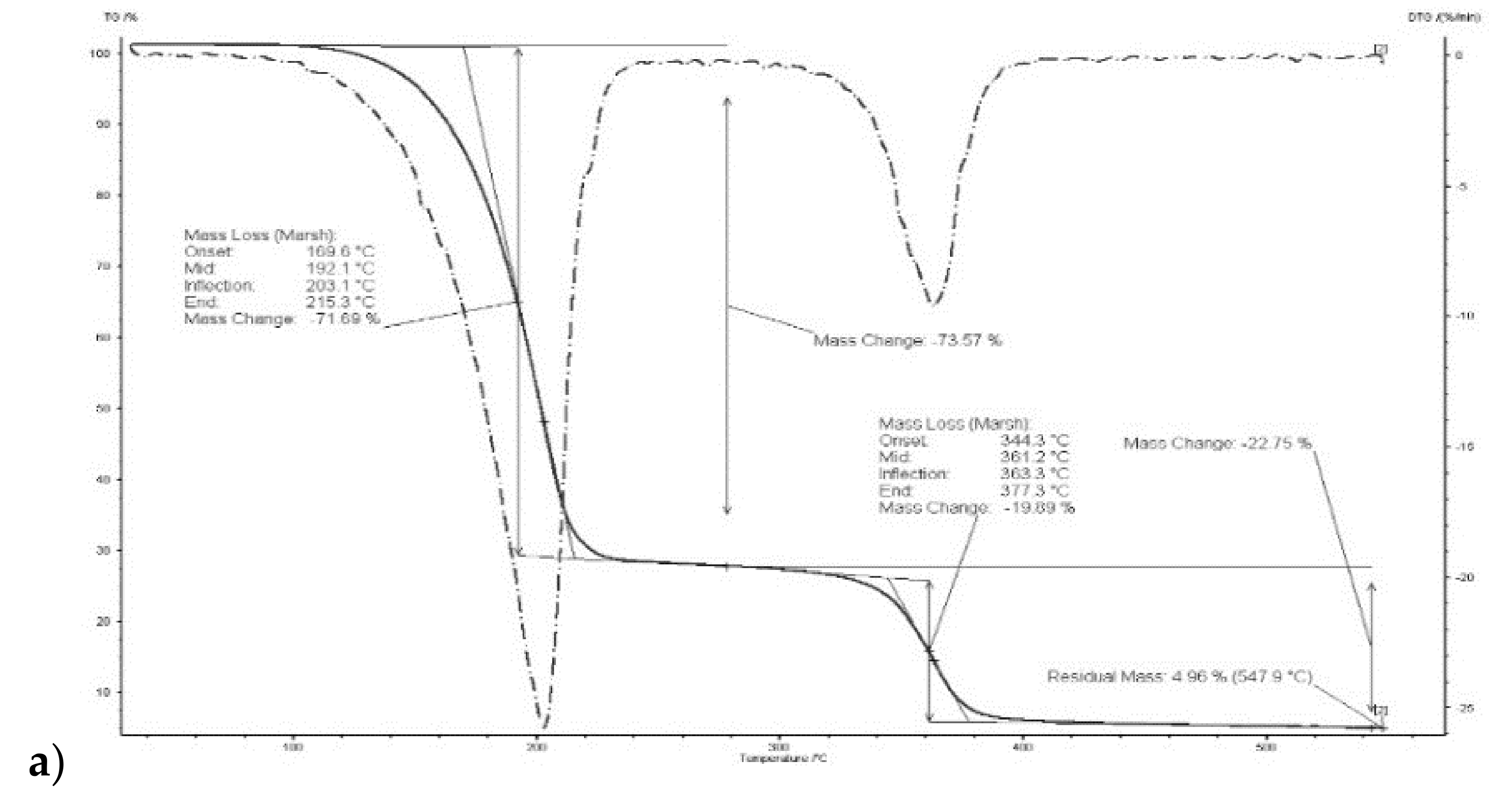
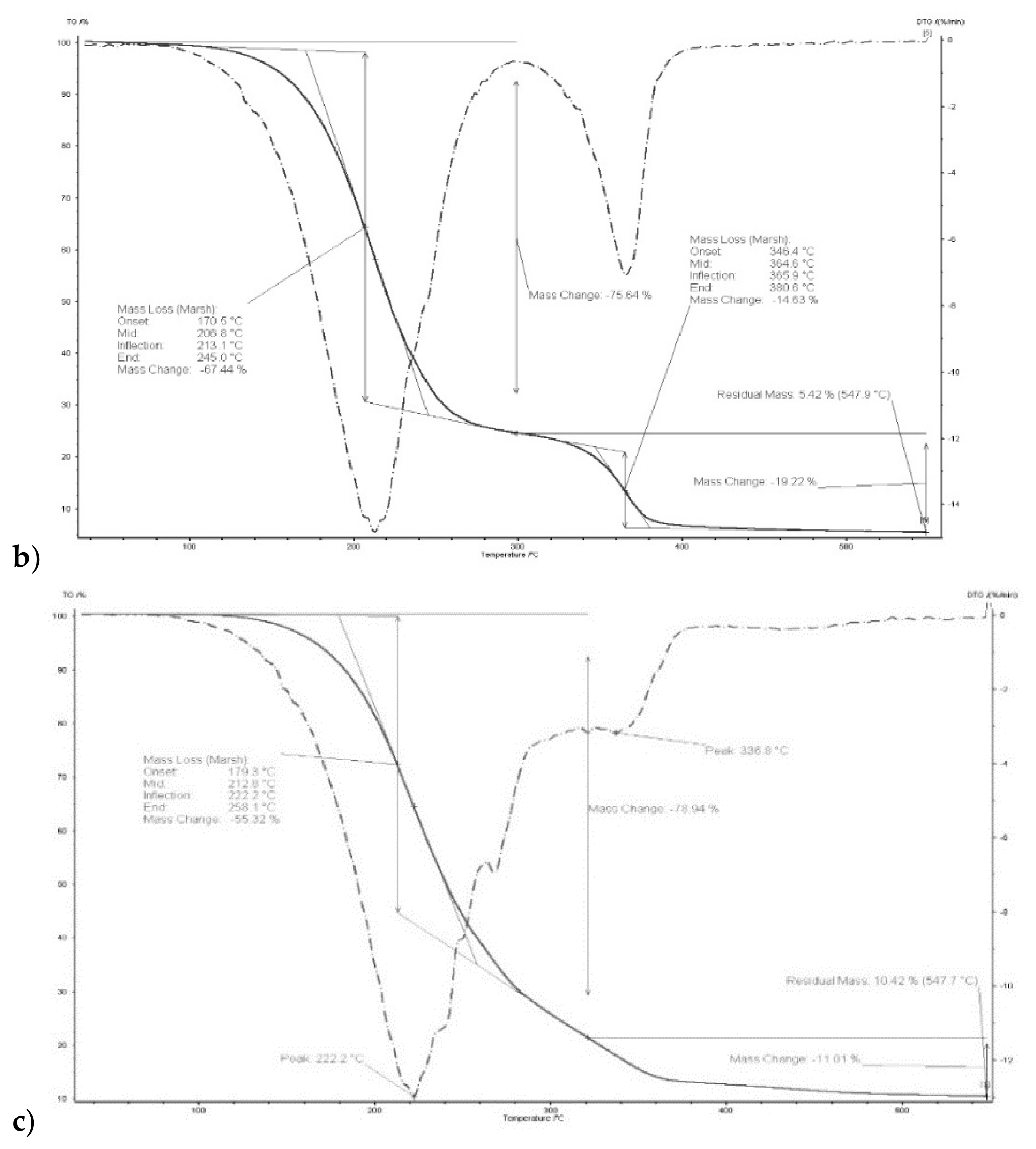
The thermal stability of the polymers depends, among other things, on their internal structure, cross-linking and the presence of aromatic rings and degradable functional groups. It was demonstrated in [46] that degradation of a CTA membrane matrix proceeds in two steps: the former in the range of 292–320 °C (main degradation), the latter in the range of 450–476 °C (carbonization). According to other reports, degradation of a CTA membrane is a single-step process occurs at 300 °C [47] or 350 °C [48].
Thermograms of membrane made of CTA – o-NPPE with 1-decyl-imidazole (5) (Figure 5) indicates that degradation of the membrane proceeds in two steps. For membrane before transport in the first step, at 208.8 °C, the weight loss is 67.44%, in the second at 364.6 °C the weight loss is 14.63%. For this membrane residual mass at 547.9 °C is 5.42%. Moreover, it was reported in [37] that degradation of the CTA—o-NPPE membrane with 1-hexylimidazole proceeds in two steps at 211.3 and 360.7 °C. Thus, the results obtained enable the conclusion that polymer inclusion membranes CTA—o-NPPE which contain imidazole derivatives show high thermal stability (up to approx. 200 °C).
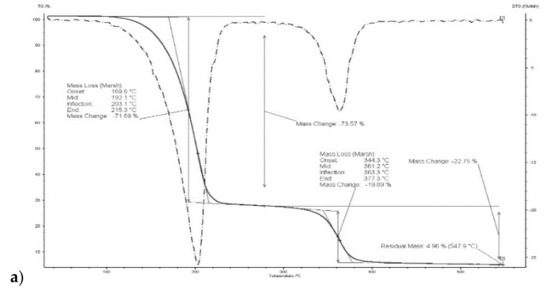
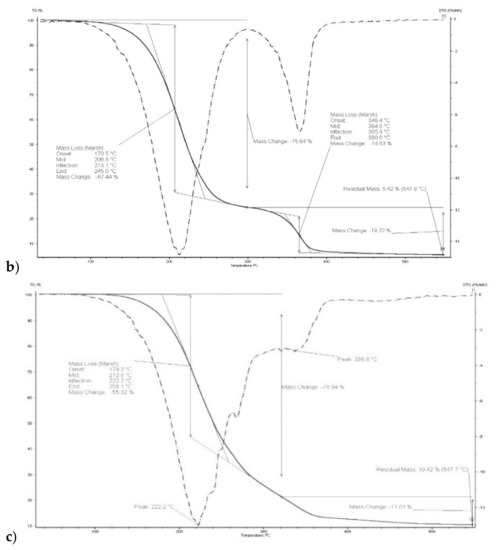
Figure 5.
Thermograms of CTA + o-NPPE (a), CTA + o-NPPE + carrier (5) (b), CTA + o-NPPE + carrier (5) after process (c).
3.2. Transport of Zn(II) and Mn(II) ions across PIMs
Findings on the transport of Zn(II) and Mn(II) ions from equimolar sulphate solutions across PIMs containing 1-alkyl-imidazole (1–5) as an ion carrier are discussed below. The studies were carried out using two-component solutions containing metal ions, each with a concentration of 0.001 mol/dm3. The initial flux values and selectivity coefficients for the transport of metal ions across the PIMs are shown in Table 2.

Table 2.
Initial fluxes, selectivity order and selectivity coefficients for competitive transport of Zn(II) and Mn(II) ions across PIM doped with 1-alkylimidazole; membrane: 2.6 cm3 o-NPPE /1g CTA and 1.0 mol/dm3 carriers calculated on plasticizer, pH of the feed phase—5.6, receiving phase—deionized water.
As indicated by the data shown in Table 2, for all investigated PIMs, the initial flux value for the transport of Zn(II) ions is higher than for Mn(II) ions, and it increases with increasing hydrophobicity of the carrier molecule. This means that in the case of a PIM doped with 1-decyl-imidazole (5), the initial flux has the highest value. In the case of a PIM doped with 1-hexylimidazole (1), the Zn(II)/Mn(II) selectivity coefficient (S) has the highest value.
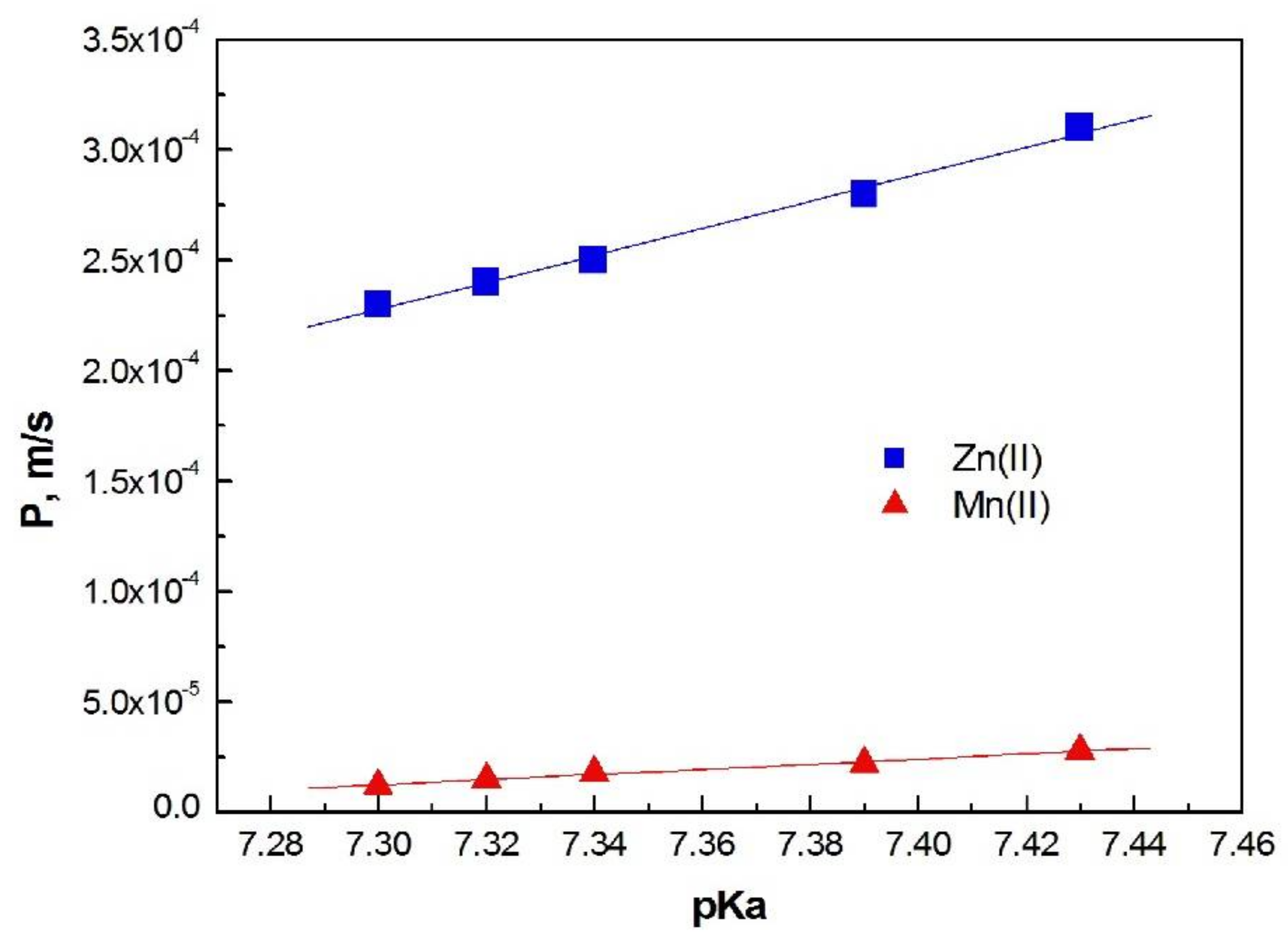
From Equation (3), the permeability coefficient of both ions was calculated for each investigated membrane. Figure 6 shows the dependence of membrane permeability coefficients (P) on the dissociation constant (pKa) of the carrier molecules used in these membranes for Zn(II) and Mn(II) ions.
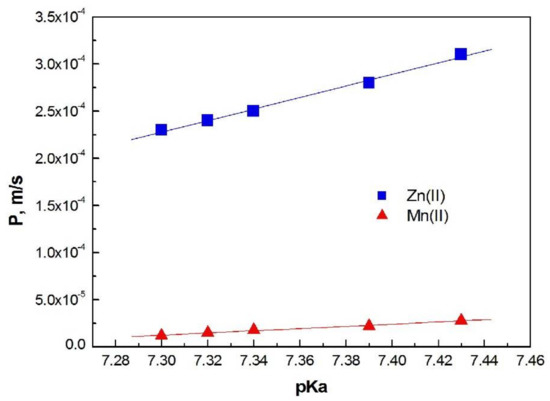
Figure 6.
The dependence of the pKa 1-alkylimidazole molecules vs. permeability coefficients (P) for Zn(II) and Mn(II) ions transported across PIMs.
For both ions, the permeability coefficients increase with an increase in the basicity of carrier molecules (increased pKa value); however, for Mn (II) ions this increase is smaller.
The values of permeability coefficient (P) increase linearly with increasing basicity of the carrier. A similar linear correlation for 1-alkylimidazoles was obtained in the case of chloride solutions [25]; this is in agreement with literature reports.
A PIM doped with 1-decyl-imidazole (5) is the most permeable for Zn(II) and Mn(II) ions. The membrane (5) also has the highest effective pore size (Table 2).
3.3. Recovery of Metal
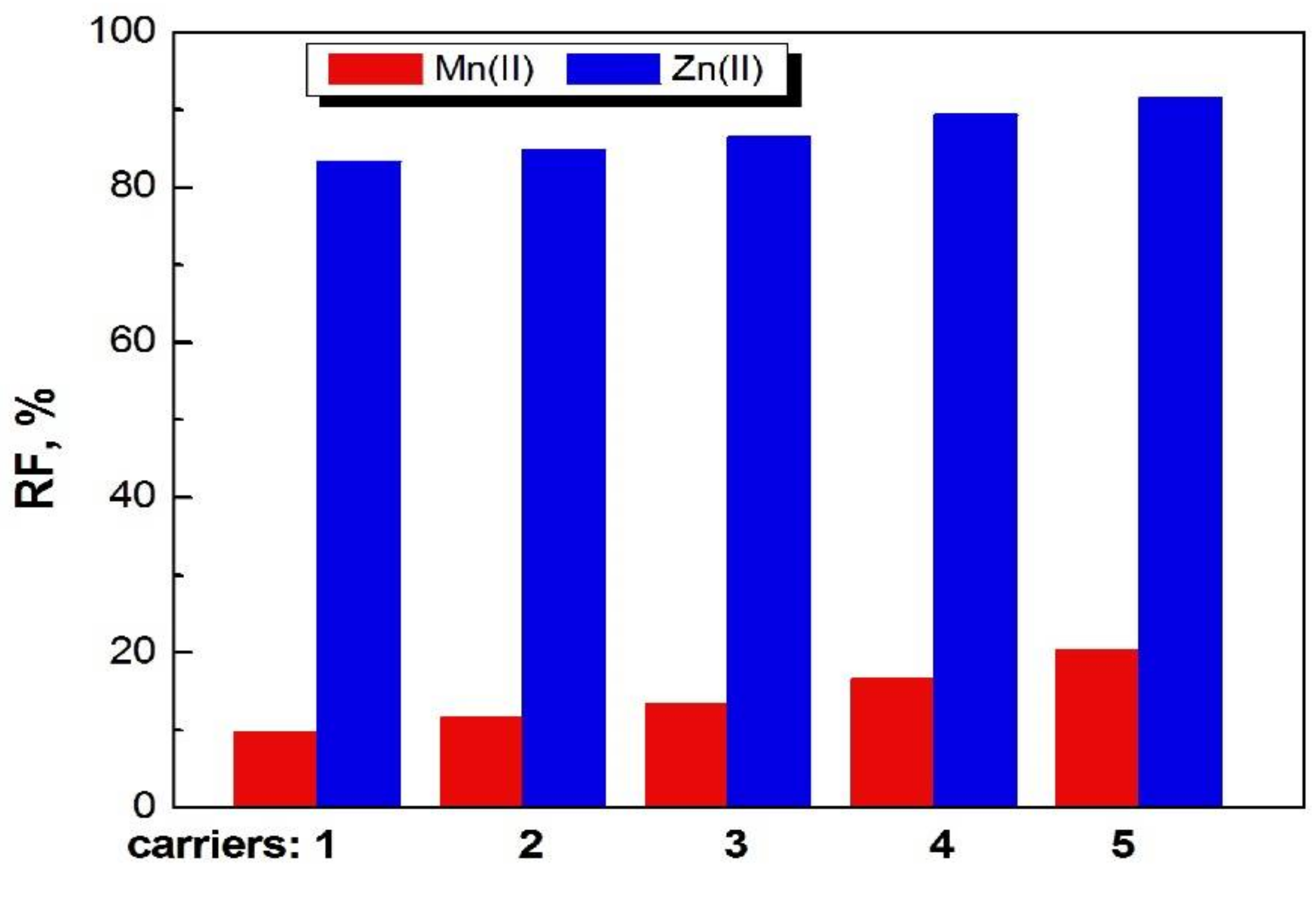
The recovery factors (RF) of Zn(II) and Mn(II) ions as a result of transport by PIMs doped with 1-alkylimidazoles from their equimolar sulphate solutions into deionized water is shown in Figure 7.
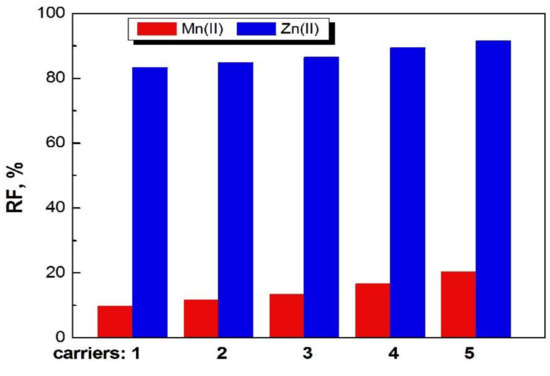
Figure 7.
Recovery factors of Mn(II) and Zn(II) ions from feed phase after 24 h in transport across PIM with 1–5 compounds at concentration 1.0 mol/dm3.
The recovery factors for both ions increase in the series: 1<2<3<4< 5.
In the case of Zn(II) ions, the recovery factors are much higher than for Mn(II) ions.
The best result for Zn(II) removal after 24 h was 92.5% for PIMs doped with 1-decyl-imidazole (5).
4. Conclusions
Zinc(II) ions can be effectively separated from equimolar aqueous solutions of zinc and manganese sulphates by using transport across polymer inclusion membranes doped with 1-alkylimidazoles (alkyl = hexyl, heptyl, octyl, nonyl, decyl). The initial fluxes of Zn(II) and Mn(II) ions increase with an increase in the basicity of carrier (1-alkylimidazole) molecules. The highest initial flux of Zn(II) ions (2.65 µmol/m2∙s) was found for PIMs doped with 1-decyl-imidazole, whereas the best Zn(II)/Mn(II) selectivity coefficients equal to 19.7 were found for 1-hexyl-imidazole.
The best result for Zn(II) removal after 24 h was 92.5% for PIMs doped with 1-decyl-imidazole. The microstructure of the membrane has an impact on the transport process. The roughness, the effective size of the pores and the tortuosity of the investigated membranes increase with an increase in the hydrophobicity of carrier molecules, so that the best membranes for Zn(II)-Mn(II) separation are PIMs doped with 1-decyl-imidazole, but for this carrier, the Zn(II)/Mn(II) separation coefficient is the lowest.
It can be assumed that strongly hydrophobic 1-alkylimidazoles (alkyl > decyl) can be more effective in the separation of zinc and manganese ions in membrane techniques.
Author Contributions
M.U. prepared the membranes, performed and analysed the transport studies. E.R-L. analysed the date of SEM, AFM and TG-DTG images of membranes. M.U. wrote introduction. E.R-L. discussed the results and conclusions.
Funding
The financial support of the Ministry of Science and Higher Education Republic of Poland (BS 13/2013) is gratefully acknowledged.
Acknowledgments
The research (SEM, AFM, TG-DTG) were carried out with the equipment-specialized analytical laboratories of the Poznan Science and Technology Park of the Adam Mickiewicz University Foundation in Poznan, Faculty of Chemistry of Nicolaus Copernicus University in Torun and the Faculty of Chemical Technology and Engineering of UTP University of Science and Technology in Bydgoszcz, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lead and Zinc Statistics. Available online: http://www.ilzsg.org/static/statistics.aspx (4 December 2018).
- Jafari, H.; Abdollahi, H.; Gharabaghi, M.; Balesini, A.A. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. Sep. Purif. Technol. 2018, 197, 210–219. [Google Scholar] [CrossRef]
- Cole, P.M.; Sole, K.C. Zinc solvent extraction in the process industries. Miner. Process. Extr. Metall. Rev. 2003, 24, 91–137. [Google Scholar] [CrossRef]
- Gotfryd, L.; Chmielarz, A.; Szołomicki, Z. Recovery of zinc from arduous wastes using solvent extraction technique. Part II. Pilot plant tests. Physicochem. Probl. Miner. Process. 2011, 47, 249–258. [Google Scholar]
- Charewicz, W.; Holowiecka, B.A.; Walkowiak, W. Selective flotation of zinc(II) and silver(I) ions from dilute aqueous solutions. Sep. Sci. Technol. 1999, 34, 2447–2460. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Bartsch, R.A. Ion Flotation of Zinc(II) and Cadmium(II) with Proton-Ionizable Lariat Ethers—The effect of cavity size. Sep. Purif. Technol. 2006, 48, 264–269. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Brandt, K.; Porwolik-Czomperlik, I. Ion flotation of zinc(II) and cadmium(II) in the presence of side-armed diphosphaza-16-crown-6ethers. Sep. Sci. Technol. 2003, 38, 633–645. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Ulewicz, M.; Walkowiak, W.; Girek, T.; Jablonska, J. The effect of tautomeric rearrangement on the separation of Zn(II) and Cd(II) in ion flotation process with 4-thiazolidinone derivatives. Miner. Eng. 2002, 15, 677–682. [Google Scholar] [CrossRef]
- Kozlowski, C.; Ulewicz, M.; Walkowiak, W. Separation of zinc and cadmium ions from aqueous chloride solutions by ion flotation and liquid membranes. Physicochem. Probl. Miner. Process. 2000, 34, 141–151. [Google Scholar]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E-S.Z. Removal of zinc ions from aqueous solution using a cation exchange resin. Chem. Eng. Res. Des. 2013, 91, 165–173. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V. Recovery of zinc from aqueous solutions by ion exchange process—A review. J. Metall. Mater. Sci. 2005, 47, 119–128. [Google Scholar]
- Sobianowska-Turek, A.; Ulewicz, M.; Sobianowska, K. Ion flotation and solvent sublation of zinc(II) and manganese(II) in the presence of proton-ionizable lariat ethers. Physicochem. Probl. Miner. Process. 2016, 52, 1048–1060. [Google Scholar] [CrossRef]
- Cheng, Q.; Dong, H. Solvent Sublation Using Dithizone as a Ligand for Determination of Trace Elements in Water Samples. Microchim. Acta 2005, 150, 59–65. [Google Scholar] [CrossRef]
- Dabek, L. Sorption of zinc ions from aqueous solutions on regenerated activated carbons. J. Hazard. Mater. 2003, 101, 191–201. [Google Scholar] [CrossRef]
- Kononova, O.N.; Kuzetsova, M.A.; Melnikov, A.M.; Karplyakova, N.S.; Kononov, Y.S. Sorption recovery of copper(II) and zinc(II) from aqueous chloride solutions. J. Serb. Chem. Soc. 2014, 79, 1037–1049. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Amado, F.D.R.; Bischoff, M.R.; Ferreira, C.A.; Bernardes, A.M.; Ferreira, J.Z. Transport of zinc complexes through an anion exchange membrane. Desalination 2008, 227, 241–252. [Google Scholar] [CrossRef]
- Gasser, M.S.; El-Hefny, N.E.; Rizk, S.E.; Daoud, J.A. Transfer and Separation of Zn(II)/Co(II) by Supported Liquid Membrane Containing CYANEX 925 in Kerosene as Carrier. J. Phys. Sci. 2013, 24, 63–81. [Google Scholar]
- Ulewicz, M.; Kozłowski, C.; Walkowiak, W. Removal of Zn(II), Cd(II) and Cu(II) ions by polymer inclusion membrane with side-armed diphosphaza-16-crown-6-ethers. Physicochem. Probl. Miner. Process 2004, 38, 131–138. [Google Scholar]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of 1-alkylimidzoles for selective separation of zinc ions in the transport process across a polymeric inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Kosciuszko, A.; Gierszewska, M.; Ziuziakowski, K. The influence of the morphology and mechanical properties of polymer inclusion membranes (PIMs) on zinc ion separation from aqueous solutions. Polymers 2018, 10, 134. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kozłowski, C.A.; Koziol, J.J. Transport of Zn(II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers. Sep. Purif. Technol. 2007, 57, 430–434. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Nowicki, M.; Wisniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 132, 42319–42329. [Google Scholar] [CrossRef]
- Ulewicz, M.; Sadowska, K.; Biernat, J.F. Selective transport of Pb(II) across polymer inclusion membrane using imidazole azocrown ethers as carriers. Physicochem. Probl. Miner. Process. 2007, 41, 133–143. [Google Scholar]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130. [Google Scholar]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer and supported membranes with 1-decyl-4-methylimidazole for pertraction of transition metal ions. Sep. Sci. Technol. 2014, 49, 1713–1721. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membrane (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. The application of membrane extraction in the separation of zinc and cadmium ions. Desalin. Water Treat. 2018, 128, 140–147. [Google Scholar] [CrossRef]
- Reddy, T.B. Linden’s Handbook of Batteries, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2011; ISBN 9780071624213. [Google Scholar]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Bernades, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Veloso, L.R.S.; Rodrigues, L.E.O.C.; Ferreira, D.A.; Magalhäes, F.S.; Mensur, M.B. Development of a hydrometallurgical route for the recovery of zinc and manganese from spent alkaline batteries. J. Power Sources 2005, 152, 295–302. [Google Scholar] [CrossRef]
- Bartsch, R.A.; Way, J.D. Chemical Separation with Liquid Membranes; ASC: Washington, DC, USA, 1996; ISBN 9780841234475. [Google Scholar]
- Bond, A.H.; Dietz, M.L.; Rogers, R.D. Metal-Ion Separation and Preconcentration; Progress and Opportunities; ACS: Washington, DC, USA, 1999; ISBN 0-8412-3594-5. [Google Scholar]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 44–451. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Proc. Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective transport of Cu(II) across a polymer inclusion membrane with 1-alkylimidazole from nitrate solutions. Sep. Sci. Technol 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Inês, M.; Almeida, G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci 2012, 415—416, 9–23. [Google Scholar] [CrossRef]
- Pernak, J.; Krysiski, J.; Skrzypczak, A. Bakterizide wirkung von iminiumverbindungen. Tenside Surfactants Deterg. 1987, 24, 276–286. [Google Scholar]
- Lenarcik, B.; Ojczenasz, P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid—Base properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Wolf, J.R.; Strieder, W. Surface and void tortuosities for a random fiber bed: Overlapping, parallel cylinders of several radii. J. Membr. Sci. 1990, 49, 103–115. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymer plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mater. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; San Miguel, E.R.; Muhammed, M.; Gyves, J. Transport characterization of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celikas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) thought polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Benosmane, N.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Selective transport of metal ions across polymer inclusion membranes containing calix[4]resorcinares. Sep. Purif. Technol. 2009, 65, 211–219. [Google Scholar] [CrossRef]
- Resina, M.; Macanas, J.; de Gyvesb, J.; Munoz, M. Development and characterization of hybrid membranes based on an organic matrix modified with silanes for metal separation. J. Membr. Sci. 2007, 289, 150–158. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).