Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Composite Materials
2.3. Characterizations of the Composite Materials
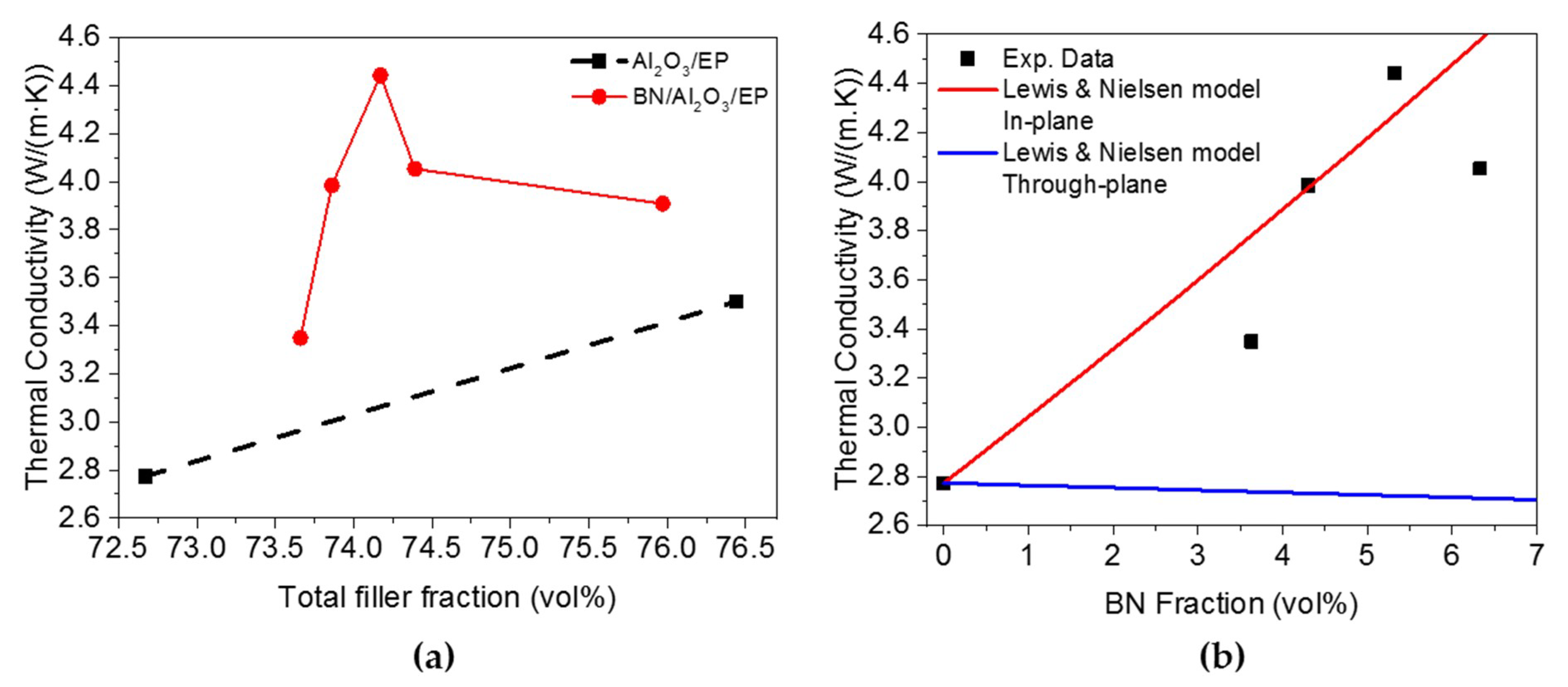
3. Results and Discussion
3.1. Characteristics of Fillers
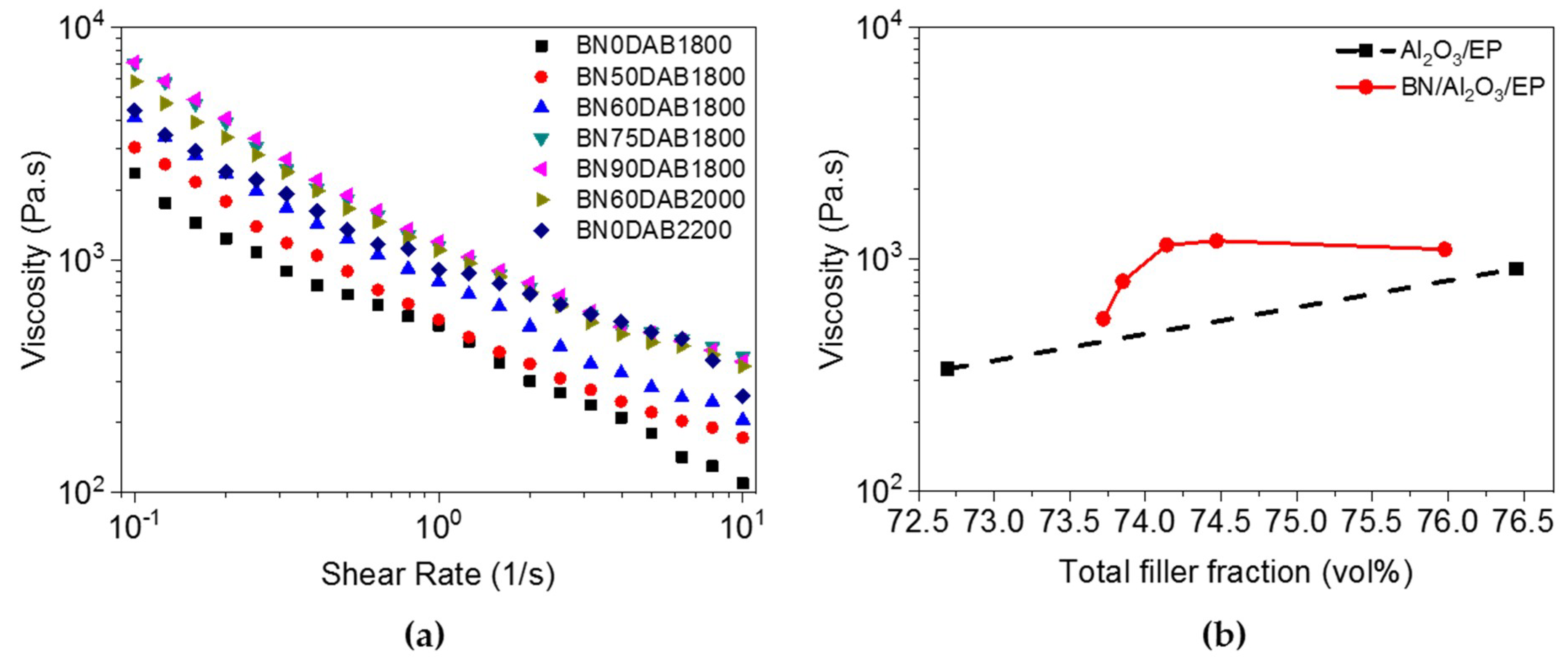
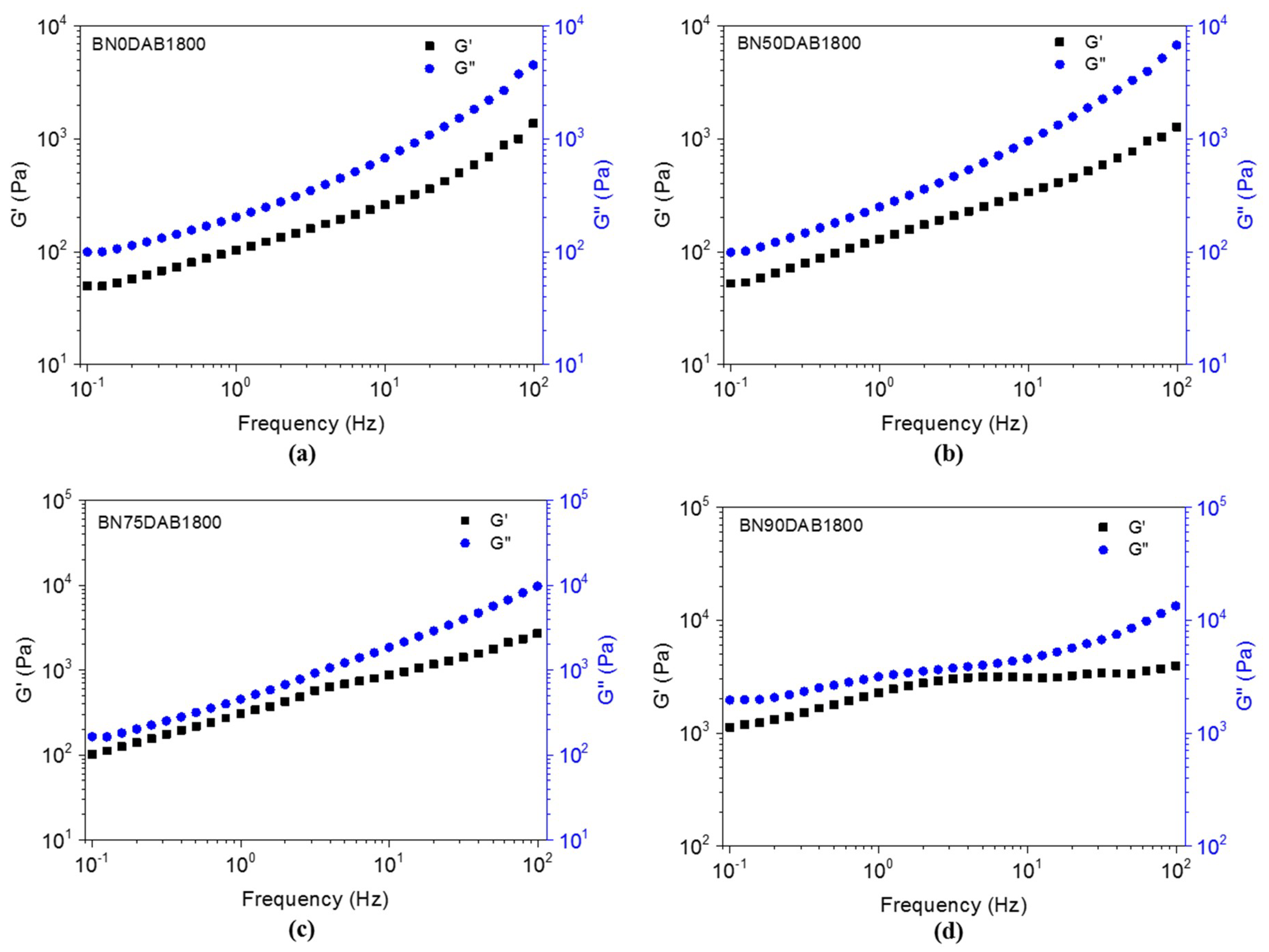
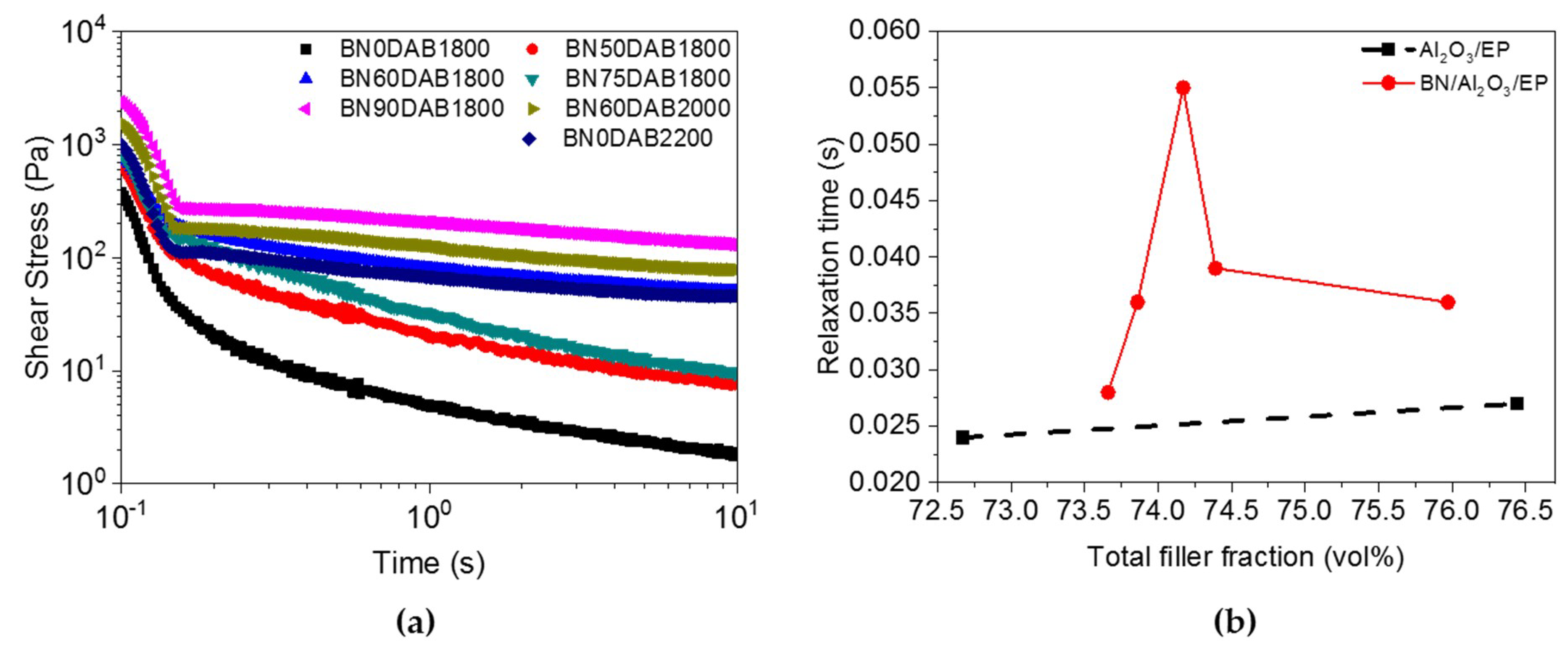
3.2. Rheological Properties of the Composite Materials before Cure
3.3. Morphology of the Composite Materials
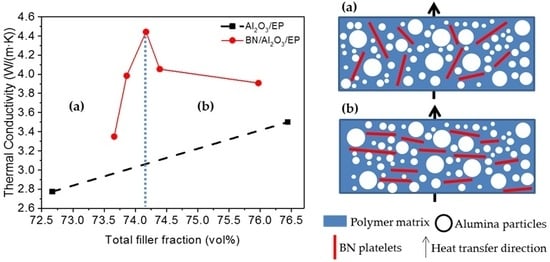
3.4. Thermal Conductivity of the Composite Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ho, T.H.; Wang, C.S. Modification of epoxy resin with siloxane containing phenol aralkyl epoxy resin for electronic encapsulation application. Eur. Polym. J. 2001, 37, 267–274. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polymer 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Tai, H.J.; Wang, J.B.; Chen, J.H.; Chou, H.L. Synthesis and properties of vinyl siloxane modified cresol novolac epoxy for electronic encapsulation. J. Appl. Polym. Sci. 2001, 79, 652–661. [Google Scholar] [CrossRef]
- Wang, C.S.; Mendoza, A. 2,6-dibromo-3,5-dimethyl-4-hydroxybenzyl ether and epoxy systems—Their application in electronic packaging. Polym. Bull. 1991, 25, 279–286. [Google Scholar] [CrossRef]
- Dueramae, I.; Jubsilp, C.; Takeichi, T.; Rimdusit, S. High thermal and mechanical properties enhancement obtained in highly filled polybenzoxazine nanocomposites with fumed silica. Compos. Part B Eng. 2014, 56, 197–206. [Google Scholar] [CrossRef]
- Heo, G.Y.; Park, S.J. Effect of coupling agents on thermal, flow, and adhesion properties of epoxy/silica compounds for capillary underfill applications. Powder Technol. 2012, 230, 145–150. [Google Scholar] [CrossRef]
- Ng, F.C.; Abas, A.; Gan, Z.L.; Abdullah, M.Z.; Ani, F.C.; Ali, M.Y.T. Discrete phase method study of ball grid array underfill process using nano-silica filler-reinforced composite-encapsulant with varying filler loadings. Microelectron. Reliab. 2017, 72, 45–64. [Google Scholar] [CrossRef]
- Weltevreden, E.R.; Tesarski, S.J.; Wymyslowski, A.; Erinc, M.; Gielen, A.W.J. A multi-scale approach of the thermo-mechanical properties of silica-filled epoxies used in electronic packaging. Microelectron. Reliab. 2012, 52, 1300–1305. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, Z.Q.; Wong, C.P. Study and characterization on the nanocomposite underfill for flip chip applications. IEEE Trans. Compon. Packag. Technol. 2006, 29, 190–197. [Google Scholar] [CrossRef]
- Teh, P.L.; Mariatti, M.; Wagiman, A.N.R.; Beh, K.S. Effect of curing agent on the properties of mineral silica filled epoxy composites. Polym. Compos. 2008, 29, 27–36. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Effect of Al2O3 addition on thermo-electrical properties of polymer composites: An experimental investigation. Polym. Compos. 2015, 36, 102–112. [Google Scholar] [CrossRef]
- Jeong, U.S.; Lee, Y.J.; Shin, D.G.; Lim, H.M.; Mun, S.Y.; Kwon, W.T.; Kim, S.R.; Kim, Y.H.; Shim, K.B. Highly thermal conductive alumina plate/epoxy composite for electronic packaging. Trans. Electr. Electron. Mater. 2015, 16, 351–354. [Google Scholar] [CrossRef]
- Kim, D.J.; Kang, P.H.; Nho, Y.C. Characterization of mechanical properties of gamma Al2O3 dispersed epoxy resin cured by gamma-ray radiation. J. Appl. Polym. Sci. 2004, 91, 1898–1903. [Google Scholar]
- McGrath, L.M.; Parnas, R.S.; King, S.H.; Schroeder, J.L.; Fischer, D.A.; Lenhart, J.L. Investigation of the thermal, mechanical, and fracture properties of alumina-epoxy composites. Polymer 2008, 49, 999–1014. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, S.M.; Shanefield, D.J.; Cannon, W.R. Enhanced thermal conductivity of polymer matrix composite via high solids loading of aluminum nitride in epoxy resin. J. Am. Ceram. Soc. 2008, 91, 1169–1174. [Google Scholar] [CrossRef]
- Yu, S.Z.; Hing, P.; Hu, X. Thermal conductivity of polystyrene-aluminum nitride composite. Compos. Part A Appl. Sci. Manuf. 2002, 33, 289–292. [Google Scholar] [CrossRef]
- Xu, Y.S.; Chung, D.D.L.; Mroz, C. Thermally conducting aluminum nitride polymer-matrix composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1749–1757. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Wang, H.; Wang, L.; Yu, K.; Lin, Z.D.; He, L.; Bai, Y.Y. Fabrication and characterization of aluminum nitride polymer matrix composites with high thermal conductivity and low dielectric constant for electronic packaging. Mater. Sci. Eng. B 2012, 177, 892–896. [Google Scholar] [CrossRef]
- Isarn, I.; Massagues, L.; Ramis, X.; Serra, A.; Ferrando, F. New BN-epoxy composites obtained by thermal latent cationic curing with enhanced thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2017, 103, 35–47. [Google Scholar] [CrossRef]
- Donnay, M.; Tzavalas, S.; Logakis, E. Boron nitride filled epoxy with improved thermal conductivity and dielectric breakdown strength. Compos. Sci. Technol. 2015, 110, 152–158. [Google Scholar] [CrossRef]
- Du, B.X.; Xiao, M.; Zhang, J.W. Effect of thermal conductivity on tracking failure of epoxy/BN composite under pulse strength. In Proceedings of the IEEE International Conference on Solid Dielectrics, Bologna, Italy, 30 June–4 July 2013; pp. 607–610. [Google Scholar] [CrossRef]
- Wang, Z.B.; Iizuka, T.; Kozako, M.; Ohki, Y.; Tanaka, T. Development of epoxy/BN composites with high thermal conductivity and sufficient dielectric breakdown strength part I -Sample Preparations and Thermal Conductivity. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1963–1972. [Google Scholar] [CrossRef]
- Xiao, M.; Du, B.X. Effects of high thermal conductivity on temperature rise of epoxy cast windings for power transformer. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2413–2420. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Mcnamara, A.; Liu, Y.; Moon, K.S.; Wong, C.P. Exfoliated hexagonal boron nitride-based polymer nanocomposite with enhanced thermal conductivity for electronic encapsulation. Compos. Sci. Technol. 2014, 90, 123–128. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of bn-filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Acocella, M.R.; Corcione, C.E.; Giuri, A.; Maggio, M.; Guerra, G.; Maffezzoli, A. Catalytic activity of oxidized carbon black and graphene oxide for the crosslinking of epoxy resins. Polymers 2017, 9, 133. [Google Scholar] [CrossRef]
- Corcione, C.E.; Maffezzoli, A. Transport properties of graphite/epoxy composites: Thermal, permeability and dielectric characterization. Polym. Test. 2013, 32, 880–888. [Google Scholar] [CrossRef]
- Acocella, M.R.; Corcione, C.E.; Giuri, A.; Maggio, M.; Maffezzoli, A.; Guerra, G. Graphene oxide as a catalyst for ring opening reactions in amine crosslinking of epoxy resins. RSC Adv. 2016, 6, 23858–23865. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.J.; Ye, Y.S.; Xue, Z.G.; Xue, Y.; Xie, X.L.; Mai, Y.W. High-performance epoxy/silica coated silver nanowire composites as underfill material for electronic packaging. Compos. Sci. Technol. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Zha, J.W.; Zhu, T.X.; Wu, Y.H.; Wang, S.J.; Li, R.K.Y.; Dang, Z.M. Tuning of thermal and dielectric properties for epoxy composites filled with electrospun alumina fibers and graphene nanoplatelets through hybridization. J. Mater. Chem. C 2015, 3, 7195–7202. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with a binary-particle system of aluminum oxide and aluminum nitride fillers. Compos. Part B Eng. 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Sanada, K.; Tada, Y.; Shindo, Y. Thermal conductivity of polymer composites with close-packed structure of nano and micro fillers. Compos. Part A Appl. Sci. Manuf. 2009, 40, 724–730. [Google Scholar] [CrossRef]
- Wetzel, B.; Haupert, F.; Zhang, M.Q. Epoxy nanocomposites with high mechanical and tribological performance. Compos. Sci. Technol. 2003, 63, 2055–2067. [Google Scholar] [CrossRef]
- Hong, J.P.; Yoon, S.W.; Hwang, T.; Oh, J.S.; Hong, S.C.; Lee, Y.; Nam, J.D. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.H.; Li, W.Z.; Wang, Q.W.; Datye, A.; Wu, K.H. Thermal properties of CNT-Alumina nanocomposites. Compos. Sci. Technol. 2008, 68, 2178–2183. [Google Scholar] [CrossRef]
- Lee, G.W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Part A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.Q.; Yin, L.Q.; Zhao, J.C.; Xia, L.G.; Chen, L.F. Exceptionally high thermal conductivity of thermal grease: Synergistic effects of graphene and alumina. Int. J. Therm. Sci. 2015, 91, 76–82. [Google Scholar] [CrossRef]
- Su, J.L.; Xiao, Y.; Ren, M. Enhanced thermal conductivity in epoxy nanocomposites with hybrid boron nitride nanotubes and nanosheets. Phys. Status Solidi A 2013, 210, 2699–2705. [Google Scholar] [CrossRef]
- Sichel, E.K.; Miller, R.E.; Abrahams, M.S.; Buiocchi, C.J. Heat-capacity and thermal-conductivity of hexagonal pyrolytic boron-nitride. Phys. Rev. B 1976, 13, 4607–4611. [Google Scholar] [CrossRef]
- Yu, C.P.; Zhang, J.; Li, Z.; Tian, W.; Wang, L.J.; Luo, J.; Li, Q.L.; Fan, X.D.; Yao, Y.G. Enhanced through-plane thermal conductivity of boron nitride/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 25–31. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef]
- Kuang, Z.Q.; Chen, Y.L.; Lu, Y.L.; Liu, L.; Hu, S.; Wen, S.P.; Mao, Y.Y.; Zhang, L.Q. Fabrication of highly oriented hexagonal boron nitride nanosheet/elastomer nanocomposites with high thermal conductivity. Small 2015, 11, 1655–1659. [Google Scholar] [CrossRef]
- Cho, H.B.; Tokoi, Y.; Tanaka, S.; Suematsu, H.; Suzuki, T.; Jiang, W.H.; Niihara, K.; Nakayama, T. Modification of BN nanosheets and their thermal conducting properties in nanocomposite film with polysiloxane according to the orientation of BN. Compos. Sci. Technol. 2011, 71, 1046–1052. [Google Scholar] [CrossRef]
- Lim, H.S.; Oh, J.W.; Kim, S.Y.; Yoo, M.J.; Park, S.D.; Lee, W.S. Anisotropically alignable magnetic boron nitride platelets decorated with iron oxide nanoparticles. Chem. Mater. 2013, 25, 3315–3319. [Google Scholar] [CrossRef]
- Yuan, C.; Duan, B.; Li, L.; Xie, B.; Huang, M.Y.; Luo, X.B. Thermal conductivity of polymer-based composites with magnetic aligned hexagonal boron nitride platelets. ACS Appl. Mater. Interfaces 2015, 7, 13000–13006. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Liu, Y.; Raghavan, S.; Moon, K.S.; Sitaraman, S.K.; Wong, C.P. Magnetic alignment of hexagonal boron nitride platelets in polymer matrix: Toward high performance anisotropic polymer composites for electronic encapsulation. ACS Appl. Mater. Interfaces 2013, 5, 7633–7640. [Google Scholar] [CrossRef]
- Farris, R.J. Prediction of the viscosity of multimodal suspensions from unimodal viscosity data. Trans. Soc. Rheol. 1968, 12, 281–301. [Google Scholar] [CrossRef]
- Han, C.D. Rheology and Processing of Polymeric Materials; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Kumlutas, D.; Tavman, I.H.; Coban, M.T. Thermal conductivity of particle filled polyethylene composite materials. Compos. Sci. Technol. 2003, 63, 113–117. [Google Scholar] [CrossRef]
- Hill, R.F.; Supancic, P.H. Thermal conductivity of platelet-filled polymer composites. J. Am. Ceram. Soc. 2002, 85, 851–857. [Google Scholar] [CrossRef]




| Sample Codes | YD-128 | HMPA | KBM-303 | EMI | Alumina | BN | Total Filler |
|---|---|---|---|---|---|---|---|
| BN0DAB1800 | 13.34 | 12.18 | 1.50 | 0.29 | 72.69 | 0 | 72.69 |
| BN50DAB1800 | 12.86 | 11.71 | 1.43 | 0.28 | 70.09 | 3.63 | 73.72 |
| BN60DAB1800 | 12.76 | 11.67 | 1.44 | 0.28 | 69.55 | 4.30 | 73.85 |
| BN75DAB1800 | 12.61 | 11.52 | 1.45 | 0.28 | 68.82 | 5.32 | 74.14 |
| BN90DAB1800 | 12.50 | 11.37 | 1.40 | 0.27 | 68.14 | 6.33 | 74.47 |
| BN60DAB2000 | 11.89 | 10.82 | 1.32 | 0.25 | 71.96 | 4.02 | 75.98 |
| BN0DAB2200 | 11.48 | 10.52 | 1.30 | 0.25 | 76.45 | 0 | 76.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, V.-D.; Lee, D.-I.; Park, J.-H.; Lee, D.-S. Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride. Polymers 2019, 11, 597. https://doi.org/10.3390/polym11040597
Mai V-D, Lee D-I, Park J-H, Lee D-S. Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride. Polymers. 2019; 11(4):597. https://doi.org/10.3390/polym11040597
Chicago/Turabian StyleMai, Van-Dung, Dae-Il Lee, Jun-Hong Park, and Dai-Soo Lee. 2019. "Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride" Polymers 11, no. 4: 597. https://doi.org/10.3390/polym11040597
APA StyleMai, V.-D., Lee, D.-I., Park, J.-H., & Lee, D.-S. (2019). Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride. Polymers, 11(4), 597. https://doi.org/10.3390/polym11040597