Complementary Assessment of Commercial Photoluminescent Pigments Printed on Cotton Fabric
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Luminescent Samples by Screen-Printing
2.3. Polydimethylsiloxane (PDMS) Coating
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Spectroscopy
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
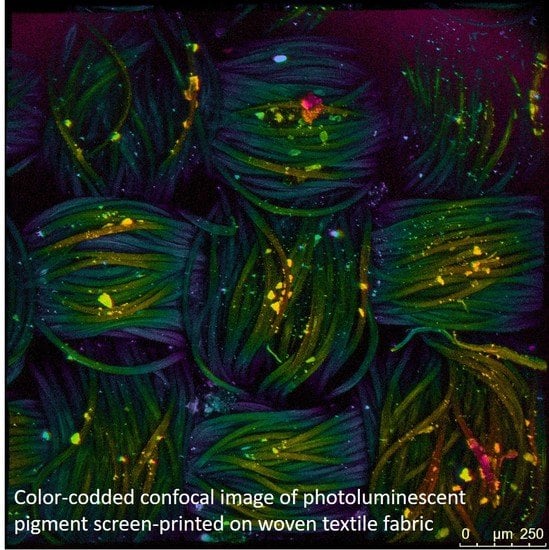
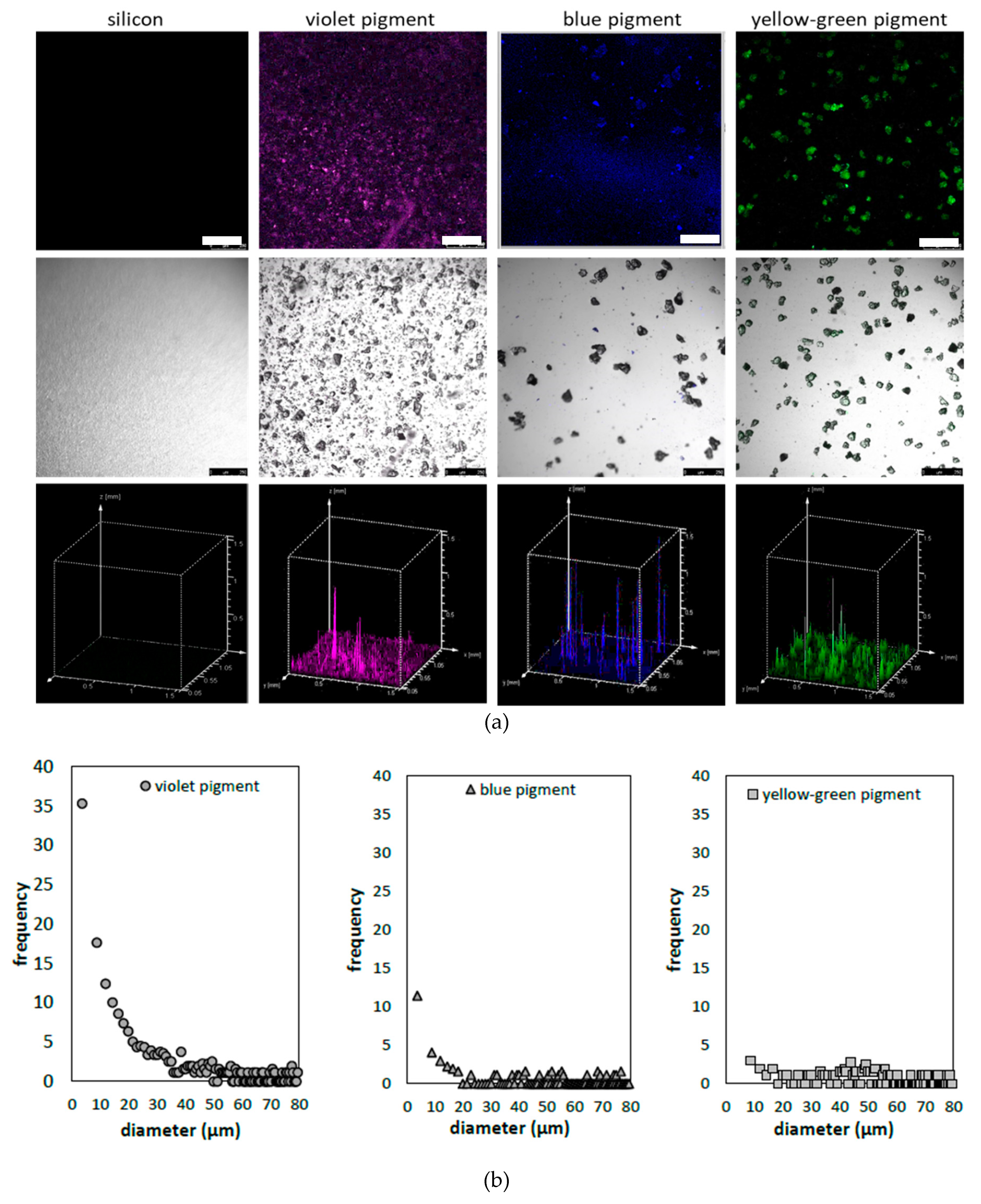
2.6. Confocal Fluorescent Microscopy (CFM)
2.7. Optical Properties
2.7.1. Ultraviolet–Visible (UV–Vis) Spectroscopy: Absorbance and Fluorescence Intensity Measurement
2.7.2. Reflectance
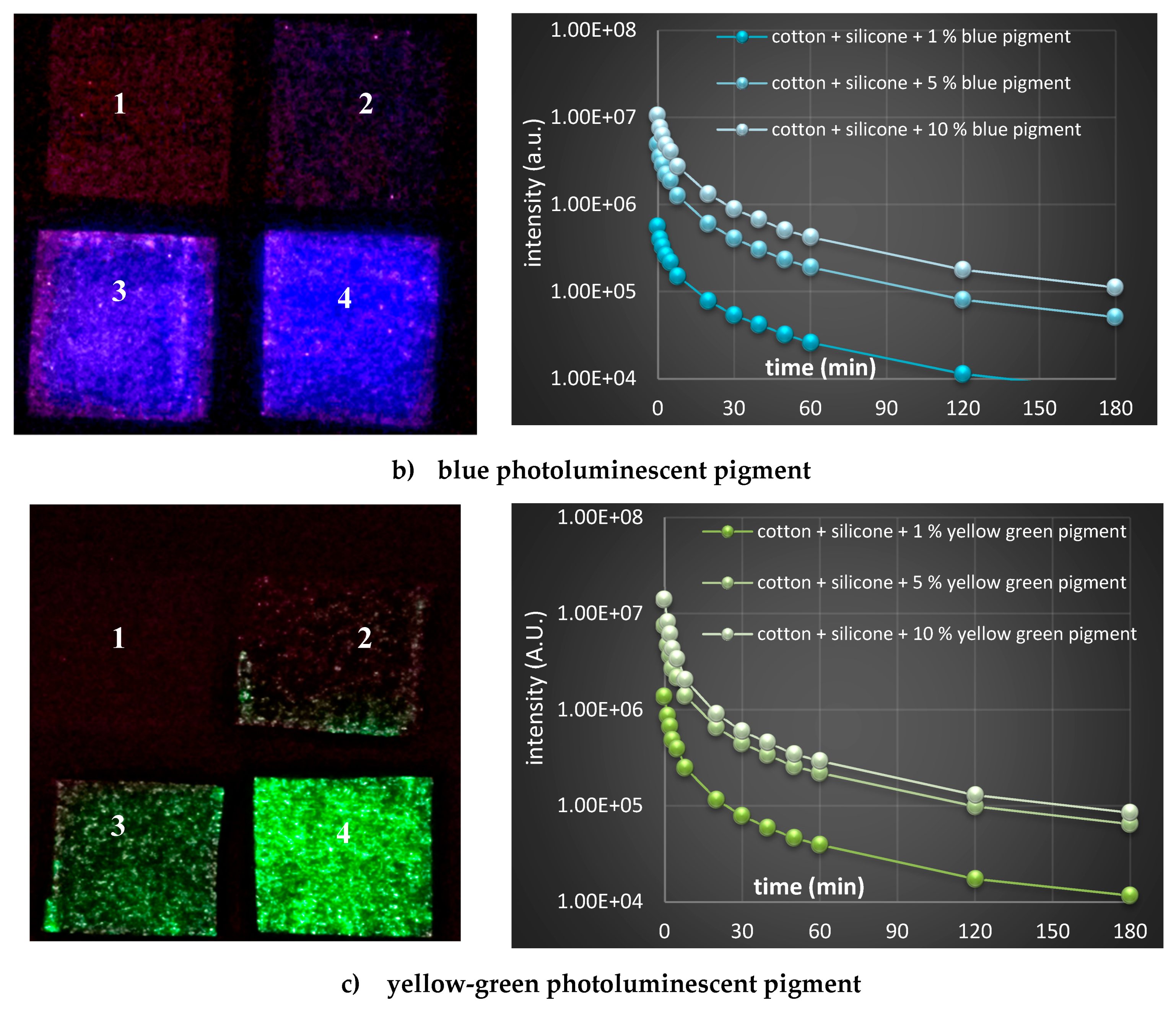
2.7.3. Afterglow Decay Kinetics
2.8. PDMS Coating Stability
3. Results and Discussion
3.1. Characterization of Photoluminescent Pigments
3.2. Characterization of Luminescent Pigment Printed on Cotton Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aspland, J.R. Chapter 14: Pigments as Textile Colorants: Pigmenting or Pigmentation. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.404.7996&rep=rep1&type=pdf (accessed on 13 May 2019).
- Kooroshnia, M. Designing a Two-Phase Glow-in-the-Dark Pattern on Textiles; Textile and Design Lab and Colab at Auckland University of Technology: Auckland, New Zealand, 2014. [Google Scholar]
- Van, P.M. Development and Research of Smart Functional Clothing Textiles. Available online: https://expertise.hogent.be/files/14910378/Smart_Functional_Clothing_Textiles.pdf (accessed on 19 July 2019).
- Yan, M.Y.; Ma, X.H.; Yang, Y.J.; Wang, X.; Cheong, W.C.; Chen, Z.H.; Xu, X.H.; Huang, Y.J.; Wang, S.; Lian, C.; et al. Biofabrication Strategy for Functional Fabrics. Nano Lett. 2018, 18, 6017–6021. [Google Scholar] [CrossRef] [PubMed]
- Salter, S.C.; Gardner, C.L.; Surman, J.J. Photoluminescent Logo for Vehicle Trim and Fabric. U.S. Patent No. 9,539,939, August 2014. [Google Scholar]
- Khattab, T.A.; Rehan, M.; Hamouda, T. Smart textile framework: Photochromic and fluorescent cellulosic fabric printed by strontium aluminate pigment. Carbohydr. Polym. 2018, 195, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, G. Inorganic Pigments; Walter de Gruyter GmbH: Berlin, Germany, 2017; pp. 274–284. [Google Scholar]
- Paterson, A.S.; Raja, B.; Garvey, G.; Kolhatkar, A.; Hagström, A.E.V.; Kourentzi, K.; Lee Randall, T.; Willson, R.C. Persistent Luminescence Strontium Aluminate Nanoparticles as Reporters in Lateral Flow Assays. Anal. Chem. 2014, 86, 9481–9488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gfroerer, T.H. Photoluminescence in Analysis of Surfaces and Interfaces. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Fernández-Osorio, A.; Rivera, C.E.; Vázquez-Olmos, A.; Chávez, J. Luminescent ceramic nano-pigments based on terbium-doped zinc aluminate: Synthesis, properties and performance. Dye. Pigment. 2015, 119, 22–29. [Google Scholar] [CrossRef]
- Khattab, T.A.; Rehan, M.; Hamdy, Y.; Shaheen, T.I. Facile Development of Photoluminescent Textile Fabric via Spray Coating of Eu(II)-Doped Strontium Aluminate. Ind. Eng. Chem. Res. 2018, 57, 11483–11492. [Google Scholar] [CrossRef]
- Mori, A.; Ohishi, Y.; Sudo, S. Erbium-doped tellurite glass fibre laser and amplifier. Electron. Lett. 1997, 10, 863. [Google Scholar] [CrossRef]
- Naftaly, M.; Jha, A. Nd3+-doped fluoroaluminate glasses for a 1.3 μm amplifier. J. Appl. Phys. 2000, 87, 2098–2104. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, C.; Song, Y.; Zheng, Y.; Zhao, K. Synthesis and luminescence properties of Er-doped and Er/Yb-codoped CoAl2O4 pigments. Ceram. Int. 2018, 44, 12909–12916. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S. Photoluminescence and energy transfer studies of Dy3+ ions doped lithium lead alumino borate glasses for w-LED and laser applications. J. Lumin. 2017, 192, 832–841. [Google Scholar] [CrossRef]
- Madhukar Reddy, C.; Deva Prasad Raju, B.; John Sushma, N.; Dhoble, N.S.; Dhoble, S.J. A review on optical and photoluminescence studies of RE3+ (RE=Sm, Dy, Eu, Tb and Nd) ions doped LCZSFB glasses. Renew. Sustain. Energy Rev. 2015, 51, 566–584. [Google Scholar] [CrossRef]
- Escudero, A.; Becerro, A.I.; Carrillo-Carrión, C.; Núñez, N.O.; Zyuzin, M.V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017, 6, 881–921. [Google Scholar] [CrossRef]
- Comelli, D.; D’Andrea, C.; Valentini, G.; Cubeddu, R.; Colombo, C.; Toniolo, L. Fluorescence lifetime imaging and spectroscopy as tools for nondestructive analysis of works of art. Appl. Opt. 2004, 43, 2175–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Structural analysis of microparticles by confocal laser scanning microscopy. AAPS PharmSciTech 2000, 1, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, J.P.; Swift, K.M.; Ruan, Q.; Perfetto, S.; Gratton, E.; Tetin, S.Y. Simplified confocal microscope for counting particles at low concentrations. Rev. Sci. Instrum. 2013, 84, 74301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordowitz, J.R.; Montgomery, B.L. Exploiting the autofluorescent properties of photosynthetic pigments for analysis of pigmentation and morphology in live Fremyella diplosiphon cells. Sensors 2010, 10, 6969–6979. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Widiyastuti, W.; Ogi, T.; Lenggoro, W.I.; Okuyama, K. Correlations between Crystallite/Particle Size and Photoluminescence Properties of Submicrometer Phosphors. Chem. Mater. 2007, 19, 1723–1730. [Google Scholar] [CrossRef]
- Swart, H.C. Surface sensitive techniques for advanced characterization of luminescent Materials. Materials 2017, 10, 906. [Google Scholar] [CrossRef]
- Rao, J.C.; Wang, Y.J.; Wang, W.; Ke, H.; Li, Y.C.; Zhao, Y.; Diao, Z.L.; Jia, D.C.; Zhou, Y. Mechanism of superior luminescent and high-efficiency photocatalytic properties of Eu-doped calcium aluminate by low-cost self-propagating combustion synthesis technique. Sci. Rep. 2017, 7, 2906. [Google Scholar] [CrossRef] [Green Version]
- Burnell-Jones, P. Luminescent Gel Coats and Moldable Resins. U.S. Patent No. 6,207,077, 27 March 2001. [Google Scholar]
- Sepahvandi, A.; Eskandari, M.; Moztarzadeh, F. Fabrication and characterization of SrAl2O4: Eu2 + Dy3 +/CS-PCL electrospun nanocomposite scaffold for retinal tissue regeneration. Mater. Sci. Eng. C 2016, 66, 306–314. [Google Scholar] [CrossRef]
- Dutczak, D.; Jüstel, T.; Ronda, C.; Meijerink, A. Eu2+ luminescence in strontium aluminates. Phys. Chem. Chem. Phys. 2015, 17, 15236–15249. [Google Scholar] [CrossRef]
- Sternig, A.; Bernardi, J.; McKenna, K.; Diwald, O. Surface-specific visible light luminescence from composite metal oxide nanocrystals. J. Mater. Sci. 2015, 50, 8153–8165. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.T.; Selvam, N.C.S.; Adinaveen, T.; Kennedy, L.J.; Vijaya, J.J. Strontium(II)-added CoAl2O4 nanocatalysts for the selective oxidation of alcohols. React. Kinet. Mech. Catal. 2012, 106, 379–394. [Google Scholar] [CrossRef]
- Yerpude, A.N.; Dhoble, S.J.; Ramesh, B.; Raju, B.D.P. Photoluminescence And Decay Properties Of Sm3+ And Dy3+ In SrAl4O7 Phosphor. Adv. Mater. Lett. 2015, 6, 1111–1115. [Google Scholar] [CrossRef]
- Saharin, N.S.B.; Ahmad, N.; Tajuddin, H.; Tamuri, A.R. Thermoluminescence Properties of Aluminium Oxide doped Strontium, Lithium and Germanium prepared by Combustion Synthesis method. EPJ Web Conf. 2017, 156, 1. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Yi, L.; Zhang, X.; Huang, S. Size-Dependent Optical Properties of Nanoscale and Bulk Long Persistent Phosphor SrAl2O4: Eu2+, Dy3+. J. Nanomater. 2015, 2015, 1–7. [Google Scholar]
- Yan, Y.; Ge, M.; Li, Y.; Kumar, D. Morphology and spectral characteristics of a luminous fiber containing a rare earth strontium aluminate. Text. Res. J. 2012, 82, 1819–1826. [Google Scholar] [CrossRef]
- Bite, I.; Krieke, G.; Zolotarjovs, A.; Laganovska, K.; Liepina, V.; Smits, K.; Auzins, K.; Grigorjeva, L.; Millers, D.; Skuja, L. Novel method of phosphorescent strontium aluminate coating preparation on aluminum. Mater. Des. 2018, 160, 794–802. [Google Scholar] [CrossRef]
- Peng, T.; Yang, H.; Pu, X.; Hu, B.; Jiang, Z.; Yan, C. Combustion synthesis and photoluminescence of SrAl2O4: Eu, Dy phosphor nanoparticles. Mater. Lett. 2004, 58, 352–356. [Google Scholar] [CrossRef]
- Adam, J.; Metzger, W.; Koch, M.; Rogin, P.; Coenen, T.; Atchison, J.S.; König, P. Light Emission Intensities of Luminescent Y₂O₃:Eu and Gd₂O₃:Eu Particles of Various Sizes. Nanomater. 2017, 7, 26. [Google Scholar] [CrossRef]
- Launner, P.J.; Arkles, B. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations; Gelest Inc.: Morrisville, PA, USA, 2013. [Google Scholar]
- Burres, E. Measuring Optic Brighteners in Ambient Water Samples Using a Fluorometer. Available online: https://www.waterboards.ca.gov/water_issues/programs/swamp/docs/cwt/guidance/3414.pdf (accessed on 19 July 2019).
- Prasad, V.; Semwogerere, D.; Weeks, E.R. Confocal microscopy of colloids. J. Phys. Condens. Matter 2007, 19, 25. [Google Scholar] [CrossRef]
- Kirkbride, K.P.; Tridico, S.R. The application of laser scanning confocal microscopy to the examination of hairs and textile fibers: An initial investigation. Forensic Sci. Int. 2010, 195, 28–35. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorgieva, S.; Virant, N.; Ojstršek, A. Complementary Assessment of Commercial Photoluminescent Pigments Printed on Cotton Fabric. Polymers 2019, 11, 1216. https://doi.org/10.3390/polym11071216
Gorgieva S, Virant N, Ojstršek A. Complementary Assessment of Commercial Photoluminescent Pigments Printed on Cotton Fabric. Polymers. 2019; 11(7):1216. https://doi.org/10.3390/polym11071216
Chicago/Turabian StyleGorgieva, Selestina, Natalija Virant, and Alenka Ojstršek. 2019. "Complementary Assessment of Commercial Photoluminescent Pigments Printed on Cotton Fabric" Polymers 11, no. 7: 1216. https://doi.org/10.3390/polym11071216
APA StyleGorgieva, S., Virant, N., & Ojstršek, A. (2019). Complementary Assessment of Commercial Photoluminescent Pigments Printed on Cotton Fabric. Polymers, 11(7), 1216. https://doi.org/10.3390/polym11071216