Application of PEG-Covered Non-Biodegradable Polyelectrolyte Microcapsules in the Crustacean Circulatory System on the Example of the Amphipod Eulimnogammarus verrucosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microcapsules
2.3. Animal Sampling and Maintenance
2.4. Injection into the Circulatory System of Amphipods
2.5. Fluorescent Microscopy and Spectroscopy of Amphipods
2.6. Determination of Biochemical Stress Response Markers
2.7. Monitoring the Healing of the Injection Wound
2.8. Determination of the Phenoloxidase Activity
2.9. Primary Culture of Amphipod Hemocytes
2.10. Analysis of Hemocyte Aggregation after Contact with Microcapsules
2.11. Preparation of the Histological Section
2.12. Statistical Analysis
3. Results and Discussion
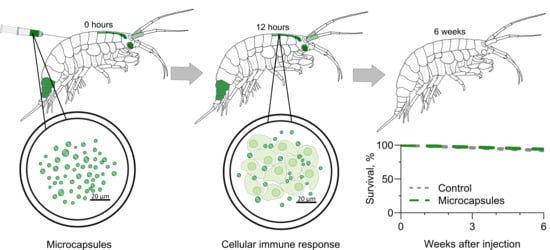
3.1. Visibility of the Microcapsules inside Amphipods: Distribution and Autofluorescence
3.2. Reaction of the E. verrucosus Organism to the Injection of Microcapsules
3.3. Amphipod Immune Response to the Microcapsules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Timin, A.S.; Gould, D.J.; Sukhorukov, G.B. Multi-layer microcapsules: Fresh insights and new applications. Expert Opin. Drug Deliv. 2017, 14, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Carmagnola, I.; Nardo, T.; Chiono, V. Layer-by-layer assembly for biomedical applications in the last decade. Nanotechnol. 2015, 26, 422001. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Bui, T.; Nguyen, V.; Bui, T.; Tran, T.; Phan, Q.; Hoang, T. Adsorption of polyelectrolyte onto nanosilica synthesized from rice husk: Characteristics, mechanisms, and application for antibiotic removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Tran, T.T.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-controlled synthesis of vaterite calcium carbonate by the mixing method: Aiming for nanosized particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Svenskaya, Y.I.; Fattah, H.; Inozemtseva, O.A.; Ivanova, A.G.; Shtykov, S.N.; Gorin, D.A.; Parakhonskiy, B.V. Key parameters for size-and shape-controlled synthesis of vaterite particles. Cryst. Growth Des. 2017, 18, 331–337. [Google Scholar] [CrossRef]
- Kreft, O.; Javier, A.M.; Sukhorukov, G.B.; Parak, W.J. Polymer microcapsules as mobile local pH-sensors. J. Mater. Chem. 2007, 17, 4471–4476. [Google Scholar] [CrossRef]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.U.; Pham, T.T.; Nguyen, T.A.H.; Nguyen, T.K.T.; Vu, N.D.; Le, T.S.; Vu, C.M.; Kobayashi, M. Adsorption of poly (styrenesulfonate) onto different-sized alumina particles: Characteristics and mechanisms. Colloid Polym. Sci. 2019, 297, 13–22. [Google Scholar] [CrossRef]
- Wattendorf, U.; Kreft, O.; Textor, M.; Sukhorukov, G.B.; Merkle, H.P. Stable stealth function for hollow polyelectrolyte microcapsules through a poly (ethylene glycol) grafted polyelectrolyte adlayer. Biomacromolecules 2008, 9, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sadovoy, A.; Teh, C.; Korzh, V.; Escobar, M.; Meglinski, I. Microencapsulated bio-markers for assessment of stress conditions in aquatic organisms in vivo. Laser Phys. Lett. 2012, 9, 542. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, N.I.; MacCormack, T.J. Ecophysiological perspectives on engineered nanomaterial toxicity in fish and crustaceans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 193, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gurkov, A.; Shchapova, E.; Bedulina, D.; Baduev, B.; Borvinskaya, E.; Meglinski, I.; Timofeyev, M. Remote in vivo stress assessment of aquatic animals with microencapsulated biomarkers for environmental monitoring. Sci. Rep. 2016, 6, 36427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and biosensors based on polyelectrolyte microcapsules containing fluorescent dyes and enzymes. Anal. Bioanal. Chem. 2013, 405, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Takhteev, V.V.; Berezina, N.A.; Sidorov, D.A. Checklist of the Amphipoda (Crustacea) from continental waters of Russia, with data on alien species. Arthropoda Sel. 2015, 24, 335–370. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Karnaukhov, D.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Simple and effective administration and visualization of microparticles in the circulatory system of small fishes using kidney injection. J. Vis. Exp. 2018, 136, e57491. [Google Scholar] [CrossRef]
- Gurkov, A.; Borvinskaya, E.; Shchapova, E.; Timofeyev, M. Restraining small decapods and amphipods for in vivo laboratory studies. Crustaceana 2018, 91, 517–525. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Meglinski, I.; Timofeyev, M. Distribution of PEG-coated hollow polyelectrolyte microcapsules after introduction into the circulatory system and muscles of zebrafish. Biol. Open 2018, 7, bio030015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedulina, D.S.; Evgen’ev, M.B.; Timofeyev, M.A.; Protopopova, M.V.; Garbuz, D.G.; Pavlichenko, V.V.; Luckenbach, T.; Shatilina, Z.M.; Axenov-Gribanov, D.V.; Gurkov, A.N.; et al. Expression patterns and organization of the hsp70 genes correlate with thermotolerance in two congener endemic amphipod species (Eulimnogammarus cyaneus and E. verrucosus) from Lake Baikal. Mol. Ecol. 2013, 22, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Bedulina, D.S.; Zimmer, M.; Timofeyev, M.A. Sub-littoral and supra-littoral amphipods respond differently to acute thermal stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Axenov-Gribanov, D.; Bedulina, D.; Shatilina, Z.; Jakob, L.; Vereshchagina, K.; Lubyaga, Y.; Gurkov, A.; Shchapova, E.; Luckenbach, T.; Lucassen, M.; et al. Thermal preference ranges correlate with stable signals of universal stress markers in Lake Baikal endemic and Holarctic amphipods. PLoS ONE 2016, 11, e0164226. [Google Scholar] [CrossRef] [PubMed]
- Shchapova, E.P.; Axenov-Gribanov, D.V.; Lubyaga, Y.A.; Shatilina, Z.M.; Vereshchagina, K.P.; Madyarova, E.V.; Timofeyev, M.A. Crude oil at concentrations considered safe promotes rapid stress-response in Lake Baikal endemic amphipods. Hydrobiologia 2018, 805, 189–201. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Bilandžija, H.; Abraham, L.; Ma, L.; Renner, K.J.; Jeffery, W.R. Behavioural changes controlled by catecholaminergic systems explain recurrent loss of pigmentation in cavefish. Proc. R. Soc. B 2018, 285, 20180243. [Google Scholar] [CrossRef]
- Cornet, S.; Biard, C.; Moret, Y. Variation in immune defence among populations of Gammarus pulex (Crustacea: Amphipoda). Oecologia 2009, 159, 257–269. [Google Scholar] [CrossRef]
- Laughton, A.M.; Siva-Jothy, M.T. A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee, Apis mellifera. Apidologie 2011, 42, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Jakob, L.; Axenov-Gribanov, D.V.; Gurkov, A.N.; Ginzburg, M.; Bedulina, D.S.; Timofeyev, M.A.; Luckenbach, T.; Lucassen, M.; Sartoris, F.J.; Pörtner, H.O. Lake Baikal amphipods under climate change: Thermal constraints and ecological consequences. Ecosphere 2016, 7, 1–15. [Google Scholar] [CrossRef]
- George, S.K.; Dhar, A.K. An improved method of cell culture system from eye stalk, hepatopancreas, muscle, ovary, and hemocytes of Penaeus vanname. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, V.; Palaniandavar, M.; Periasamy, V.S.; Akbarsha, M.A. Ru(phen)2(dppz)]2+ as an efficient optical probe for staining nuclear components. J. Inorg. Biochem. 2010, 104, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Presnell, J.K.; Schreibman, M.P.; Humason, G.L. Humason’s Animal Tissue Techniques Humason. Humason’s Animal Tissue Techniques; Johns Hopkins University Press: Baltimore, MD, USA, 1997. [Google Scholar]
- Mikodina, E.V.; Sedova, M.A.; Chmilevsky, D.A.; Mikulin, A.E.; Pianova, S.V.; Poluektova, O.G. Histology for Ichthyologists: Experience and Advices; VNIRO: Moscow, Russia, 2009. [Google Scholar]
- Antipov, A.A.; Sukhorukov, G.B.; Leporatti, S.; Radtchenko, I.L.; Donath, E.; Möhwald, H. Polyelectrolyte multilayer capsule permeability control. Colloids Surf. 2002, 198, 535–541. [Google Scholar] [CrossRef]
- Déjugnat, C.; Sukhorukov, G.B. pH-responsive properties of hollow polyelectrolyte microcapsules templated on various cores. Langmuir 2004, 20, 7265–7269. [Google Scholar] [CrossRef]
- Tuan, V.V.; Dantas-Lima, J.J.; Thuong, K.V.; Li, W.; Grauwet, K.; Bossier, P.; Nauwynck, H.J. Differences in uptake and killing of pathogenic and non-pathogenic bacteria by haemocyte subpopulations of penaeid shrimp, Litopenaeus vannamei, (Boone). J. Fish Dis. 2016, 39, 163–174. [Google Scholar] [CrossRef]
- De Geest, B.G.; Vandenbroucke, R.E.; Guenther, A.M.; Sukhorukov, G.B.; Hennink, W.E.; Sanders, N.N.; Demeester, J.; De Smedt, S.C. Intracellularly degradable polyelectrolyte microcapsules. Adv. Mater. 2006, 18, 1005–1009. [Google Scholar] [CrossRef]
- Christensen, B.M.; Li, J.; Chen, C.C.; Nappi, A.J. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005, 21, 192–199. [Google Scholar] [CrossRef]
- Marek, V.; Mélik-Parsadaniantz, S.; Villette, T.; Montoya, F.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A. Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions. Free Radic. Biol. Med. 2018, 126, 27–40. [Google Scholar] [CrossRef]
- Domingues, I.; Agra, A.R.; Monaghan, K.; Soares, A.M.; Nogueira, A.J. Cholinesterase and glutathione-S-transferase activities in freshwater invertebrates as biomarkers to assess pesticide contamination. Environ. Toxicol. Chem. 2010, 29, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhu, L.; Shao, B.; Zhu, S.; Wang, J.; Xie, H.; Wang, J.H.; Wang, F.H. The effects of endosulfan on cytochrome P450 enzymes and glutathione S-transferases in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 2013, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajak, P.; Roy, S. Heat shock proteins and pesticide stress. In Regulation of Heat Shock Protein Responses; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Wong, L.L.; Do, D.T. The role of heat shock proteins in response to extracellular stress in aquatic organisms. In Heat Shock Proteins in Veterinary Medicine and Sciences; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Mohanty, B.P.; Mahanty, A.; Mitra, T.; Parija, S.C.; Mohanty, S. Heat Shock Proteins in Stress in Teleosts; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Oliver, J.D.; Loy, J.D.; Parikh, G.; Bartholomay, L. Comparative analysis of hemocyte phagocytosis between six species of arthropods as measured by flow cytometry. J. Invertebr. Pathol. 2011, 108, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Alpuche, J.; Maldonado, G.; Agundis, C.; Pereyra-Morales, A.; Zenteno, E. Immunity mechanisms in crustaceans. Innate Immun. 2009, 15, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Arizza, V.; Manachini, B.; Brivio, M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida). Insect Sci. 2015, 22, 748–760. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchapova, E.; Nazarova, A.; Gurkov, A.; Borvinskaya, E.; Rzhechitskiy, Y.; Dmitriev, I.; Meglinski, I.; Timofeyev, M. Application of PEG-Covered Non-Biodegradable Polyelectrolyte Microcapsules in the Crustacean Circulatory System on the Example of the Amphipod Eulimnogammarus verrucosus. Polymers 2019, 11, 1246. https://doi.org/10.3390/polym11081246
Shchapova E, Nazarova A, Gurkov A, Borvinskaya E, Rzhechitskiy Y, Dmitriev I, Meglinski I, Timofeyev M. Application of PEG-Covered Non-Biodegradable Polyelectrolyte Microcapsules in the Crustacean Circulatory System on the Example of the Amphipod Eulimnogammarus verrucosus. Polymers. 2019; 11(8):1246. https://doi.org/10.3390/polym11081246
Chicago/Turabian StyleShchapova, Ekaterina, Anna Nazarova, Anton Gurkov, Ekaterina Borvinskaya, Yaroslav Rzhechitskiy, Ivan Dmitriev, Igor Meglinski, and Maxim Timofeyev. 2019. "Application of PEG-Covered Non-Biodegradable Polyelectrolyte Microcapsules in the Crustacean Circulatory System on the Example of the Amphipod Eulimnogammarus verrucosus" Polymers 11, no. 8: 1246. https://doi.org/10.3390/polym11081246