Modification of Montmorillonite with Polyethylene Oxide and Its Use as Support for Pd0 Nanoparticle Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
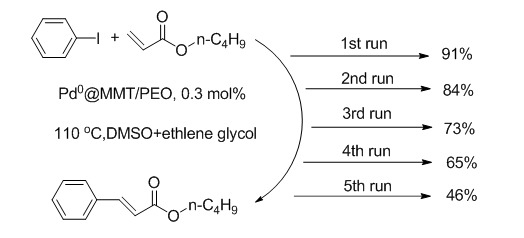
2.4. Catalytic Test
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Zampori, L.; Dotelli, G.; Stampino, P.G.; Cristiani, C.; Zorzi, F.; Finocchio, E. Thermal characterization of a montmorillonite, modified with polyethylene-glycols (PEG1500 and PEG4000), by in situ HT-XRD and FT IR: Formation of a high-temperature phase. Appl. Clay Sci. 2012, 59–60, 140–147. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Simon, G.P.; Cheng, Y.-B. Comparison of solution intercalation and melt intercalation of polymer-clay nanocomposites. Polymer 2002, 43, 4251–4260. [Google Scholar] [CrossRef]
- Strawhecker, K.E.; Manias, E. Structure and properties of poly (vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem. Mater. 2000, 12, 2943–2949. [Google Scholar] [CrossRef]
- Papp, S.; Patakfalvi, R.; Dekany, I. Metal nanoparticle formation on layer silicate lamellae. Colloid Polym. Sci. 2008, 286, 3–14. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Meirelles, L.M.A.; Raffin, F.N. Clay and polymer-based composites applied to drug release: A scientific and technological prospection. J. Pharm. Pharm. Sci. 2017, 20, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Floody, M.C.; Theng, B.K.G.; Reyes, P.; Mora, M.L. Natural nanoclays: Applications and future trends-chilean perspective. Clay Miner. 2009, 44, 161–176. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Louvel, B.; Dufrenoy, P.; Rigo, B.; Daich, A.; Waterlot, C. From conventional lewis acids to heterogeneous montmorillonite K10: Eco-friendly plant-based catalysts used as green lewis acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Atarod, M.; Alizadeh, M.; Hatamifard, A.; Sajadi, S.M. Recent advances in the application of heterogeneous nanocaalysts for Sonogashira coupling reactions. Curr. Org. Chem. 2017, 21, 708–749. [Google Scholar] [CrossRef]
- Heirati, S.Z.D.; Shirini, F.; Shojaei, A.F. Sulfonated PEG-intercalated montmorillonite [(Mt/PEG)-SO3H] as efficient and ecofriendly nanocatalyst for synthesis of alpha, alpha′-bis(substituted benzylidene) cycloalkanones. Res. Chem. Intermediat. 2017, 43, 6167–6186. [Google Scholar] [CrossRef]
- Kar, P.; Nayak, A.; Bhoi, Y.P.; Mishra, B.G. Preparation and catalytic application of sulfonated PVA-Zr-pillared clay nanocomposite materials towards one pot synthesis of hexahydropyrimidines. Microporous Mesoporous Mater. 2016, 223, 176–186. [Google Scholar] [CrossRef]
- Senarathna, K.G.C.; Randiligama, H.M.S.P.; Rajapakse, R.M.G. Preparation, characterization and oxygen reduction catalytic activities of nanocomposites of Co(II)/montmorillonite containing polypyrrole, polyaniline or poly(ethylenedioxythiophene). RSC Adv. 2016, 6, 112853–112863. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.L.; Zhou, R.; Long, M.D.; Liu, Y.F. Fabrication and application of porous Pd@Mt/PC composite as an efficient green heterogeneous catalyst for Suzuki cross-coupling reaction. Micro Nano Lett. 2018, 13, 969–973. [Google Scholar]
- Xu, M.D.; Zhao, J.; Shu, G.Q.; Liu, Q.; Zeng, M.F. Heterogeneous catalytic composites from palladium nanoparticles in montmorillonite intercalated with poly (vinyl pyrrolidone) chains. Polymers 2018, 10, 669. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, M.D.; Shu, G.Q.; Yang, Z.; Liu, Q.; Zeng, M.F.; Qi, C.Z.; Cao, X.Z.; Wang, B.Y. Positron annihilation characteristics and catalytic performances of poly (vinyl alcohol) intercalated montmorillonite supported Pd0 nanoparticles composites. Radiat. Phys.Chem. 2018, 153, 164–172. [Google Scholar] [CrossRef]
- Zeng, M.F.; Wang, Y.D.; Liu, Q.; Yuan, X.; Zuo, S.F.; Feng, R.K.; Yang, J.; Wang, B.Y.; Qi, C.Z.; Lin, Y. Encaging palladium nanoparticles in chitosan modified montmorillonite for efficient, recyclable catalysts. ACS Appl. Mater. Interfaces 2016, 8, 33157–33164. [Google Scholar] [CrossRef]
- Zampori, L.; Stampino, P.G.; Cristiani, C.; Cazzola, P.; Dotelli, G. Intercalation of poly (ethylene-oxides) in montmorillonite: Tailor-made nanocontainers for drug delivery systems. Appl. Clay Sci. 2010, 50, 266–270. [Google Scholar] [CrossRef]
- Aranda, P.; Ruiz-Hitzky, E. Poly (ethylene oxide)-silicate intercalation materials. Chem. Mater. 1992, 4, 1395–1403. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Backhouse, T.; Abood, E.; Alshaikh, H.; Clemmet, A.R.; Ellison, J.R.; Bourne, R.A.; Chamberlain, T.W.; Stones, R.; Warren, N.J.; Fairlamb, I.J.S.; Lovelock, K.R.J. Heteroatom donor-decorated polymer-immobilized ionic liquid stabilized palladium nanoparticles: efficient catalysts for room-temperature Suzuki-Miyaura cross-coupling in aqueous media. Adv. Syn. Catal. 2018, 360, 3716–3731. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Zamanbekova, A.T.; Darmenbayeva, A.S.; Auyezkhanova, A.S.; Jumekeyeva, A.I.; Talgatov, E.T. Effect of polymers on the formation of nanosized palladium catalysts and their activity and selectivity in the hydrogenation of acetylenic alcohols. Theor. Exp. Chem. 2017, 53, 265–269. [Google Scholar] [CrossRef]
- Pires, M.J.D.; Purifição, S.I.; Santos, A.S.; Marques, M.M.B. The role of PEG on Pd- and Cu-catalyzed cross-coupling reactions. Synthesis 2017, 49, 2337–2350. [Google Scholar]
- Iranpoor, N.; Firouzabadi, H.; Riazi, A.; Shakerpoor, A. Phosphorylated PEG (PPEG) as a new support for generation of nano-Pd(0): Application to the Heck–Mizoroki and Suzuki–Miyaura coupling reactions. Appl. Organomet. Chem. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Karami, K.; Moghadam, Z.K.; Hosseini-Kharat, M. Polyethylene glycol-supported recyclable NC palladacycle catalyst for Heck cross-coupling reactions. Catal. Commun. 2014, 43, 25–28. [Google Scholar] [CrossRef]
- Zeng, M.F.; Qi, C.Z.; Yang, J.; Wang, B.Y.; Zhang, X.-M. A highly efficient and stable palladium catalyst entrapped within the cross-linked chitosan membrane for Heck reactions. Ind. Eng. Chem. Res. 2014, 53, 10041–10050. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P. Polymer-salt intercalation complexes in layer silicates. Adv. Mater. 1990, 2, 545–547. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.P.; Chen, J.Y.; Li, H.L.; Cao, Y. Structure and conformation of poly(ethylene glycol) in confined space of montmorillonite. Appl. Surf. Sci. 2013, 264, 500–506. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Mullenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Waltham, MN, USA, 1979. [Google Scholar]
- Joshi, J.M.; Sodaye, H.S.; Pujari, P.K.; Srisaila, S.; Bajpai, M.B. Positron annihilation spectroscopic investigation of Al-pillared montmorillonites. Catal. Lett. 1998, 51, 109–112. [Google Scholar] [CrossRef]
- Sano, M.; Murakami, H.; Ichimura, K. Positronium in a layered-structure material: Montmorillonite. J. Radioanal. Nucl. Chem. 1999, 239, 325–328. [Google Scholar] [CrossRef]
- Consolati, G.; Natali-Sora, I.; Pelosato, R.; Quasso, F. Investigation of cation-exchanged montmorillonites by combined X-ray diffraction and positron annihilation lifetime spectroscopy. J. Appl. Phys. 2002, 91, 1928–1932. [Google Scholar] [CrossRef]
- Jasińska, B.; Koziol, A.E.; Goworek, T. Voids shape and o-Ps lifetime in molecular crystals. Acta Phys. Pol. A 1999, 95, 557–561. [Google Scholar] [CrossRef]
- Tao, S.J. Positronium annihilation in molecular substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature-dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Bradshaw, M.; Zou, J.L.; Byrne, L.; Iyer, K.S.; Stewart, S.G.; Raston, C.L. Pd(II) conjugated chitosan nanofibre mats for application in Heck cross-coupling reactions. Chem. Commun. 2011, 47, 12292–12294. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; You, H.H.; Liu, W.Y.; Lu, J.; Lu, P.; Dong, J. Pd nanoparticles encaged in nanoporous interpenetrating polymer networks: A robust recyclable catalyst for Heck reactions. React. Funct. Polym. 2011, 71, 756–765. [Google Scholar] [CrossRef]






| Sample | 2θ (°) | d001 Spacing (nm) | Interlayer Spacing (nm) |
|---|---|---|---|
| A. MMT | 7.02 | 1.25 | 0.29 |
| B. MMT/PEO (90/10) | 6.15 | 1.44 | 0.48 |
| C. MMT/PEO (80/20) | 5.78 | 1.52 | 0.56 |
| D. MMT/PEO (70/30) | 5.60 | 1.58 | 0.62 |
| E. MMT/PEO (60/40) | 4.88 | 1.81 | 0.85 |
| F. MMT/PEO (50/50) | 4.84 | 1.82 | 0.86 |
| G. Pd2+@MMT/PEO (0.2/80/20) | 5.13 | 1.72 | 0.76 |
| H. Pd0@MMT/PEO (0.2/80/20) | 5.11 | 1.73 | 0.77 |
| Samples | τ3 (ns) | I3 (%) | l (nm) |
|---|---|---|---|
| MMT/PEO (80/20) | 2.235 | 5.8 | 0.2901 |
| Pd2+@MMT/PEO (0.2/80/20) | 2.132 | 3.7 | 0.2792 |
| Pd0@MMT/PEO (0.2/80/20) | 2.220 | 4.5 | 0.2886 |

| Entry | Aromatic Halides | Acrylates | Coupling Yield a (%) |
|---|---|---|---|
| 1 | C6H5I | CH2=CHCOO(n-C4H9) | 91 |
| 2 | 4-CH3C6H4I | CH2=CHCOO(n-C4H9) | 89 |
| 3 | 3-CH3OC6H4I | CH2=CHCOO(n-C4H9) | 84 |
| 4 | 4-FC6H4I | CH2=CHCOO(n-C4H9) | 88 |
| 5 | 3-FC6H4I | CH2=CHCOO(n-C4H9) | 87 |
| 6 | C6H5I | CH2=CHCOO(t-C4H9) | 74 |
| 7 | 4-CH3C6H4I | CH2=CHCOO(t-C4H9) | 75 |
| 8 | 4-FC6H4I | CH2=CHCOO(t-C4H9) | 71 |
| 9 | C6H5Br | CH2=CHCOO(n-C4H9) | trace b |
| 10 | 3-COCH3C6H4Br | CH2=CHCOO(n-C4H9) | 45 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, G.; Zhao, J.; Zheng, X.; Xu, M.; Liu, Q.; Zeng, M. Modification of Montmorillonite with Polyethylene Oxide and Its Use as Support for Pd0 Nanoparticle Catalysts. Polymers 2019, 11, 755. https://doi.org/10.3390/polym11050755
Shu G, Zhao J, Zheng X, Xu M, Liu Q, Zeng M. Modification of Montmorillonite with Polyethylene Oxide and Its Use as Support for Pd0 Nanoparticle Catalysts. Polymers. 2019; 11(5):755. https://doi.org/10.3390/polym11050755
Chicago/Turabian StyleShu, Guiqing, Jing Zhao, Xiu Zheng, Mengdie Xu, Qi Liu, and Minfeng Zeng. 2019. "Modification of Montmorillonite with Polyethylene Oxide and Its Use as Support for Pd0 Nanoparticle Catalysts" Polymers 11, no. 5: 755. https://doi.org/10.3390/polym11050755
APA StyleShu, G., Zhao, J., Zheng, X., Xu, M., Liu, Q., & Zeng, M. (2019). Modification of Montmorillonite with Polyethylene Oxide and Its Use as Support for Pd0 Nanoparticle Catalysts. Polymers, 11(5), 755. https://doi.org/10.3390/polym11050755