Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PHMB:PU Nanofibrous Membranes via Electrospinning
2.2.1. Solution Preparation and Electrospinning Optimisation
2.2.2. Sample Preparation
2.3. Structural, Physical and Mechanical Property Characterisation
2.3.1. Morphology and Fibre Diameter of PHMB:PU Membranes via SEM
2.3.2. Surface Chemistry of PHMB:PU Membranes via ATR-FTIR
2.3.3. Static Tensile Mechanical Properties of PHMB:PU Membranes
2.3.4. Pore Size and Distribution of PHMB:PU Membranes
2.3.5. Surface Wettability of PHMB:PU Membranes
2.3.6. PHMB Release Kinetics from PHMB:PU Membranes
2.4. Biological Interaction Characterisations
2.4.1. Antimicrobial Properties of PHMB:PU Membranes
2.4.2. Human Cell Viability and Attachment on PHMB:PU Membranes
2.5. Statistical Analysis
3. Results
3.1. Initial Optimisations
3.2. Structure, Physical and Mechanical Properties
3.2.1. Morphology and Fibre Diameter of PHMB:PU Membranes via SEM
3.2.2. Surface Chemistry of PHMB:PU Membranes via ATR-FTIR
3.2.3. Tensile Mechanical Properties of PHMB:PU Membranes
3.2.4. Pore Size and Distribution of PHMB:PU Membranes
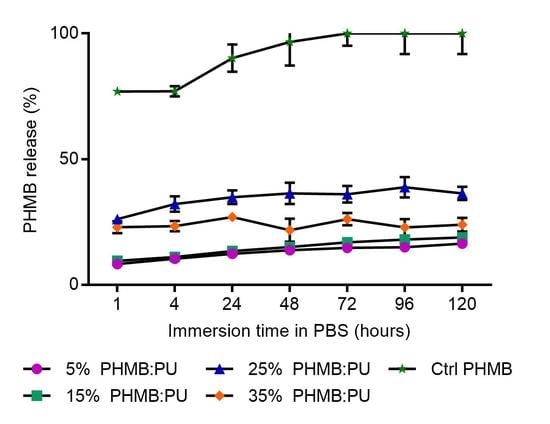
3.2.5. PHMB Release Kinetics of PHMB:PU Membranes
3.2.6. Surface Wettability of PHMB:PU Membranes
3.3. Effects on Pathogen and Host Cells
3.3.1. Antibacterial Activities of PHMB:PU Membranes
3.3.2. HaCaT Cell Responses
Human Cell Toxicity of PHMB:PU Membranes
Human Cell Attachment on PHMB:PU Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ndosi, M.; Wright-Hughes, A.; Brown, S.; Backhouse, M.; Lipsky, B.A.; Bhogal, M.; Reynolds, C.; Vowden, P.; Jude, E.B.; Nixon, J.; et al. Prognosis of the infected diabetic foot ulcer: A 12-month prospective observational study. Diabetic Med. 2018, 35, 78–88. [Google Scholar] [CrossRef]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer-a review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Antibiotic Resistance—A Threat to Global Health Security and the Case for Action. Available online: https://www.gov.uk/government/publications/antibiotic-resistance-a-threat-to-global-health-security-and-the-case-for-action (accessed on 21 May 2019).
- Mulder, G.D.; Cavorsi, J.P.; Lee, D.K. Polyhexamethylene biguanide (phmb): An addendum to current topical antimicrobials. Wounds 2007, 19, 173–182. [Google Scholar] [PubMed]
- Landis, S.J. Chronic wound infection and antimicrobial use. Adv. Skin Wound Care 2008, 21, 541–542. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Delivery Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef]
- Chhibber, S.; Gondil, V.S.; Singla, L.; Kumar, M.; Chhibber, T.; Sharma, G.; Sharma, R.K.; Wangoo, N.; Katare, O.P. Effective topical delivery of h-agnps for eradication of klebsiella pneumoniae-induced burn wound infection. AAPS Pharmscitech 2019, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.C.; Lima, I.S.D.; Morais, A.I.S.; Silva, S.O.; Carvalho, R.D.F.D.; Ribeiro, A.B.; Osajima, J.A.; Silva, E.C. Chitosan associated with chlorhexidine in gel form: Synthesis, characterization and healing wounds applications. J. Drug Delivery Sci. Technol. 2019, 49, 375–382. [Google Scholar] [CrossRef]
- Pak, C.S.; Park, D.H.; Oh, T.S.; Lee, W.J.; Jun, Y.J.; Lee, K.A.; Oh, K.S.; Kwak, K.H.; Rhie, J.W. Comparison of the efficacy and safety of povidone-iodine foam dressing (betafoam), hydrocellular foam dressing (allevyn), and petrolatum gauze for split-thickness skin graft donor site dressing. Int. Wound J. 2019, 16, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer phmb enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety (European Commission). Opinion on the Safety of Poly(Hexamethylene) Biguanide Hydrochloride (PHMB). Available online: http://ec.europa.eu/health//sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_157.pdf (accessed on 21 May 2019).
- Gilbert, P.; Pemberton, D.; Wilkinson, D. Synergism within polyhexamethylene biguanide biocide formulations. J. Appl. Bacteriol. 1990, 69, 593–598. [Google Scholar] [CrossRef]
- Gilbert, P.; Das, J.; Jones, M.; Allison, D. Assessment of resistance towards biocides following the attachment of micro-organisms to, and growth on, surfaces. J. Appl. Microbiol. 2001, 91, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafra, C.; Carrijo-Carvalho, L.; Chudzinski-Tavassi, A.; Taguchi, F.; Foronda, A.; Carvalho, F.; Freitas, D.D. Antimicrobial action of biguanides on the viability of acanthamoeba cysts and assessment of cell toxicity. Invest. Ophthalmol. Visual Sci. 2013, 54, 6363. [Google Scholar] [CrossRef] [PubMed]
- Forstner, C.; Leitgeb, J.; Schuster, R.; Dosch, V.; Kramer, A.; Cutting, K.; Leaper, D.; Assadian, O. Bacterial growth kinetics under a novel flexible methacrylate dressing serving as a drug delivery vehicle for antiseptics. Int. J. Mol. Sci. 2013, 14, 10582–10590. [Google Scholar] [CrossRef]
- Kramer, A.; Roth, B.; Müller, G.; Rudolph, P.; Klöcker, N. Influence of the antiseptic agents polyhexanide and octenidine on fl cells and on healing of experimental superficial aseptic wounds in piglets. Skin Pharmacol. Physiol. 2004, 17, 141–146. [Google Scholar] [CrossRef]
- Eberlein, T.; Haemmerle, G.; Signer, M.; Gruber-Moesenbacher, U.; Traber, T.; Mittlboeck, M.; Abel, M.; Strohal, R. Comparison of phmb-containing dressing and silver dressings in patients with critically colonised or locally infected wounds. J. Wound Care 2012, 21, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Siadat, S.; Mokhtari, J. Fabrication of novel antimicrobial bio-fibres using silk wastage, study of poly (hexamethylene) biguanide, and silver nanoparticles interaction. J. Nat. Fibres 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Song, W.; Mitchell, G.; Burugapalli, K. Electrospinning for biomedical applications. In Electrospinning: Principles, Practics and Possibilities; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Llorens, E.; Calderón, S.; Valle, L.D.; Puiggalí, J. Polybiguanide (phmb) loaded in pla scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater. Sci. Eng. C 2015, 50, 74–84. [Google Scholar] [CrossRef]
- Waring, M.; Bielfldt, S.; Mätzold, K.; Wilhelm, K.; Butcher, M. An evaluation of the skin stripping of wound dressing adhesives. J. Wound Care 2011, 20, 412, 414, 416–422. [Google Scholar] [CrossRef]
- Wang, N.; Burugapalli, K.; Song, W.; Zheng, Y.; Ma, Y.; Wu, Z.; Li, K. Electrospun co-axial polyurethane-gelatin nano-fibrous coating for implantable glucose biosensor. Biofabrication 2014, 6, 015002. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Burugapalli, K.; Song, W.; Hall, J.; Moussy, F.; Wu, Z.; Li, K. Tailored fibro-porous structure of electrospun polyurethane membranes, their size-dependent properties and trans-membrane glucose diffusion. J. Membr. Sci. 2013, 247, 207–217. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, J.; Stiadle, J.; Wang, X.; Wang, L.; Li, Q.; Shen, C.; Thibeault, S.L.; Turng, L. Electrospun nanofibrous thermoplastic polyurethane/poly(glycerol sebacate) hybrid scaffolds for vocal fold tissue engineering applications. Mater. Sci. Eng. C 2019, 94, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Akduman, C.; Özgüney, I.; Kumbasar, E.P.A. Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater. Sci. Eng. C 2016, 64, 383–390. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Oprea, A.E.; Trusca, R.; Surdu, A.V.; Ficai, A.; Holban, A.M.; Lordache, F.; Paduraru, A.V.; Filip, D.G.; Altun, E.; et al. Production and characterization of antimicrobial electrospun nanofibers containing polyurethane, zirconium oxide and zeolite. Bionanoscience 2018, 8, 154–165. [Google Scholar] [CrossRef]
- Huang, C.C.; Rwei, S.P.; Jang, S.C.; Tsen, W.C.; Chuang, F.S.; Ku, T.H.; Chow, J.D.; Chen, C.C.; Shu, Y.C. Antibacterial of silver-containing polydimethylsiloxane urethane nanofibrous, hollow fibrous, using the electrospinning process. J. Nanosci. Nanotechnol. 2017, 17, 1975–1982. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P. Electrospun polyurethane nanofibrous composite impregnated with metallic copper for wound-healing application. 3 Biotech 2018, 8, 327. [Google Scholar] [CrossRef]
- Shababdoust, A.; Ehsani, M.; Shokrollahi, P.; Zandi, M. Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog. Biomater. 2018, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.; Sharma, U.; Mikos, A. Electrospinning of polymeric nanofibres for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and chemical characterization of poly(hexamethylene biguanide) hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Küstersa, M.; Beyera, S.; Kutschera, S.; Schlesingera, H.; Gerhartz, M. Rapid, simple and stability-indicating determination of polyhexamethylene biguanide in liquid and gel-like dosage forms by liquid chromatography with diode-array detection. J. Pharm. Anal. 2013, 3, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Akhtar, N.; Ghauri, M.A.; Rajoka, M.I.; Khalid, Z.M.; Hussain, I. Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res. Lett. 2012, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care (New Rochelle) 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Agache, P.; Monneur, C.; Leveque, J.; Rigal, J.D. Mechanical properties and young’s modulus of human skin in vivo. Arch. Dermatol. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef] [PubMed]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worsley, A.; Vassileva, K.; Tsui, J.; Song, W.; Good, L. Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control. Polymers 2019, 11, 915. https://doi.org/10.3390/polym11050915
Worsley A, Vassileva K, Tsui J, Song W, Good L. Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control. Polymers. 2019; 11(5):915. https://doi.org/10.3390/polym11050915
Chicago/Turabian StyleWorsley, Anna, Kristin Vassileva, Janice Tsui, Wenhui Song, and Liam Good. 2019. "Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control" Polymers 11, no. 5: 915. https://doi.org/10.3390/polym11050915
APA StyleWorsley, A., Vassileva, K., Tsui, J., Song, W., & Good, L. (2019). Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control. Polymers, 11(5), 915. https://doi.org/10.3390/polym11050915