Development of a High-Performance Adhesive with a Microphase, Separation Crosslinking Structure Using Wheat Flour and a Hydroxymethyl Melamine Prepolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydroxymethyl Melamine Prepolymer (HMP)
2.3. Preparation of Adhesives
2.4. Preparation of the Plywood Sample
2.5. Sedimentation Volume Evaluation
2.6. Apparent Viscosity Measurement
2.7. Attenuated Total Reflectance–Fourier Transforms Infrared (ATR–FTIR) Spectroscopy Investigation
2.8. Wet Shear Strength Measurement
2.9. Thermogravimetric (TG) Measurement
2.10. Scanning Electron Microscopy (SEM)
3. Results and Discussion
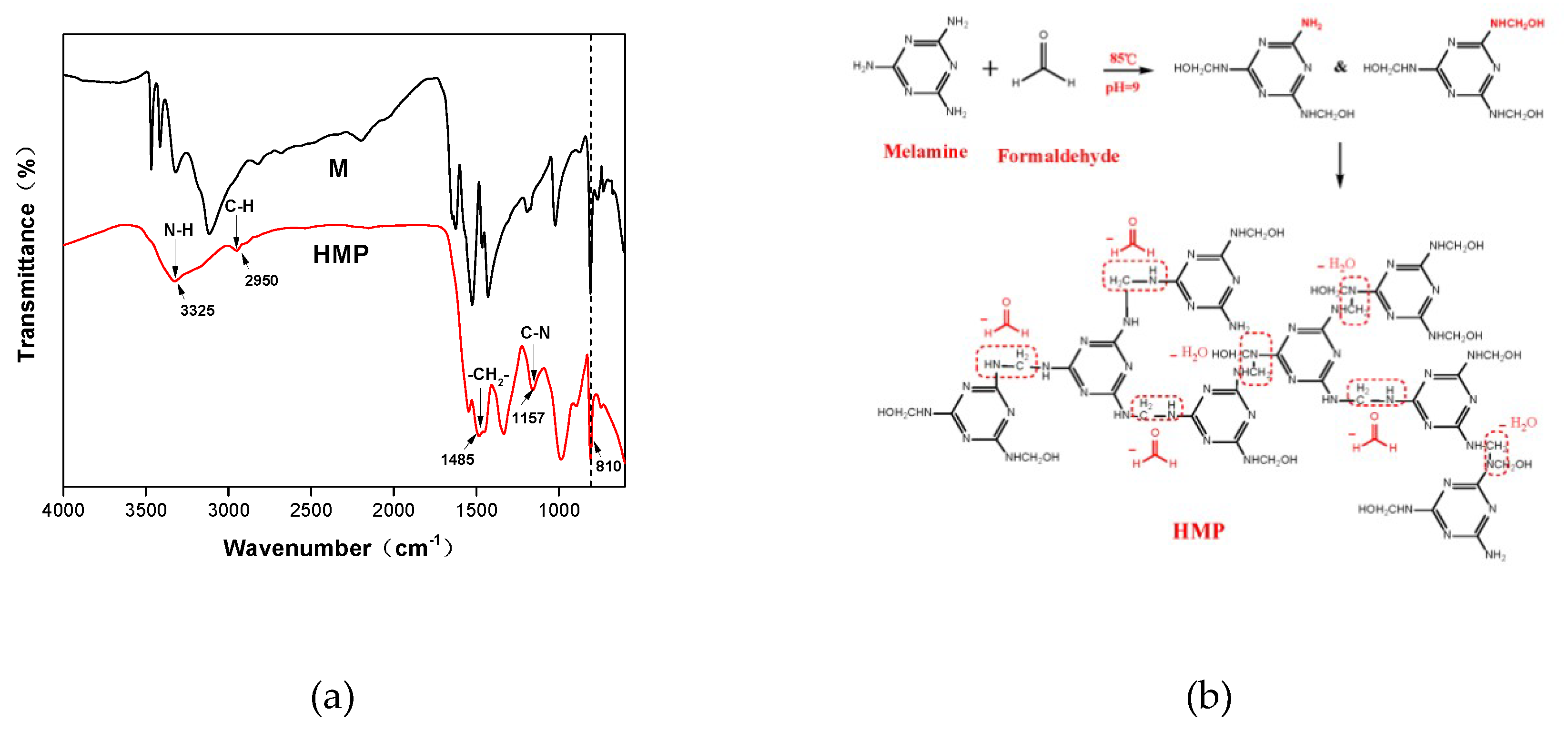
3.1. HMP Analysis
3.2. ATR-FTIR Analysis
3.3. Sedimentation Volume Analysis
3.4. Apparent Viscosity Measurement
3.5. TG Analysis
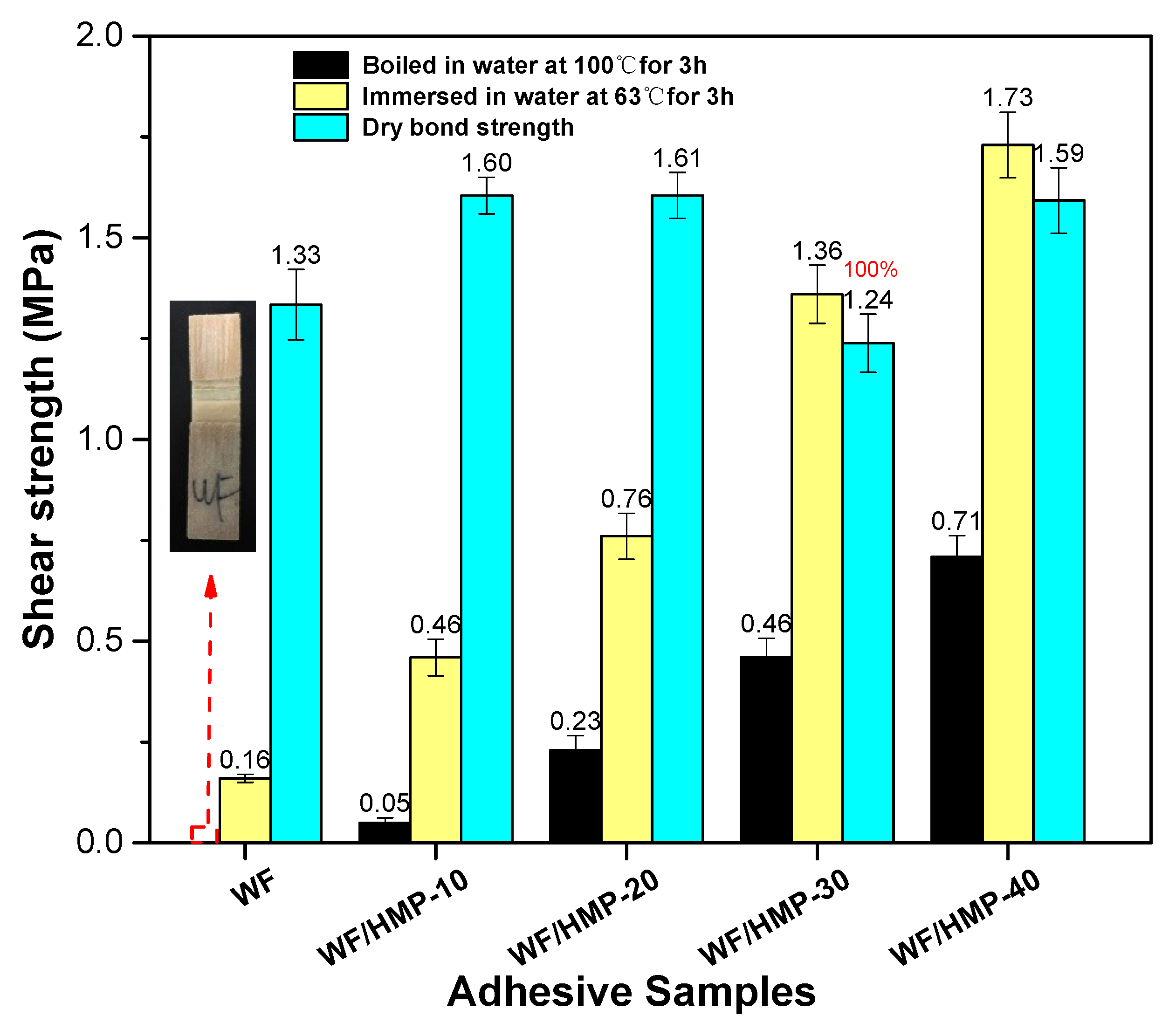
3.6. Plywood Shear Strength Measurement
3.7. SEM Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, R.; Wang, X.; Cheng, M. Preparation and characterization of potato starch film with various size of nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Suriyatem, R.; Auras, R.; Rachtanapun, C.; Rachtanapun, P. Biodegradable rice starch/carboxymethyl chitosan films with added propolis extract for potential use as active food packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Shi, J.-Y.; Xu, W. Synthesis and characterization of starch-based aqueous polymer isocyanate wood adhesive. Bioresources 2015, 10, 7653–7666. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Gu, Z.; Hong, Y.; Cheng, L. Preparation, characterization and properties of starch-based wood adhesive. Carbohydr. Polym. 2012, 88, 699–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Cheng, H.; Huang, W. Preparation, characterization and properties of an organic siloxane-modified cassava starch-based wood adhesive. J. Adhes. 2017, 94, 278–293. [Google Scholar] [CrossRef]
- Junxi, L.; Qiong, S.; Yamin, Z.; Yanbin, W. Theoretical insights into three types of oxidized starch-based adhesives: Chemical stability, water resistance, and shearing viscosity from a molecular viewpoint. J. Chem. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Ren, J.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y.; Li, Z. Digestion rate of tapioca starch was lowed through molecular rearrangement catalyzed by 1,4-α-glucan branching enzyme. Food Hydrocoll. 2018, 84, 117–124. [Google Scholar] [CrossRef]
- Guo, L. Sweet potato starch modified by branching enzyme, β-amylase and transglucosidase. Food Hydrocoll. 2018, 83, 182–189. [Google Scholar] [CrossRef]
- Worzakowska, M. Starch-g-poly(phenyl acrylate) copolymers-synthesis, characterization, and physicochemical properties. Starch Stärke 2017, 69, 1700027. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical properties of starch adhesives enhanced by esterification modification with dodecenyl succinic anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263. [Google Scholar] [CrossRef]
- Qiao, Z.; Gu, J.; Lv, S.; Cao, J.; Tan, H.; Zhang, Y. Preparation and properties of normal temperature cured starch-based wood adhesive. Bioresources 2016, 11, 4839–4849. [Google Scholar] [CrossRef]
- Wen, B.X.; Shi, J.Y.; Shu, M.W. Study on heat aging properties of starch based aqueous polymer isocyanate adhesive for wood. Adv. Mater. Res. 2014, 933, 138–143. [Google Scholar]
- Xu, P.; Zeng, Q.; Cao, Y.; Ma, P.; Dong, W.; Chen, M. Interfacial modification on polyhydroxyalkanoates/starch blend by grafting in-situ. Carbohydr. Polym. 2017, 174, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mahto, V. Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. Pet. Sci. 2017, 14, 765–779. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ullah, I.; Wang, P.K.; Javaid, A.B.; Hu, C.; Zhang, M.; Ahamd, I.; Xiong, H.; Wang, Z. Synthesis and characterization of starch-g-poly(vinyl acetate- co -butyl acrylate) bio-based adhesive for wood application. Int. J. Biol. Macromol. 2018, 114, 1186–1193. [Google Scholar]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Luo, J.; Luo, J.; Gao, Q.; Li, J. A soybean meal-based wood adhesive improved by a diethylene glycol diglycidyl ether: Properties and performance. RSC Adv. 2016, 6, 74186–74194. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Zhang, J.; Bai, Y.; Gao, Q.; Li, J.; Li, L. A New flexible soy-based adhesive enhanced with neopentyl glycol diglycidyl ether: Properties and application. Polymers 2016, 8, 346. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Li, K.; Gao, Q.; Li, J. Toughening improvement to a soybean meal-based bioadhesive using an interpenetrating acrylic emulsion network. J. Mater. Sci. 2016, 51, 9330–9341. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Gao, Q.; Li, J. Effects of polyisocyanate on properties and pot life of epoxy resin cross-linked soybean meal-based bioadhesive. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Bo, F.; Zhang, L.P.; Gao, Z.H.; Zhang, Y.; Shi, J.; Li, J. Formulation of a novel soybean protein-based wood adhesive with desired water resistance and technological applicability. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Gao, Q.; Zhang, S.; Li, J. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crops Prod. 2014, 59, 35–40. [Google Scholar] [CrossRef]
- Hamdi, G.; Ponchel, G. Enzymatic degradation of epichlorohydrin crosslinked starch microspheres by α-amylase. Pharm. Res 1996, 16, 1867–1875. [Google Scholar]
- Zhang, Y.; Zhang, M.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. Preparation and characterization of a soy protein-based high-performance adhesive with a hyperbranched cross-linked structure. Chem. Eng. J. 2018, 354, 1032–1041. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Qin, Z.; Chen, H.; Gao, Q.; Li, J. Mechanical and thermal properties of microcrystalline cellulose-reinforced soy protein isolate–gelatin eco-friendly films. RSC Adv. 2015, 5, 56518–56525. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Bai, Y.; Gao, Q.; Li, J. A high performance soy protein-based bio-adhesive enhanced with a melamine/epichlorohydrin prepolymer and its application on plywood. RSC Adv. 2016, 6, 67669–67676. [Google Scholar] [CrossRef]
- Meng, Z.; Yi, Z.; Chen, M.; Qiang, G.; Li, J. A high-performance and low-cost soy flour adhesive with a hydroxymethyl melamine prepolymer. Polymers 2018, 10, 909. [Google Scholar]
- Xu, Y.; Xu, Y.; Zhu, W.; Zhang, W.; Gao, Q.; Li, J. Improve the performance of soy protein-based adhesives by a polyurethane elastomer. Polymers 2018, 10, 1016. [Google Scholar] [CrossRef]
- Srivatsan, D.T.S. Wood Adhesives. Adv. Manuf. Process. 2012, 27, 1. [Google Scholar] [CrossRef]
- Ming, G.; Duan, H.; Xia, M.; Sun, G.; Sun, W.; Yu, L.; Lucia, L. A novel fabrication of monodisperse melamine–formaldehyde resin microspheres to adsorb lead (II). Chem. Eng. J. 2016, 288, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Q.; Li, L.S.; Xu, L. Removal of perfluorooctanoic acid from water with economical mesoporous melamine-formaldehyde resin microsphere. Chem. Eng. J. 2017, 320, 501–509. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, X.; Zhang, Y.; Tang, S.; Guo, C.; Wu, J.; Lau, R. Anionic and cationic dyes adsorption on porous poly-melamine-formaldehyde polymer. Chem. Eng. Res. Des. 2016, 114, 258–267. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, L.; Lei, Q.; Yang, F.; Wu, D. Monodispersed or narrow-dispersed melamine-formaldehyde resin polymer colloidal spheres: Preparation, size-control, modification, bioconjugation and particle formation mechanism. J. Mater. Chem. B 2012, 1, 204–212. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, X.; Ma, P.; Chen, M.; Dong, W.; Hoch, M.; Lemstra, P.J. Design of nano-starch-reinforced ethyl-co-vinyl acetate elastomers by simultaneously constructing interfacial bonding and novel reversible matrix crosslinking. Chem. Eng. J. 2018, 346, 497–505. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Tjandra, R.; Cousins, A.; Sy, S.; Yu, A.; Berry, R.M.; Tam, K.C. Nitrogen-enriched porous carbon nanorods templated by cellulose nanocrystals as high performance supercapacitor electrode. J. Mater. Chem. A 2015, 3, 23768–23777. [Google Scholar] [CrossRef]
- Liu, Y. Starch Production and Its Deep Processing Technology; China Light Industry Press: Bejing, China, 2001. [Google Scholar]
- Qi, G.; Sun, X.S. Peel adhesion properties of modified soy protein adhesive on a glass panel. Ind. Crops Prod. 2010, 32, 208–212. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Fei, P.; Jin, W.; Xiong, H.; Wang, Z. Enhancing the performance of starch-based wood adhesive by silane coupling agent(KH570). Int. J. Biol. Macromol. 2017, 104, 137. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Ying, L.; Gao, Z.; Gu, J. Nano-scale blocking mechanism of MMT and its effects on the properties of polyisocyanate-modified soybean protein adhesive. Ind. Crops Prod. 2014, 57, 35–42. [Google Scholar] [CrossRef]





| Sample | Adhesive Formulation | |||
|---|---|---|---|---|
| Wheat Flour (g) | Deionized Water (g) | HMP (g) | ||
| 0 | HMP adhesive | 0 | 0 | 100 |
| 1 | WF adhesive | 35 | 65 | – |
| 2 | WF/HMP–10 adhesive | 35 | 55 | 10 |
| 3 | WF/ HMP–20 adhesive | 35 | 45 | 20 |
| 4 | WF/ HMP–30 adhesive | 35 | 35 | 30 |
| 5 | WF/ HMP–40 adhesive | 35 | 25 | 40 |
| Sample | 0 | 1 | 2 | 3 | 4 | 5 |
| Sedimentation volume (mL) | −16 ± 0.02 a | −15.5 ± 0.03 a | −16.5 ± 0.05 a | −17.25 ± 0.02 a | −18 ± 0.06 a | −18.5 ± 0.07 a |
| Sample | 0 | 1 | 2 | 3 | 4 |
| Initial viscosity (mPa·s) | 2283 | 5258 | 8212 | 13,220 | 36,960 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, Y.; Li, J.; Gao, Q. Development of a High-Performance Adhesive with a Microphase, Separation Crosslinking Structure Using Wheat Flour and a Hydroxymethyl Melamine Prepolymer. Polymers 2019, 11, 893. https://doi.org/10.3390/polym11050893
Zhang J, Zhang Y, Li J, Gao Q. Development of a High-Performance Adhesive with a Microphase, Separation Crosslinking Structure Using Wheat Flour and a Hydroxymethyl Melamine Prepolymer. Polymers. 2019; 11(5):893. https://doi.org/10.3390/polym11050893
Chicago/Turabian StyleZhang, Jieyu, Yi Zhang, Jianzhang Li, and Qiang Gao. 2019. "Development of a High-Performance Adhesive with a Microphase, Separation Crosslinking Structure Using Wheat Flour and a Hydroxymethyl Melamine Prepolymer" Polymers 11, no. 5: 893. https://doi.org/10.3390/polym11050893





