Crowding-Activity Coupling Effect on Conformational Change of a Semi-Flexible Polymer
Abstract
:1. Introduction
2. Model and Method
3. Results and Discussion
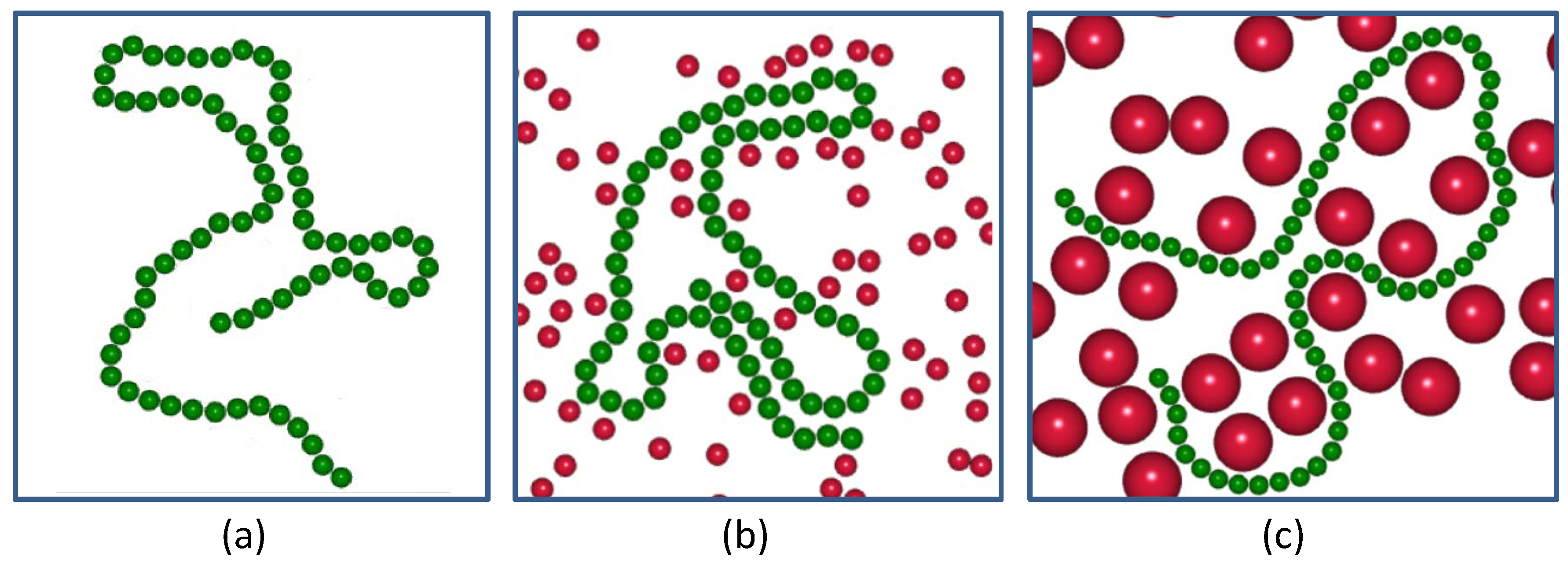
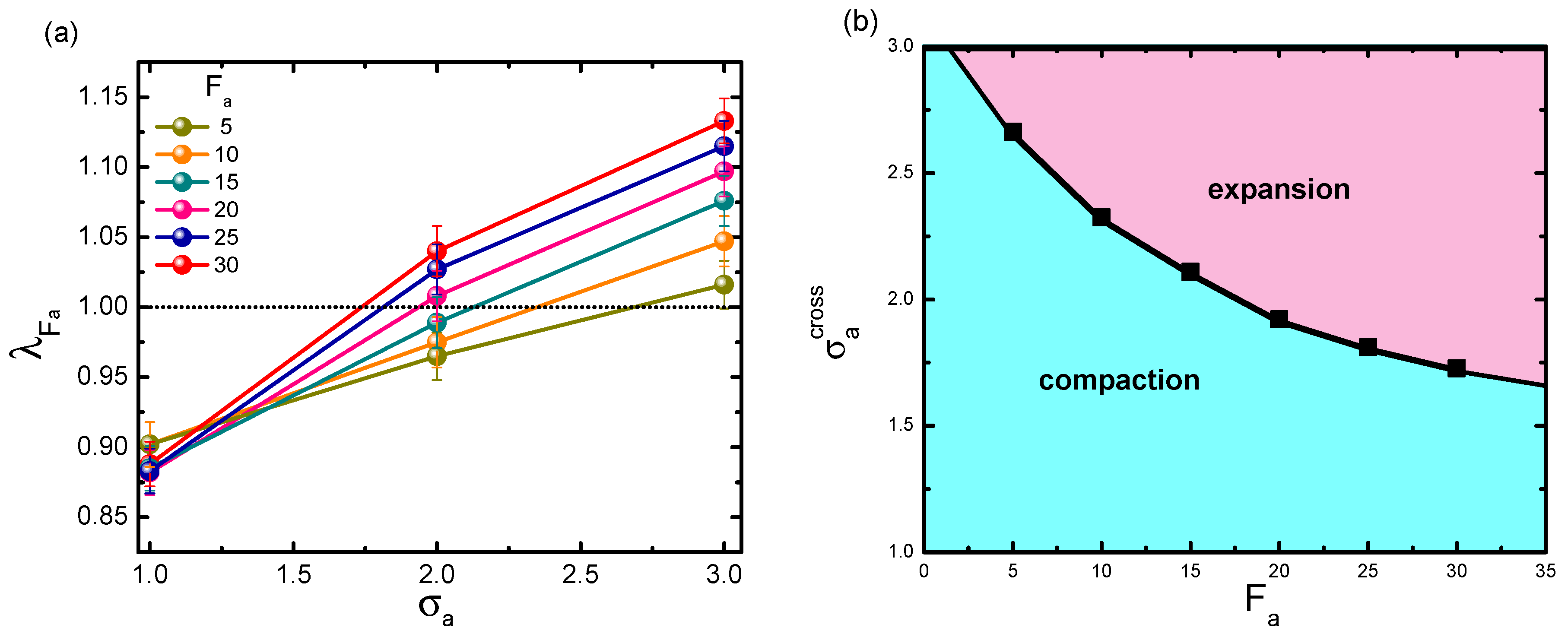
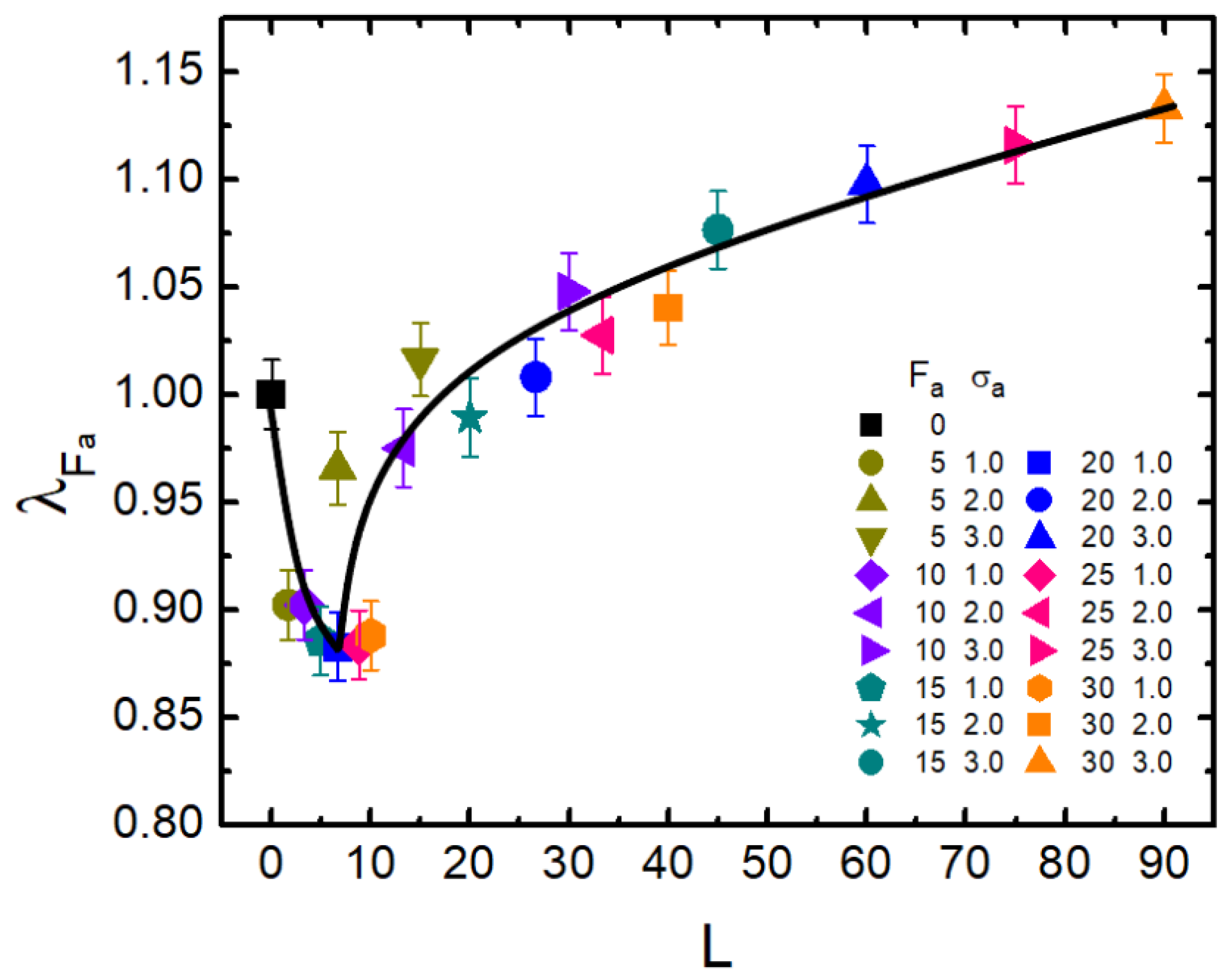
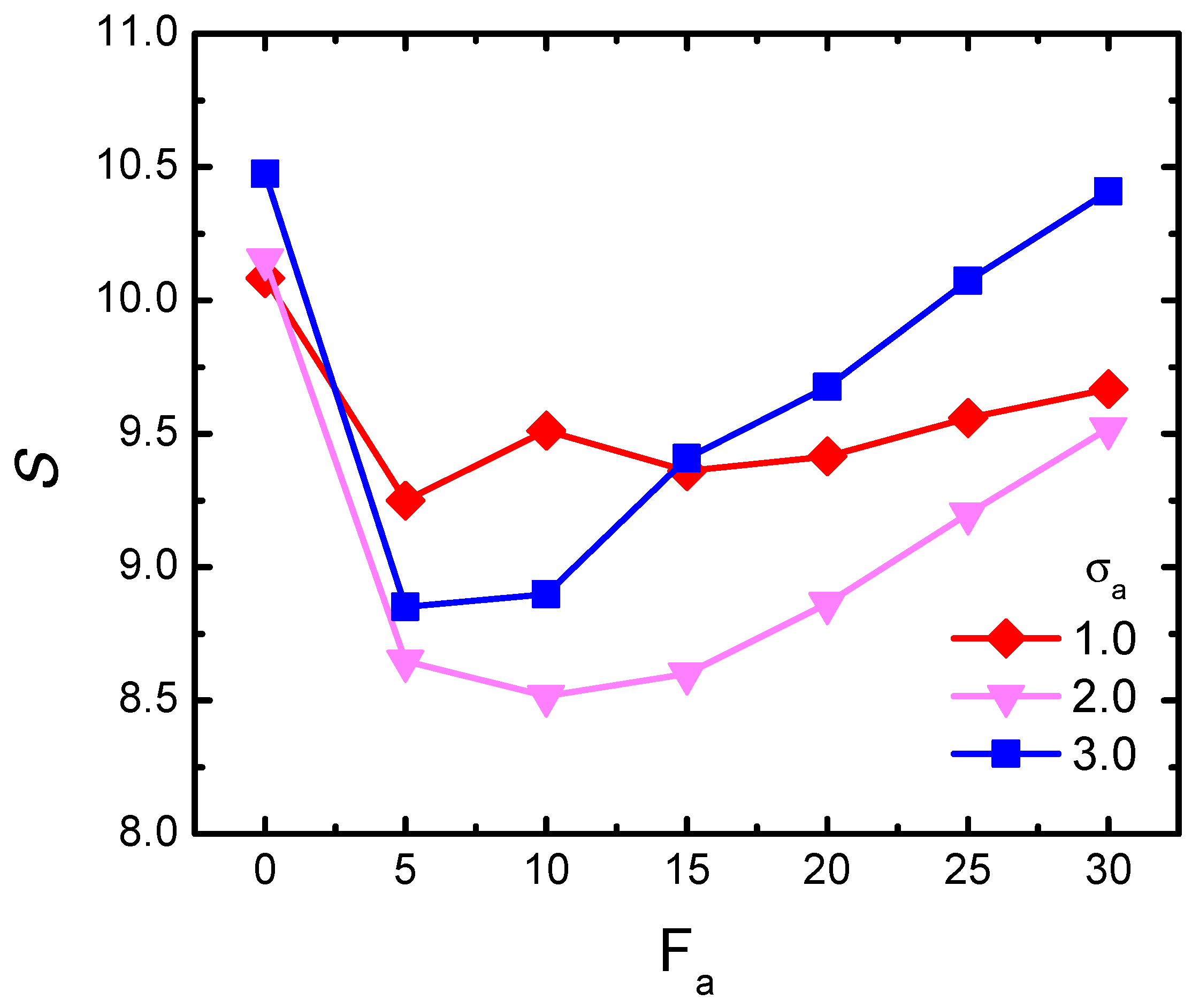
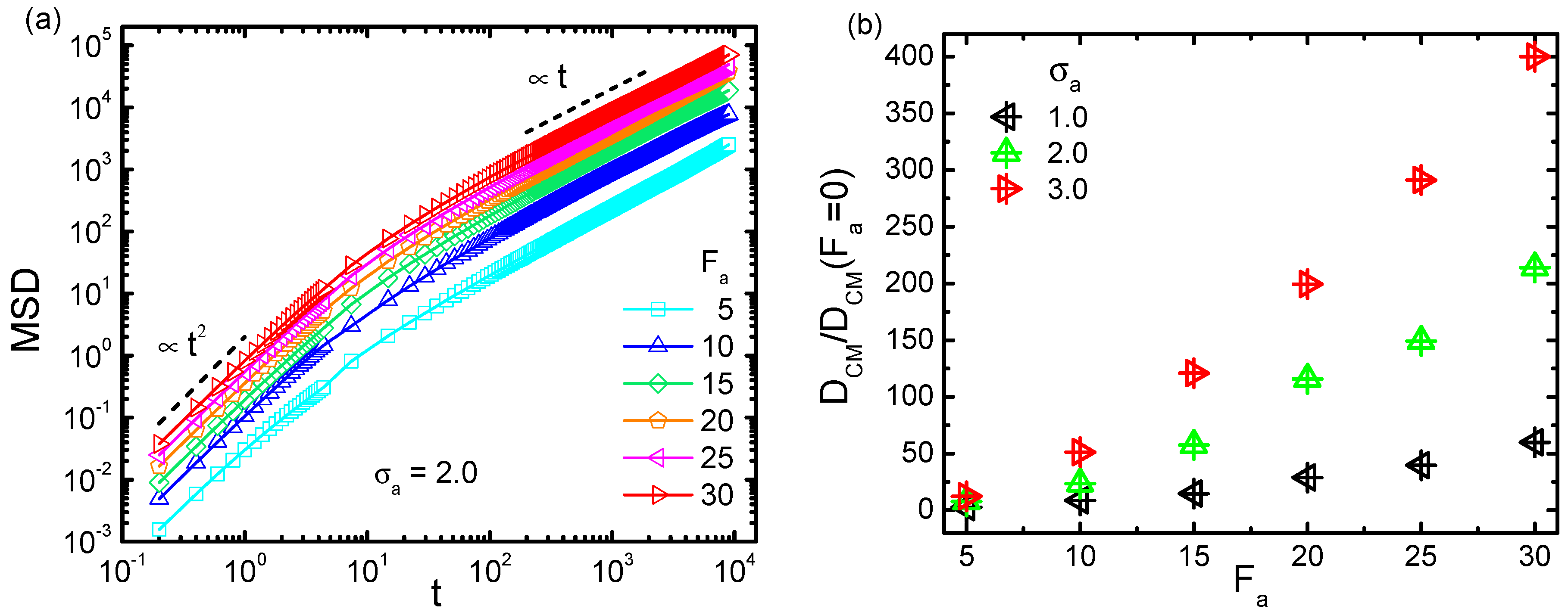
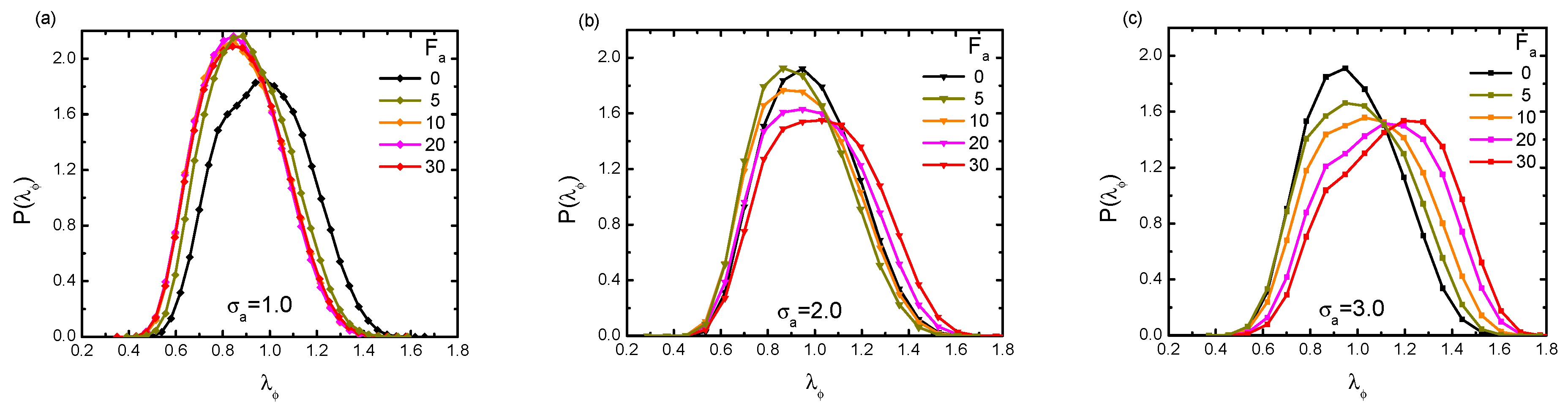
3.1. Activity Effect under Varying Crowder Sizes
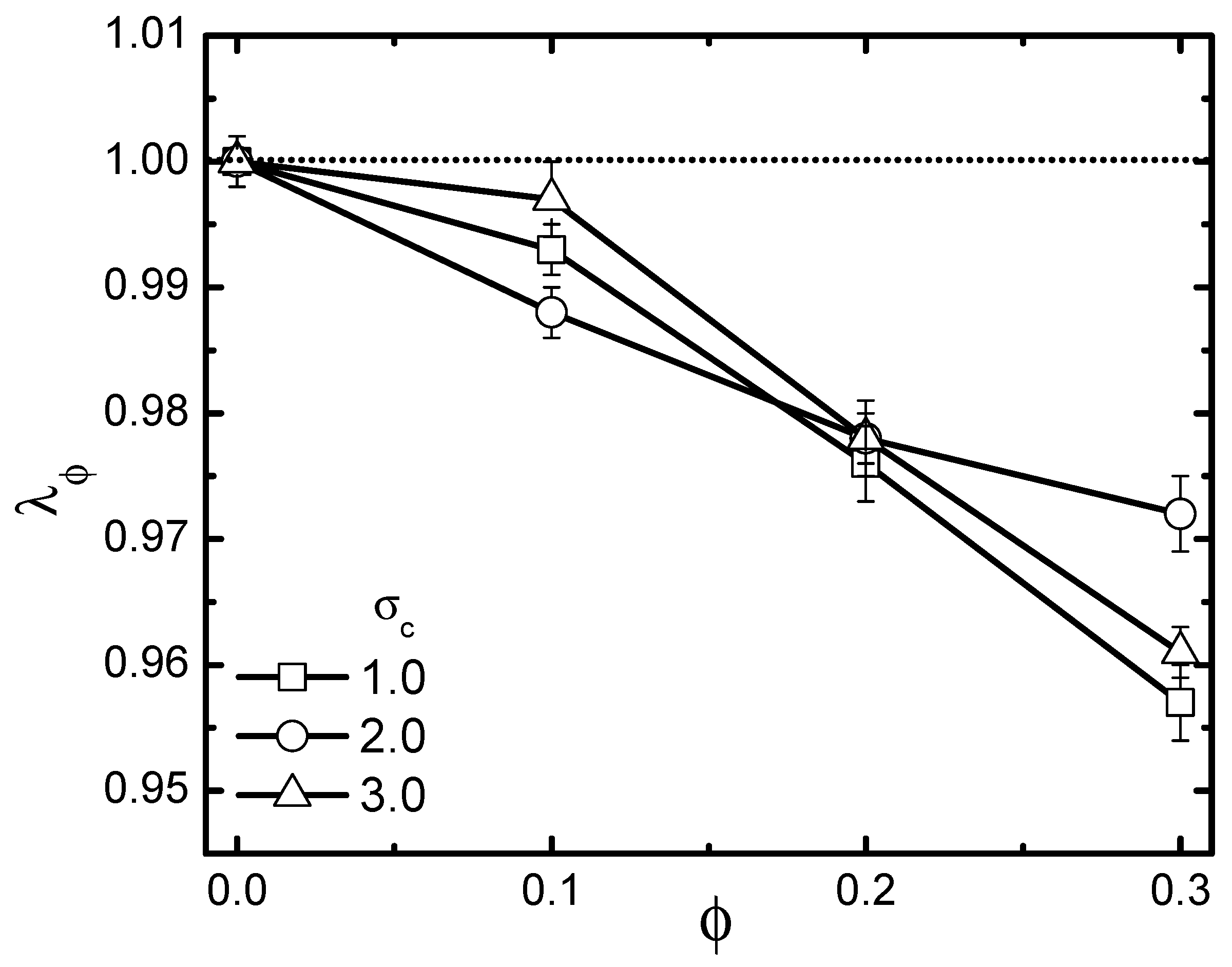
3.2. Activity-Crowding Coupling Effect
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ramaswamy, S. The Mechanics and Statistics of Active Matter. Annu. Rev. Condens. Matter Phys. 2010, 1, 323–345. [Google Scholar] [CrossRef] [Green Version]
- Ten Hagen, B.; van Teeffelen, S.; Löwen, H. Brownian motion of a self-propelled particle. J. Phys. Condens. Matter 2011, 23, 194119–194132. [Google Scholar] [CrossRef] [PubMed]
- Romanczuk, P.; Bär, M.E.W.; Lindner, B.; Schimansky-Geier, L. Active Brownian particles. Eur. Phys. J. Spec. Top. 2012, 202, 1–162. [Google Scholar] [CrossRef] [Green Version]
- Elgeti, J.; Winkler, R.G.; Gompper, G. Physics of microswimmers—Single particle motion and collective behavior: A review. Rep. Prog. Phys. 2015, 78, 056601–056652. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, C.; Di Leonardo, R.; Löwen, H.; Reichhardt, C.; Volpe, G.; Volpe, G. Active particles in complex and crowded environments. Rev. Mod. Phys. 2016, 88, 045006–045056. [Google Scholar] [CrossRef]
- Jülicher, F.; Kruse, K.; Prost, J.; Joanny, J.F. Active behavior of the Cytoskeleton. Phys. Rep. 2007, 449, 3–28. [Google Scholar] [CrossRef]
- Mizuno, D.; Tardin, C.; Schmidt, C.F.; MacKintosh, F.C. Nonequilibrium mechanics of active cytoskeletal networks. Science 2007, 315, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Koenderink, G.H.; MacKintosh, F.C.; Weitz, D.A. Nonequilibrium Microtubule Fluctuations in a Model Cytoskeleton. Phys. Rev. Lett. 2008, 100, 118104–118108. [Google Scholar] [CrossRef]
- Aranson, I.S.; Tsimring, L.S. Self-organization of microtubules and motors. Nature 1997, 389, 305–308. [Google Scholar]
- Kruse, K.; Joanny, J.F.; Jülicher, F.; Prost, J.; Sekimoto, K. Asters, Vortices, and Rotating Spirals in Active Gels of Polar Filaments. Phys. Rev. Lett. 2004, 92, 078101–078105. [Google Scholar] [CrossRef] [PubMed]
- Dölger, J.; Bohr, T.; Andersen, A. An analytical model of flagellate hydrodynamics. Phys. Scr. 2017, 92, 044003–044013. [Google Scholar] [CrossRef]
- Riedel, I.H.; Kruse, K.; Howard, J. A Self-Organized Vortex Array of Hydrodynamically Entrained Sperm Cells. Science 2005, 309, 300–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S.C.; Spakowitz, A.J.; Theriot, J.A. Bacterial Chromosomal Loci Move Subdiffusively through a Viscoelastic Cytoplasm. Phys. Rev. Lett. 2010, 104, 238102–238106. [Google Scholar] [CrossRef] [PubMed]
- Lauga, E.; Powers, T.R. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 2009, 72, 096601–096638. [Google Scholar] [CrossRef]
- Zöttl, A.; Stark, H. Emergent behavior in active colloids. J. Phys. Condens. Matter 2016, 28, 253001–253029. [Google Scholar] [CrossRef]
- Vicsek, T.; Zafeiris, A. Collective motion. Phys. Rep. 2012, 517, 71–140. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.C.; Joanny, J.F.; Ramaswamy, S.; Liverpool, T.B.; Prost, J.; Rao, M.; Simha, R.A. Hydrodynamics of soft active matter. Rev. Mod. Phys. 2013, 85, 1143–1189. [Google Scholar] [CrossRef] [Green Version]
- Jülicher, F.; Grill, S.W.; Salbreux, G. Hydrodynamic theory of active matter. Rep. Prog. Phys. 2018, 81, 076601–076629. [Google Scholar] [CrossRef]
- Needleman, D.; Dogic, Z. Active matter at the interface between materials science and cell biology. Nat. Rev. Mater. 2017, 2, 17048–17062. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W. Nano/Microscale Motors: Biomedical Opportunities and Challenges. ACS Nano 2012, 6, 5745–5751. [Google Scholar] [CrossRef] [PubMed]
- Rivas, G.; Minton, A.P. Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem. Sci. 2016, 41, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Koenderink, G.H.; MacKintosh, F.C.; Weitz, D.A. Cytoplasmic diffusion: Molecular motors mix it up. J. Cell Biol. 2008, 183, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I.; Kharchenko, V.O.; Metzler, R. How molecular motors work in the crowded environment of living cells: Coexistence and efficiency of normal and anomalous transport. PLoS ONE 2014, 9, e91700–e91707. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Weiss, M.; Elsner, M.; Kartberg, F.; Nilsson, T. Anomalous subdiffusion is a measure for cytoplasmic crowding in living cells. Biophys. J. 2004, 87, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- McGuffee, S.R.; Elcock, A.H. Diffusion, Crowding & Protein Stability in a Dynamic Molecular Model of the Bacterial Cytoplasm. PLoS Comput. Biol. 2010, 6, 1–18. [Google Scholar]
- Metzler, R.; Jeon, J.H.; Cherstvy, A. Non-Brownian diffusion in lipid membranes: Experiments and simulations. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 2451–2467. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef]
- Cheung, M.S.; Klimov, D.; Thirumalai, D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 4753–4758. [Google Scholar] [CrossRef] [Green Version]
- Denesyuk, N.A.; Thirumalai, D. Crowding promotes the switch from hairpin to pseudoknot conformation in human telomerase RNA. J. Am. Chem. Soc. 2011, 133, 11858–11861. [Google Scholar] [CrossRef]
- Dupuis, N.F.; Holmstrom, E.D.; Nesbitt, D.J. Molecular-crowding effects on single-molecule RNA folding/unfolding thermodynamics and kinetics. Proc. Natl. Acad. Sci. USA 2014, 111, 8464–8469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloomfield, V.A. DNA condensation. Curr. Opin. Struct. Biol. 1996, 6, 334–341. [Google Scholar] [CrossRef]
- Shin, J.; Cherstvy, A.G.; Metzler, R. Kinetics of polymer looping with macromolecular crowding: Effects of volume fraction and crowder size. Soft Matter 2015, 11, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Backman, V.; Szleifer, I. Crowding-induced structural alterations of random-loop chromosome model. Phys. Rev. Lett. 2011, 106, 168102–168106. [Google Scholar] [CrossRef]
- Stuhrmann, B.; Soares e Silva, M.; Depken, M.; MacKintosh, F.C.; Koenderink, G.H. Nonequilibrium fluctuations of a remodeling in vitro cytoskeleton. Phys. Rev. E 2012, 86, 020901–020906. [Google Scholar] [CrossRef]
- Kaiser, A.; Löwen, H. Unusual swelling of a polymer in a bacterial bath. J. Chem. Phys. 2014, 141, 044903–044911. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Valeriani, C.; Cacciuto, A. Activity-induced collapse and reexpansion of rigid polymers. Phys. Rev. E 2014, 90, 062312–062316. [Google Scholar] [CrossRef]
- Soranno, A.; Koenig, I.; Borgia, M.B.; Hofmann, H.; Zosel, F.; Nettels, D.; Schuler, B. Single-molecule spectroscopy reveals polymer effects of disordered proteins in crowded environments. Proc. Natl. Acad. Sci. USA 2014, 111, 4874–4879. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Pincus, P.A.; Hyeon, C.; Thirumalai, D. Effects of Macromolecular Crowding on the Collapse of Biopolymers. Phys. Rev. Lett. 2015, 114, 068303–068308. [Google Scholar] [CrossRef]
- Shin, J.; Cherstvy, A.G.; Kim, W.K.; Metzler, R. Facilitation of polymer looping and giant polymer diffusivity in crowded solutions of active particles. New J. Phys. 2015, 17, 113008–113021. [Google Scholar] [CrossRef]
- Shin, J.; Cherstvy, A.G.; Kim, W.K.; Zaburdaev, V. Elasticity-based polymer sorting in active fluids: A Brownian dynamics study. Phys. Chem. Chem. Phys. 2017, 19, 18338–18347. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, S.J.; Howse, J.R. In pursuit of propulsion at the nanoscale. Soft Matter 2010, 6, 726–738. [Google Scholar] [CrossRef]
- Howse, J.R.; Jones, R.A.L.; Ryan, A.J.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-Motile Colloidal Particles: From Directed Propulsion to Random Walk. Phys. Rev. Lett. 2007, 99, 048102–048106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.C.; Hummer, G. System-Size Dependence of Diffusion Coefficients and Viscosities from Molecular Dynamics Simulations with Periodic Boundary Conditions. J. Phys. Chem. B 2004, 108, 15873–15879. [Google Scholar] [CrossRef]
- Desai, T.G.; Keblinski, P.; Kumar, S.K.; Granick, S. Modeling Diffusion of Adsorbed Polymer with Explicit Solvent. Phys. Rev. Lett. 2007, 98, 218301–218305. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, J.; Gaspari, G. The aspherity of random walks. J. Phys. A Math. Gen. 1986, 19, L191–L195. [Google Scholar] [CrossRef]
- Dima, R.I.; Thirumalai, D. Asymmetry in the shapes of folded and denatured states of proteins. J. Phys. Chem. B 2004, 108, 6564–6570. [Google Scholar] [CrossRef]
- Vandebroek, H.; Vanderzande, C. Dynamics of a polymer in an active and viscoelastic bath. Phys. Rev. E 2015, 92, 060601–060607. [Google Scholar] [CrossRef]
- Kaiser, A.; Babel, S.; ten Hagen, B.; von Ferber, C.; Löwen, H. How does a flexible chain of active particles swell? J. Chem. Phys. 2015, 142, 124905–124914. [Google Scholar] [CrossRef]
- Briscoe, W.H. Depletion forces between particles immersed in nanofluids. Curr. Opin. Colloid Interface Sci. 2015, 20, 46–53. [Google Scholar] [CrossRef]
- Chen, X.; Dong, X.; Be’er, A.; Swinney, H.L.; Zhang, H.P. Scale-Invariant Correlations in Dynamic Bacterial Clusters. Phys. Rev. Lett. 2012, 108, 148101–148106. [Google Scholar] [CrossRef] [PubMed]
- Wensink, H.H.; Dunkel, J.; Heidenreich, S.; Drescher, K.; Goldstein, R.E.; LCondenswen, H.; Yeomans, J.M. Meso-scale turbulence in living fluids. Proc. Natl. Acad. Sci. USA 2012, 109, 14308–14313. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zhang, B.; Zhao, N. Crowding-Activity Coupling Effect on Conformational Change of a Semi-Flexible Polymer. Polymers 2019, 11, 1021. https://doi.org/10.3390/polym11061021
Cao X, Zhang B, Zhao N. Crowding-Activity Coupling Effect on Conformational Change of a Semi-Flexible Polymer. Polymers. 2019; 11(6):1021. https://doi.org/10.3390/polym11061021
Chicago/Turabian StyleCao, Xiuli, Bingjie Zhang, and Nanrong Zhao. 2019. "Crowding-Activity Coupling Effect on Conformational Change of a Semi-Flexible Polymer" Polymers 11, no. 6: 1021. https://doi.org/10.3390/polym11061021
APA StyleCao, X., Zhang, B., & Zhao, N. (2019). Crowding-Activity Coupling Effect on Conformational Change of a Semi-Flexible Polymer. Polymers, 11(6), 1021. https://doi.org/10.3390/polym11061021




