Recycled-Oil-Based Polyurethane Modified with Organic Silicone for Controllable Release of Coated Fertilizer
Abstract
:1. Introduction
2. Experimental
2.1. Materials
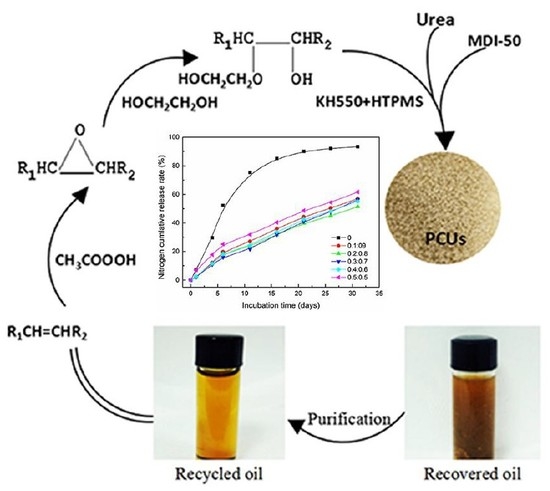
2.2. Purification of Recovered Oil
2.3. Premodification of Recycled Oil
2.4. Preparation of KH550/HTPMS Modified PCUs
2.5. Nitrogen Release from PCUs
2.6. Characterization
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6.2. Liquid Nuclear Magnetic Resonance (NMR) Spectrometer Analysis
2.6.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.6.4. Water Contact Angles (WCAs) Analysis
2.6.5. Scanning Electron Microscopy (SEM) Analysis
2.6.6. Determination of Water Permeability
2.6.7. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Structural Analysis of the PUs and the K-H-PUs
3.2. Surface Elemental Composition Analyses
3.3. Thermal Stability Analyses
3.4. Water Contact Angle
3.5. Macroscopic and Microscopic Morphology
3.6. Water Permeability of the Polymer Coating Films
3.7. Release Profiles of PCU
3.8. Surface Microscopic Morphology of Coated Materials before and after PCUs Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiang, Y.; Ji-Yun, J.; Ping, H.E.; Ming, Z.L. Recent Advances in Technology of Increasing Fertilizer Use Efficiency. Sci. Agric. Sin. 2008, 41, 450–459. [Google Scholar]
- Rashidzadeh, A.; Olad, A.; Reyhanitabar, A. Hydrogel/clinoptilolite nanocomposite-coated fertilizer: Swelling, water-retention and slow-release fertilizer properties. Polym. Bull. 2015, 72, 2667–2684. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.W.; Branham, B.E.; Mulvaney, R.L. Response of turfgrass to urea-based fertilizers formulated to reduce ammonia volatilization and nitrate conversion. Biol. Fertil. Soils 2013, 49, 51–60. [Google Scholar] [CrossRef]
- Salman, O.A.; Hovakeemian, G.; Khraishi, N. Polyethylene-coated urea. 2. Urea release as affected by coating material, soil type and temperature. Ind. Eng. Chem. Res. 1989, 28, 633–638. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation—A review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Choudhury, A.T.M.A.; Kennedy, I.R. Nitrogen Fertilizer Losses from Rice Soils and Control of Environmental Pollution Problems. Commun. Soil Sci. Plant Anal. 2005, 36, 1625–1639. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in controlled-release fertilizers. Adv. Agron. 2001, 71, 1–49. [Google Scholar]
- Zhao, C. Present situation and progress of research on slow/controlled release fertilize. Phosphate Compd. Fertil. 2007, 22, 14–16. [Google Scholar]
- Khan, S.; Hanjra, M.A. Footprints of water and energy inputs in food production global perspectives. Food Policy 2009, 34, 130–140. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Jarosiewicz, A. Use of polysulfone in controlled-release NPK fertilizer formulations. J. Agric. Food Chem. 2002, 50, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Liu, B.Y.; Yang, L.; Wang, T.J.; Kan, C.Y. Fabrication of graphene oxide/polymer latex composite film coated on KNO3 fertilizer to extend its release duration. Chem. Eng. J. 2017, 311, 318–325. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landon, J.R. Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Landevaluation in the Tropics and Subtropics; Routledge: Abingdon, UK, 2014. [Google Scholar]
- Lesturgez, G.; Poss, R.; Noble, A.; Grunberger, O.; Chintachao, W.; Tessier, D. Soil acidification without p H drop under intensivecropping systems in Northeast Thailand. Agric. Ecosyst. Environ. 2006, 114, 239–248. [Google Scholar] [CrossRef]
- Randall, P.J.; Abaidoo, R.C.; Hocking, P.J.; Sanginga, N. Mineral nutrient uptake and removal bycowpea, soybean and maize cultivars in West Africa, and implications for carbon cycleeffects on soil acidification. Exp. Agric. 2006, 42, 475–494. [Google Scholar] [CrossRef]
- Ma, W.J.; Meng, X.H.; Ma, Y.Y.; Wan, J.L. Synthesis and Properties of Cationic Surfactant Based on Trench Oil. Jiangxi Chem. Ind. 2018, 5, 126–129. [Google Scholar]
- Meng, X.H.; Ma, W.J.; Ma, Y.Y.; Wan, J.L. Study on Cleaning Agent for Industrial Production of Gutter Oil. Jiangxi Chem. Ind. 2018, 5, 157–160. [Google Scholar]
- Zhai, L.J.; Niu, Y.L.; Jia, J.J.; Chen, L.J.; Li, G.F. Study on Preparation of Biodiesel from Waste Oil by Sulfonation of Activated Carbon. Guangdong Chem. Ind. 2018, 45, 40–41, 43. [Google Scholar]
- Chen, W.H.; Xie, X.; Li, Y. Preparation of biodiesel from gutter oil catalyzed by solid superacid . Shandong Chem. 2018, 47, 3–4. [Google Scholar]
- Li, Y.; Liu, W.; Ma, Y.; Gao, Y.; Yang, X. Preparation of Super Absorbent Polymer Utilizing Corn Husks. Asian J. Chem. 2014, 26, 5268–5270. [Google Scholar] [CrossRef]
- Sun, W.; Ouyang, K.; Zhang, L.; Hu, Y.; Chen, C. Preparation of hydrolyzate of hogwash oil (HHO) and its application in separating diaspore from kaolinite. Miner. Eng. 2010, 23, 670–675. [Google Scholar] [CrossRef]
- Bin, Z.; Wei, Q.L. The Synthesis and Properties of Organic Silicon-Modified Epoxy Resin. Guangzhou Chem. 2002, 27, 6–9. [Google Scholar]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Mahdavi, H.; Kamyabi, A.; Shahalizade, T.; Taheri, H.A. Preparation of highly flexible cellulose acetate membranes modified by hyperbranched poly(amine ester)-epoxidized soybean oil and evaluation of its filtration properties. Cellulose 2017, 24, 5389–5402. [Google Scholar] [CrossRef]
- Mathew, A.; Kurmvanshi, S.; Mohanty, S.; Nayak, S.K. Mechanical Behavior of Castor-Oil-Based Advanced Polyurethane Functionalized with Glycidol and Siloxanes. JOM 2017, 69, 2501–2507. [Google Scholar] [CrossRef]
- Yim, Y.-J.; Rhee, K.Y.; Park, S.-J. Fracture toughness and ductile characteristics of diglycidyl ether of bisphenol-A resins modified with biodegradable epoxidized linseed oil. Compos. Part B Eng. 2017, 131, 144–152. [Google Scholar] [CrossRef]
- Oertli, J.J.; Lunt, O.R. Controlled Release of Fertilizer Minerals by Incapsulating Membranes: I Factors Influencing the Rate of Release. Soil Sci. Soc. Am. J. 1962, 26, 579–583. [Google Scholar] [CrossRef]
- Lu, J. Studies on the Preparation and Nutrient Release Characteristics of Low Molecular Weight Polylactic Acid Coated Urea. Master’s Thesis, Zhejiang University, Hangzhou, China, 2011. [Google Scholar]
- Liu, X.; Yang, Y.; Gao, B.; Li, Y. Organic silicone-modified transgenic soybean oil as bio-based coating material for controlled-release urea fertilizers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Peng, J.; Liu, L. The filler-rubber interface and reinforcement in styrene butadiene rubber composites with graphene/silica hybrids: A quantitative correlation with the constrained region. Compos. Part A Appl. Sci. Manuf. 2016, 86, 19–30. [Google Scholar] [CrossRef]
- Qing, S.A.; Dong, M.X.; Xin, L.F.; Yuan, K.L.; Zhong, W.W.; Hong, C. Analysis on Formula Raw Materials and Their Proportions of Polyurethane Elastomer by IR and 1H-NMR. Plast. Technol. 2016, 44, 39–43. [Google Scholar]
- Ma, X.; Chen, J.; Yang, Y.; Su, X.; Zhang, S.; Gao, B.; Li, Y.C. Siloxane and polyether dual modification improves hydrophobicity and interpenetrating polymer network of bio-polymer for coated fertilizers with enhanced slow release characteristics. Chem. Eng. J. 2018, 350, 1125–1134. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, G.K.; Modi, O.P.; Prasad, B.K.; Gupta, A.K. Titanium foam through powder metallurgy route using acicular urea particles as space holder. Mater. Lett. 2011, 65, 3199–3201. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Ribeiro, C.; Polito, W.L. Controlled release of nitrogen-source fertilizers by natural-oil-based poly(urethane) coatings: The kinetic aspects of urea release. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Y.Z.; Luo, H.Y.; Jiang, M.; Zhang, Q.P.; Xie, L. Synthese of degradable polurethane suing recycled-oil and the optimization of parameters for preparation of coated urea. Journal of Plant Nutrition and Fertilizer. 2018, pp. 1–8. Available online: http://kns.cnki.net/kcms/detail/11.3996.S.20181123.1722.002.html (accessed on 3 January 2018).















| Sample | Chemical Composition/% | Atom Ratio/% | |||||
|---|---|---|---|---|---|---|---|
| C | O | N | Si | O/C | N/C | Si/C | |
| PUs | 82.56 | 14.68 | 2.76 | - | 17.78 | 3.34 | - |
| K-H-PUs | 69.64 | 16.92 | 3.93 | 9.51 | 24.30 | 5.64 | 13.66 |
| PUs | K-H-PUs | ||||
|---|---|---|---|---|---|
| Linkage | Binding Energy (eV) | Atomic % | Linkage | Binding Energy (eV) | Atomic % |
| C–C | 284.80 | 43.70 | C–C | 284.80 | 44.62 |
| C–N | 286.17 | 14.80 | C–N | 286.15 | 16.05 |
| C–O | 284.20 | 28.97 | C–O | 284.21 | 24.55 |
| –COO | 288.52 | 12.53 | –COO | 289.13 | 14.78 |
| KH550:HTMS | ||||
|---|---|---|---|---|
| Content (%) | T5% (°C) | T50% (°C) | Tmax1 (°C) | Tmax2 (°C) |
| 0.1:0.9 | 188.4 | 243.4 | 244.7 | 356.7 |
| 0.2:0.8 | 195.7 | 258.7 | 248.7 | 358.7 |
| 0.3:0.7 | 190.6 | 284.6 | 241.6 | 359.6 |
| 0.4:0.6 | 190.7 | 246.7 | 242.4 | 371.4 |
| 0.5:0.5 | 192.6 | 327.6 | 238.6 | 354.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Dong, F.; Dai, J.; Zhang, Q.; Jiang, M.; Xiong, Y. Recycled-Oil-Based Polyurethane Modified with Organic Silicone for Controllable Release of Coated Fertilizer. Polymers 2019, 11, 454. https://doi.org/10.3390/polym11030454
Wang Q, Dong F, Dai J, Zhang Q, Jiang M, Xiong Y. Recycled-Oil-Based Polyurethane Modified with Organic Silicone for Controllable Release of Coated Fertilizer. Polymers. 2019; 11(3):454. https://doi.org/10.3390/polym11030454
Chicago/Turabian StyleWang, Qian, Fuping Dong, Jun Dai, Qingpo Zhang, Meng Jiang, and Yuzhu Xiong. 2019. "Recycled-Oil-Based Polyurethane Modified with Organic Silicone for Controllable Release of Coated Fertilizer" Polymers 11, no. 3: 454. https://doi.org/10.3390/polym11030454
APA StyleWang, Q., Dong, F., Dai, J., Zhang, Q., Jiang, M., & Xiong, Y. (2019). Recycled-Oil-Based Polyurethane Modified with Organic Silicone for Controllable Release of Coated Fertilizer. Polymers, 11(3), 454. https://doi.org/10.3390/polym11030454