Non-Equilibrium Potential Responses towards Neutral Orcinol Using All-Solid-State Potentiometric Sensors Integrated with Molecularly Imprinted Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Materials
2.3. Polymer Synthesis and Characterization
2.4. Membranes and Electrodes Construction
3. Results and Discussions
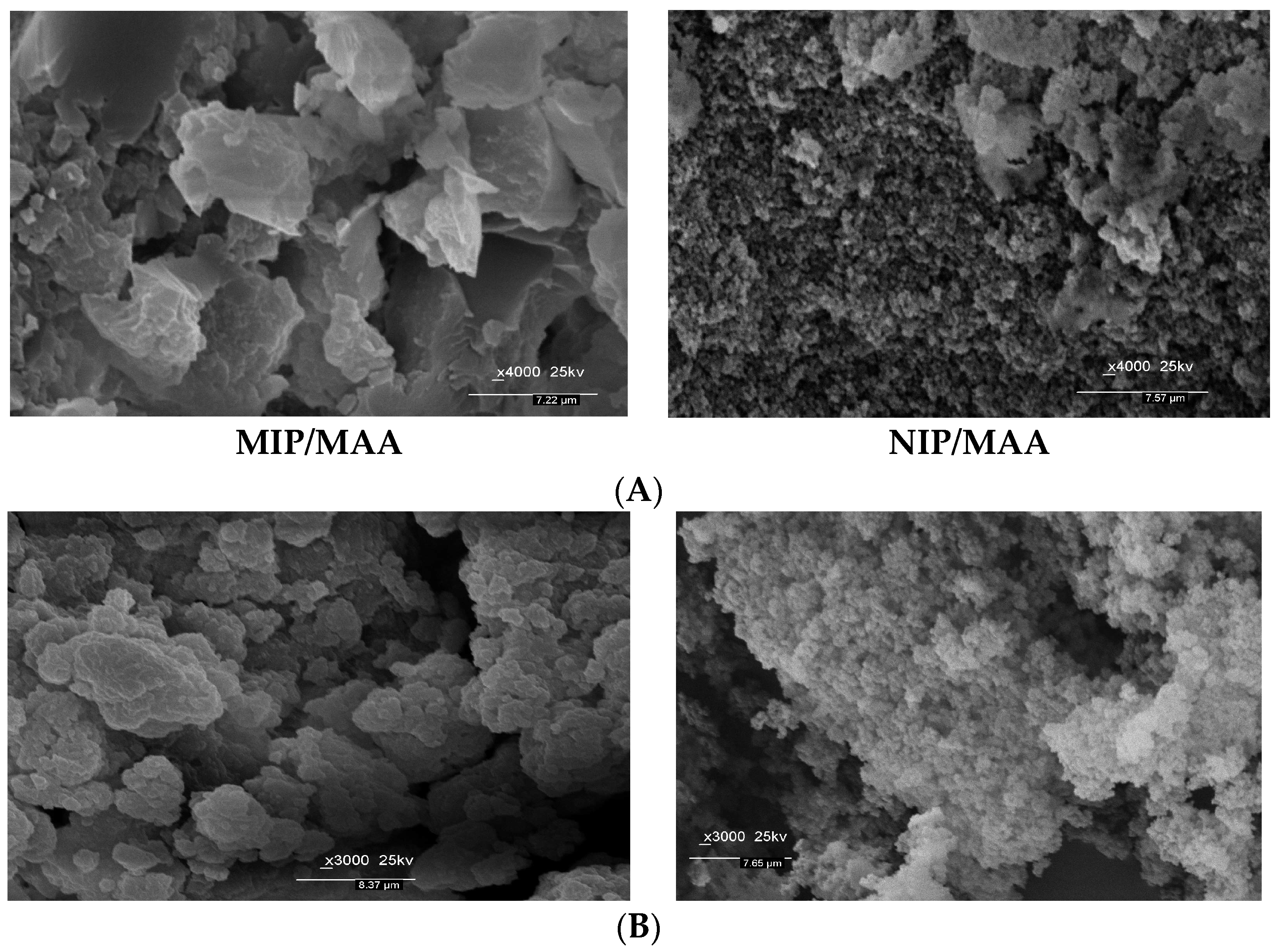
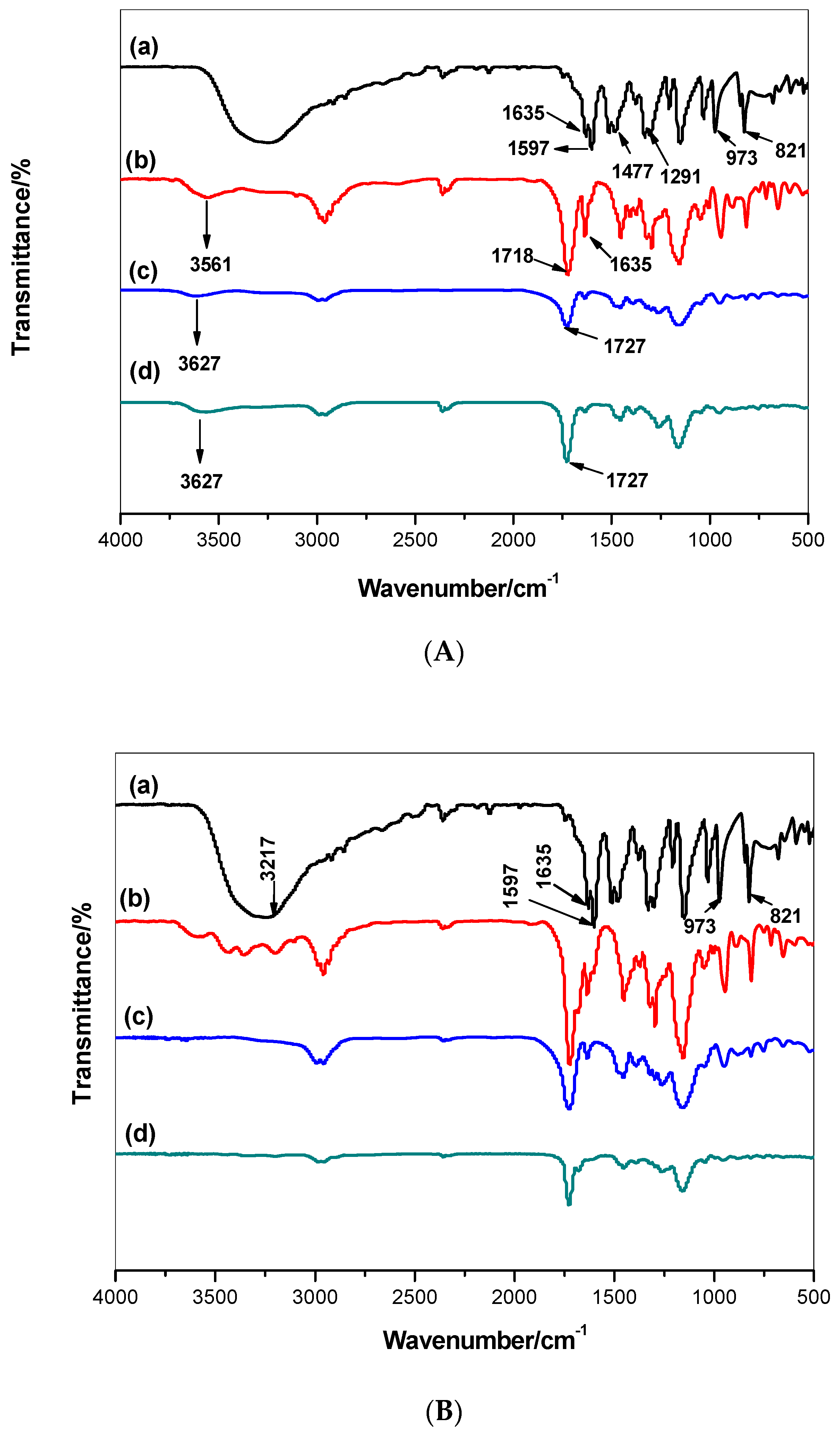
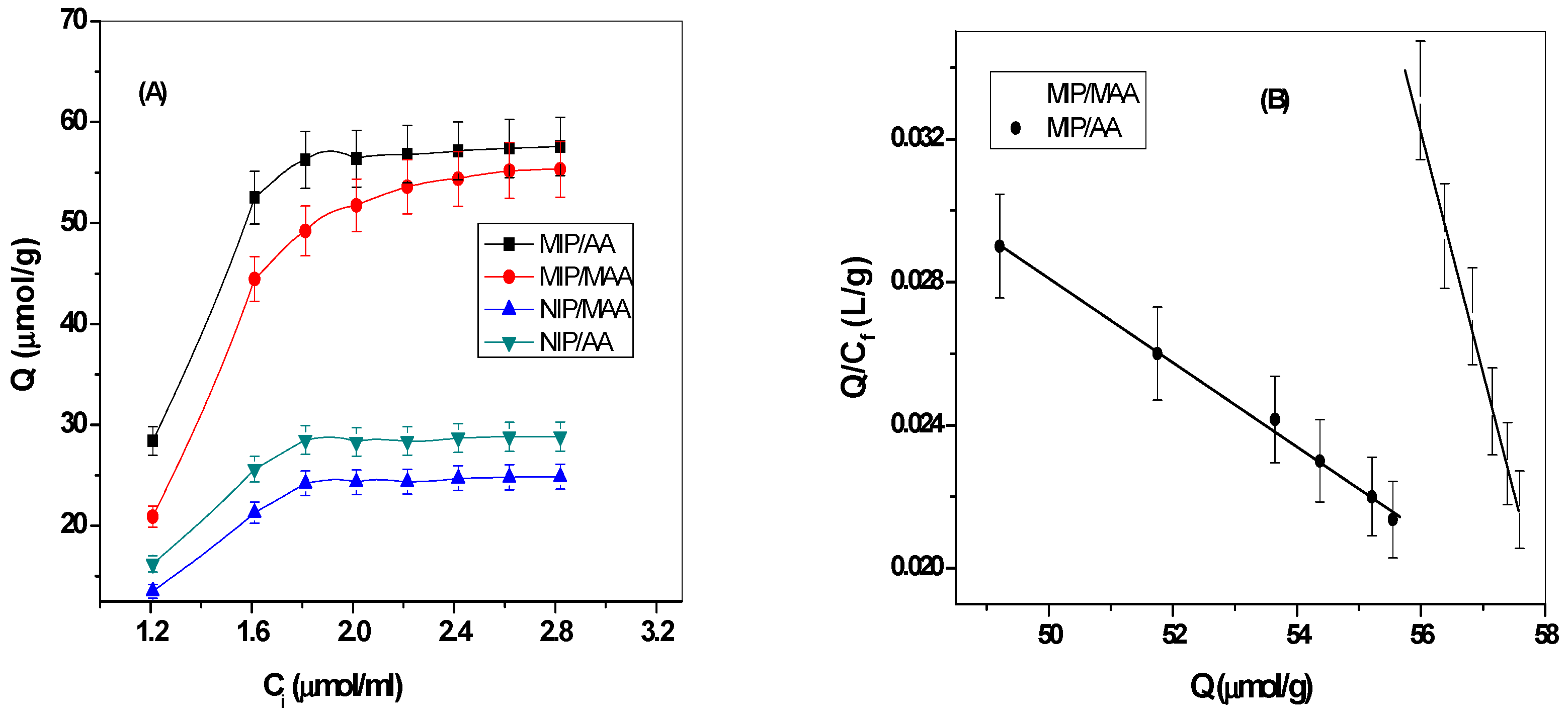
3.1. Synthesis of MIPs and Their Characterization
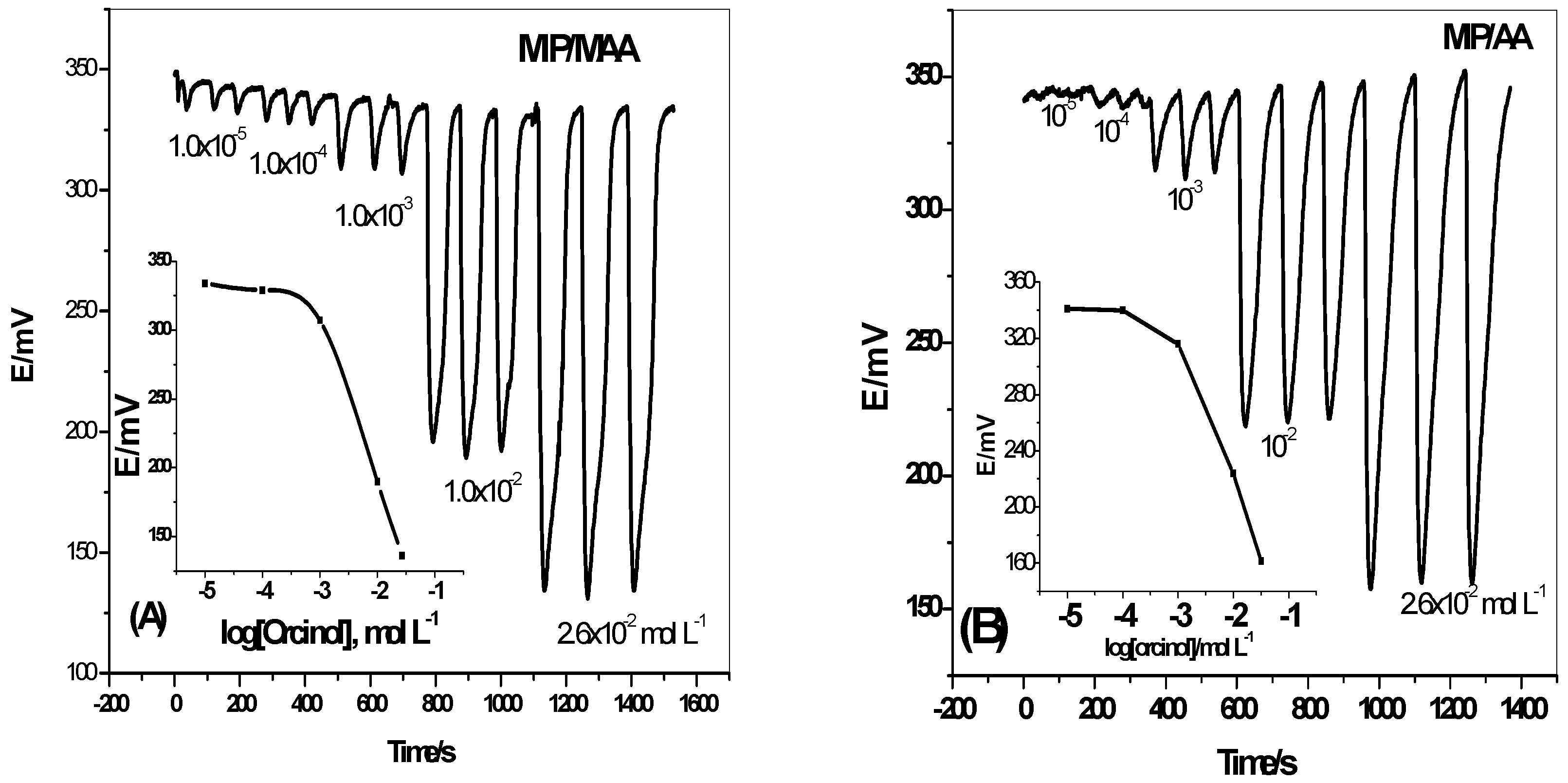
3.2. Performance Characteristics of the Sensors
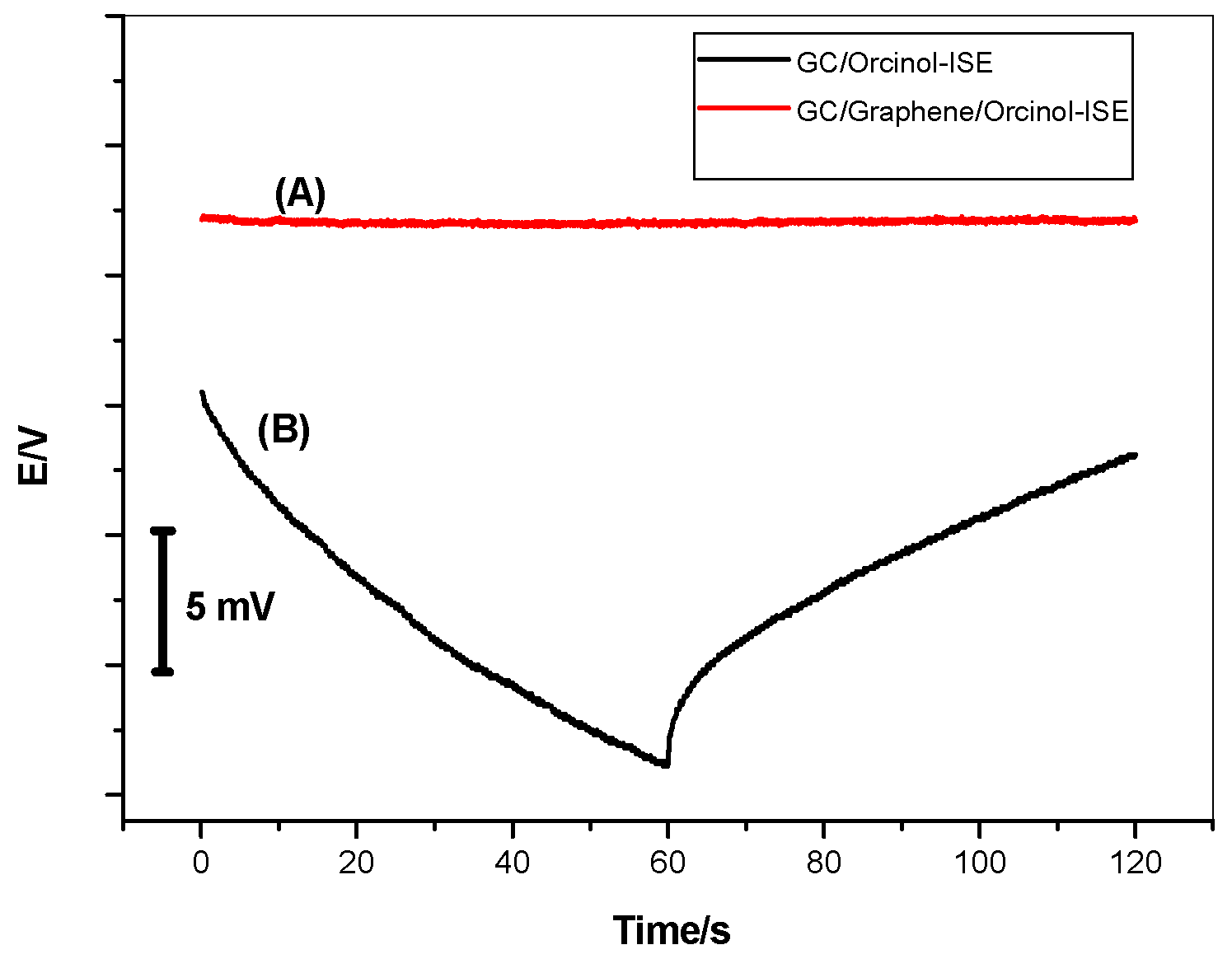
3.3. Potential Stability for MIP/MAA/Gr-ISE
3.4. Selectivity
3.5. Optimization of the Flow-Through System
3.6. Analytical Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Yin, T.; Qin, W. An all-solid-state polymeric membrane Pb2+-selective electrode with bimodal pore C60 as solid contact. Anal. Chim. Acta 2015, 876, 49–54. [Google Scholar] [CrossRef] [PubMed]
- El-Shalakany, H.H.; Hamza, M.S.; Mohamed, A.H.K. Solid-contact potentiometric sensors for reliable automatic quantification of 2,4-dichlorophenol (2,4-DCP) as a food taint. Meas. Sci. Technol. 2018, 29, 105102. [Google Scholar] [CrossRef]
- Li, J.; Yin, T.; Qin, W. An effective solid contact for an all-solid-state polymeric membrane Cd2+-selective electrode: Three-dimensional porous graphene-mesoporous platinum nanoparticle composite. Sens. Actuators B 2017, 239, 438–446. [Google Scholar] [CrossRef]
- Kamel, A.H.; Al Hamid, F.A.; Soror, T.Y.; Galal, H.R.; El Gendy, F.A. Solid contact biosensor based on man-tailored polymers for acetylcholine detection: Application to acetycholinesterase assay. Eur. Chem. Bull. 2016, 5, 266–273. [Google Scholar]
- Kamel, A.H.; Galal, H.R. Novel Planar Chip Biosensors for Potentiometric Immunoassay of Acid Phosphatase Activity Based on the Use of Ion Association Complexes as Novel Electroactive Materials. Int. J. Electrochem. Sci. 2014, 9, 5776–5787. [Google Scholar]
- Hassan, S.S.M.; Elnemma, E.M.; Mahmoud, W.H.; Mohamed, A.H.K. Continuous Potentiometric monitoring of Sildenafil Citrate (Viagra) in Pharmaceutical Preparations using Novel membrane sensors. J. Appl. Electrochem. 2006, 36, 139–146. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; El-Naby, H.A. New potentiometric sensors based on selective recognition sites for determination of ephedrine in some pharmaceuticals and biological fluids. Talanta 2013, 103, 330–336. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All Solid-State Poly(Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and Rapid Detection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Graphite Solid-Contact Mepiquat Potentiometric Sensors Based on Molecularly Imprinted Polymers and Their Application to Flow Through Analysis. Anal. Meth. 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- Kamel, A.H.; Khalifa, M.E.; Elgendy, F.A.; Abd El-Maksoud, S.A. New Plastic Membrane Sensors for Selective Determination of Pyridine as a Hazardous Pollutant: Validation and Applications to Flow Injection Analysis. Int. J. Electrochem. Sci. 2014, 9, 1663–1677. [Google Scholar]
- Kamel, A.H.; Argig, A.A.A. Automatic potentiometric system for quantification of three imidazole derivatives based on new polymeric PVC membrane sensors. Ionics 2017, 12, 466–2211. [Google Scholar] [CrossRef]
- Jaramillo, A.; Lopez, S.; Justice, J.B., Jr.; Salamone, J.D.; Neil, D.B. Acetycholine and choline ion-selective microelectrodes. Anal. Chim. Acta 1983, 146, 149–159. [Google Scholar] [CrossRef]
- Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaikoff, B. Molecularly Imprinted Polymers—Potential and Challenges in Analytical Chemistry. Anal. Chim. Acta 2005, 36, 31–39. [Google Scholar] [CrossRef]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Hassan, A.M. E Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Rubinova, N.; Chumbimunitorres, K.; Bakker, E. Solid-contact potentiometric polymer membrane microelectrodes for the detection of silver ions at the femtomole level. Sens. Actuators B Chem. 2007, 121, 135–141. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Kirf, M.K. Chemical Eyes—Visualization and Interpretation of Chemical Gradients in Stratified Water Bodies. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2014. [Google Scholar]
- Mattinen, U.; Hupa, E.; Yrjänä, V.; Bobacka, J. A new signal readout principle for solid-contact ion-selective electrodes (SC-ISEs). Anal. Chem. 2016, 88, 4369–4374. [Google Scholar]
- J´agerszki, G.; Tak´acs, A.; Bitter, I.; Gyurcs´anyi, R.E. Solid-State Ion Channels for Potentiometric Sensing. Angew. Chem. Int. Ed. 2011, 50, 1656–1659. [Google Scholar] [CrossRef]
- Lindner, E.; Gyurcsányi, R.J. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Sutter, J.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Solid-contact polymeric membrane electrodes with detection limits in the subnanomolar range. Anal. Chim. Acta 2004, 523, 53–59. [Google Scholar] [CrossRef]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; El-Galil, E.A.A.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef] [PubMed]
- Robiquet, E. Analytique des lichens de l’orseille. Ann. Chim. Phys. 1829, 42, 236–257. [Google Scholar]
- Fernell, W.R.; King, H.K. The simultaneous determination of pentose and hexose in mixtures of sugars. Analyst 1953, 78, 80–83. [Google Scholar] [CrossRef]
- Almog, R.; Shirey, T.L. A modified orcinol test for the specific determination of RNA. Anal. Biochem. 1978, 91, 130–137. [Google Scholar] [CrossRef]
- IUPAC Analytical Chemistry Division; Commission on Analytical Nomenclature. Recommendations for Nomenclature of Ion-Selective Electrodes. Pure Appl. Chem. 1976, 48, 127–132. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]
- Available online: https://Pubchem.ncbi.nlm.nih.gov (accessed on 18 July 2019).


| Parameter | Solid-Contact ISEs | Liquid-Contact ISEs | ||||
|---|---|---|---|---|---|---|
| GC/Gr/MIP/ MAA-ISE | GC/Gr/MIP/ AA-ISE | MIP/MAA | NIP/MAA | MIP/AA | NIP/AA | |
| Slope (mV) | −160 ± 3.3 | −55.8 ± 1.1 | −92.2 ± 0.7 | −35.2 ± 1.5 | −78.8 ± 1.3 | −39.4 ± 1.1 |
| Correlation coefficient, (r2) | −0.999 | −0.999 | −0.999 | −0.998 | −0.999 | −0.997 |
| Linear range, (M) | 4.1 × 10−5– 1.0 × 10−2 | 8.0 × 10−6– 1.0 × 10−2 | 5.8 × 10−4– 1.0 × 10−2 | 6.0 × 10−3– 1.0 × 10−2 | 4.4 × 10−4– 10-2 | 4.0 × 10−3– 1.0 × 10−2 |
| Detection limit, (M) | 1.7 × 10−5 | 3.3 × 10−6 | 1.4 × 10-5 | 1.5 × 10−3 | 1.1 × 10−4 | 1.0 × 10−3 |
| Response time, (s) | >10 | >10 | >10 | >10 | >10 | <10 |
| Life Span, (week) | 12 | 12 | 12 | 4 | 12 | 4 |
| Standard deviation, σv (mV) | 1.52 | 1.1 | 1 | 0.8 | 1 | 1.2 |
| Accuracy % | 99.2 | 98.1 | 98 | 99.8 | 99.4 | 97.2 |
| Repeatability, CVw (%) | 0.24 | 0.3 | 0.7 | 0.3 | 0.55 | 0.87 |
| Interfering Ion | GC/Gr/MIP/MAA-ISE | GC/Gr/MIP/AA-ISE | Log POct,w | pKa1 |
|---|---|---|---|---|
| Pyrogallol | −2.03 | −2.14 | 0.97 | 9.01 |
| I− | −0.2 | −1.12 | - | - |
| Br− | −2.33 | −1.81 | - | - |
| NO2− | −2.17 | −1.94 | - | - |
| NO3− | −1.74 | −1.81 | - | - |
| CH3COO− | −1.94 | −1.15 | - | - |
| SCN− | −0.53 | −2.04 | - | - |
| S2O3−2 | −2.88 | −2.67 | - | - |
| SO3−2 | −4.33 | −3.38 | - | - |
| PO4−3 | −3.44 | −2.04 | - | - |
| Phenol | −2.51 | −1.79 | 1.46 | 9.99 |
| 2,4 DCP | −0.3 | −1.57 | 3.06 | 7.89 |
| Picric acid | 1.56 | −0.53 | 1.44 | 0.42 |
| 3,4- dihydroxytolune | −1.60 | −1.85 | 1.37 | 10.47 |
| 4-methyl catechol | −2.10 | −2.70 | 1.37 | 9.55 |
| Resorcinol | −2.04 | −2.31 | 0.8 | 9.3 |
| Parameter | MIP/AA | MIP/MAA |
|---|---|---|
| Slope, mV/decade | −101.3 ± 1.3 | −119.1 ± 0.9 |
| Correlation coefficient, r | −0.987 | −0.999 |
| Linear range, M | 9.0 × 10−4–10−2 | 9.0 × 10−4–10−2 |
| Detection limit, M | 7.7 × 10−4 | 7.2 × 10−4 |
| Life span, week | 12 | 12 |
| Optimum flow rate, mL/min | 3.5 | 3.5 |
| Sample frequency, sample/h | 41 | 39 |
| Added, mM | * Found, mM | Recovery% |
|---|---|---|
| 0.88 | 0.89 ± 0.03 | 101.1 |
| 1.60 | 1.61 ± 0.2 | 100.6 |
| 2.40 | 2.30 ± 0.1 | 95.8 |
| 3.20 | 2.9 ± 0.3 | 90.6 |
| 4.00 | 3.7 ± 0.4 | 92.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. M. Hassan, S.; E. Amr, A.E.-G.; H. A. Elbehery, N.; A. Al-Omar, M.; H. Kamel, A. Non-Equilibrium Potential Responses towards Neutral Orcinol Using All-Solid-State Potentiometric Sensors Integrated with Molecularly Imprinted Polymers. Polymers 2019, 11, 1232. https://doi.org/10.3390/polym11081232
S. M. Hassan S, E. Amr AE-G, H. A. Elbehery N, A. Al-Omar M, H. Kamel A. Non-Equilibrium Potential Responses towards Neutral Orcinol Using All-Solid-State Potentiometric Sensors Integrated with Molecularly Imprinted Polymers. Polymers. 2019; 11(8):1232. https://doi.org/10.3390/polym11081232
Chicago/Turabian StyleS. M. Hassan, Saad, Abd El-Galil E. Amr, Nada H. A. Elbehery, Mohamed A. Al-Omar, and Ayman H. Kamel. 2019. "Non-Equilibrium Potential Responses towards Neutral Orcinol Using All-Solid-State Potentiometric Sensors Integrated with Molecularly Imprinted Polymers" Polymers 11, no. 8: 1232. https://doi.org/10.3390/polym11081232







