Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives
Abstract
:1. Introduction
2. Biodegradable Polymers and Their Classification
3. Cellulose
3.1. Cellulose Nanostructures
3.2. Modification of Cellulose Nanostructure
3.3. Preparation Methods for Cellulose Nanostructure-Based Nanocomposites
4. Role of CN in Foam Processing
- There should not be strong adhesion to the polymer matrix;
- Nucleating agents should disperse uniformly and be exfoliated. However, intercalation may also be desired for increased thermodynamic fluctuations at the matrix-filler interface;
- Their amount should be adequate to increase the number of nucleation sites. Excess nucleating particles may lead to agglomeration.
5. Methods and Parameters Associated with Cellulose Nanostructured Nanocomposite Foam Processing
5.1. Batch Processing
5.2. Injection Foaming
5.3. Extrusion Foaming
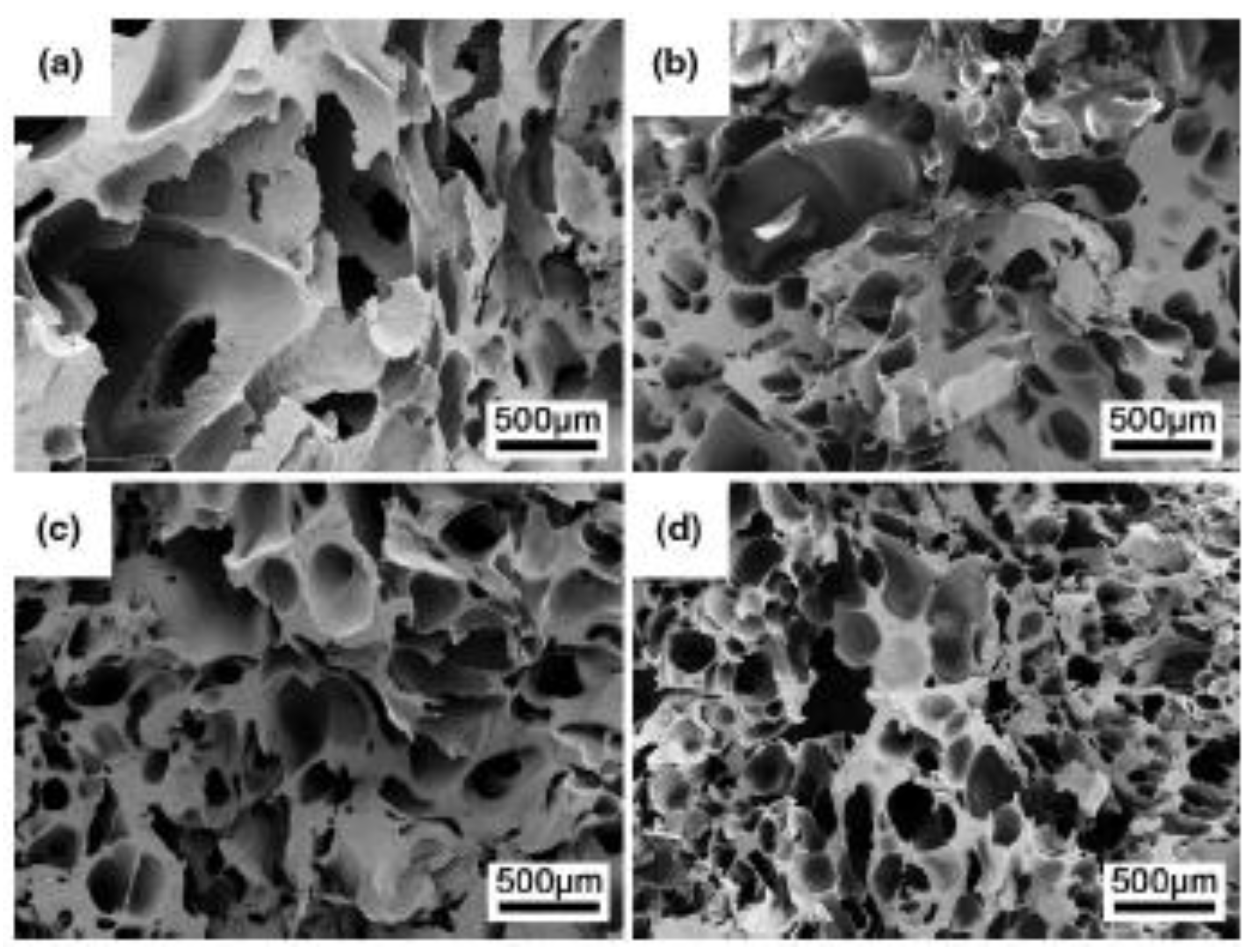
6. Influence of Cellulose Nanostructures on Crystallisation and Morphological Properties of Foam
7. Properties of Cellulose-Nanostructured Nanocomposite Foams
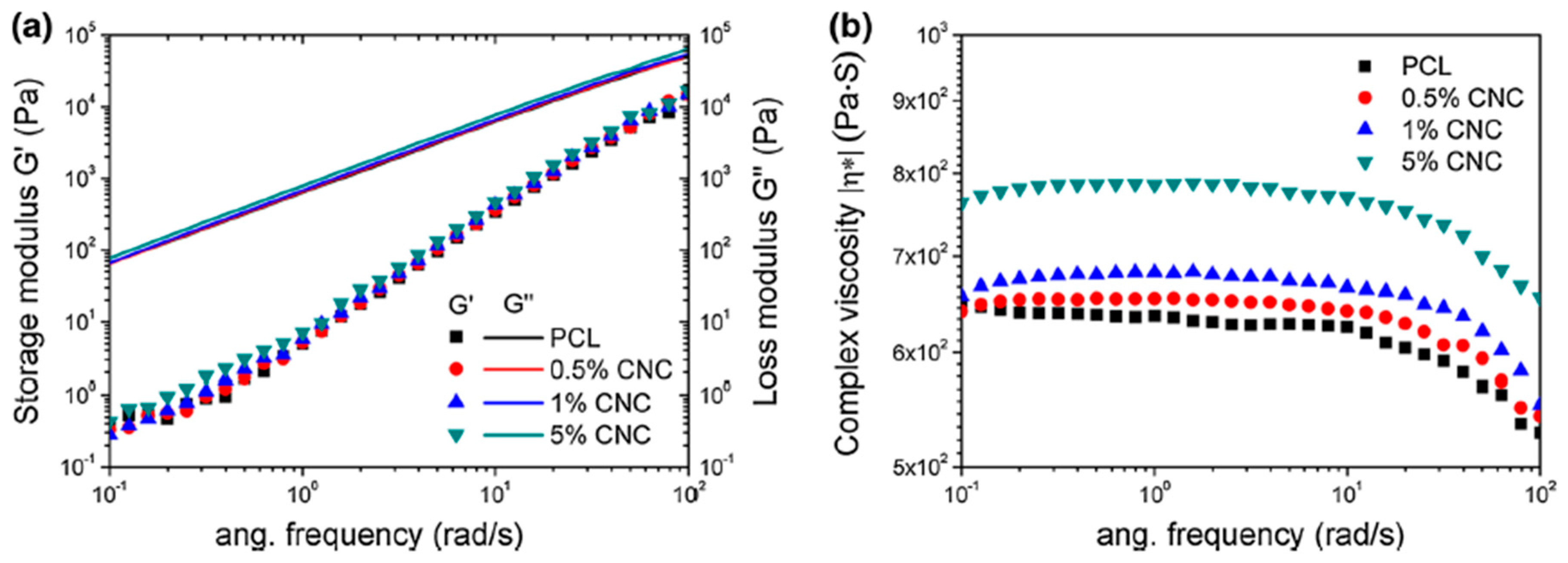
7.1. Rheological Properties
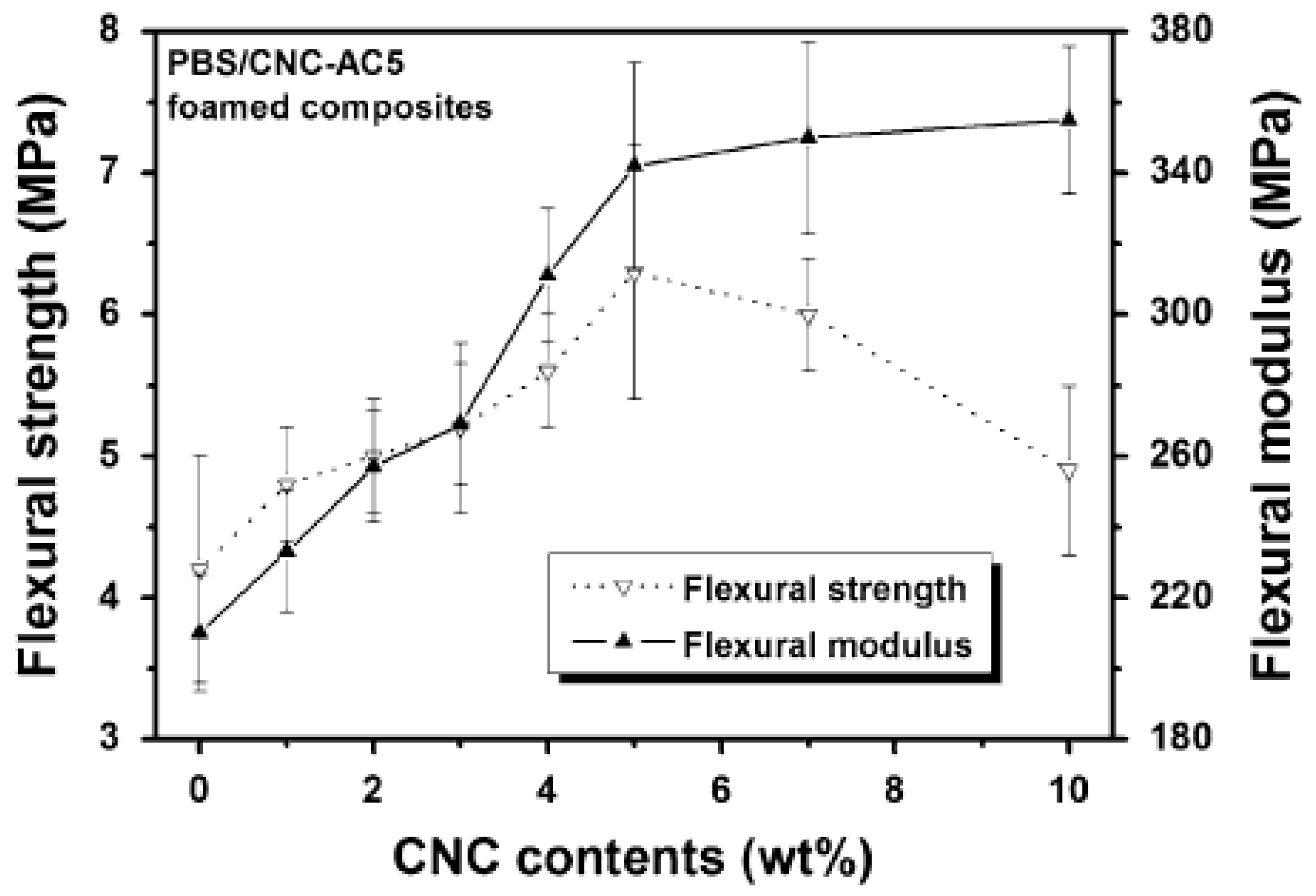
7.2. Mechanical Properties
- Matrix–filler interaction—Stronger adhesions results in large improvements in mechanical properties. Modification of CN particles in this regard is the key factor in dictating the compatibility of the two components.
- Filler concentration—Most of the improvements in mechanical properties occur at CN particle concentration less than 5 wt.%. Higher amounts lead to the weakening of the interfacial area and poor performance.
- Cell concentration—Increased number of nucleation sites by CN particles results in large cell density; therefore, the presence of a large number of voids results in low modulus.
7.3. Thermo-Mechanical Properties
7.4. Thermal Decomposition
7.5. Thermal Insulation and Flammability Properties of CN-Based Biodegradable Foams
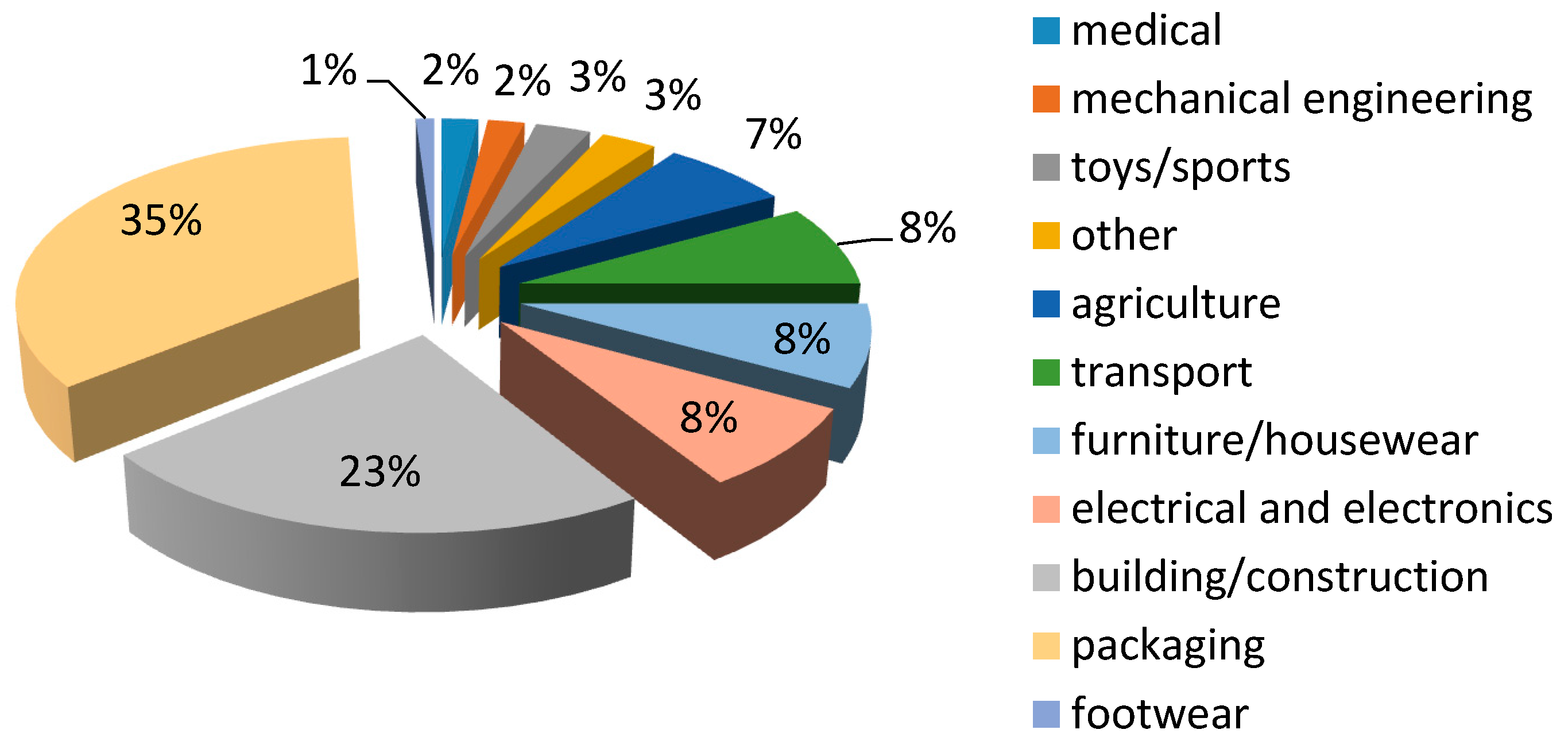
8. Application of CN-Biopolymer Foams
9. Biodegradation of CN-Based Biopolymer Foams
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ojijo, V.; Ray, S.S. Processing strategies in bionanocomposites. Prog. Polym. Sci. 2013, 38, 1543–1589. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M.; Sinharay, S.; Ray, S.S. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Rapa, M.; Popa, M.E.; Cinelli, P.; Lazzeri, A.; Burnichi, R.; Mitelut, A.; Grosu, E. Biodegradable alternative to plastics for agriculture application. Rom. Biotechnol. Lett. 2011, 16, 59–64. [Google Scholar]
- United Nations, Department of Economic and Social Affairs. Available online: https://www.un.org/development/desa/en/ (accessed on 1 May 2018).
- Nkwachukwu, O.I.; Chima, C.H.; Ikenna, A.O.; Albert, L. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. Int. J. Ind. Chem. 2013, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.J.; Zeng, C.; Cao, X.; Han, X.; Shen, J.; Xu, G. Polymer nanocomposite foams. Compos. Sci. Technol. 2005, 65, 2344–2363. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, H.; Park, C.B. Continuous processing of low-density, microcellular poly (lactic acid) foams with controlled cell morphology and crystallinity. Chem. Eng. Sci. 2012, 75, 390–399. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Nanofoaming of PMMA using a batch CO2 process: Influence of the PMMA viscoelastic behaviour. Polymers 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Szegda, D.; Duangphet, S.; Song, J.; Tarverdi, K. Extrusion foaming of PHBV. J. Cell. Plast. 2014, 50, 145–162. [Google Scholar] [CrossRef]
- Pilla, S.; Kim, S.G.; Auer, G.K.; Gong, S.; Park, C.B. Microcellular extrusion foaming of poly (lactide)/poly (butylene adipate-co-terephthalate) blends. Mater. Sci. Eng. C 2010, 30, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, M.; Nofar, M.; Park, C.; Carreau, P. Extruded PLA/clay nanocomposite foams blown with supercritical CO2. Polymers 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.B.; Carreau, P.J.; Heuzey, M.-C.; Kamal, M.R. Mechanical and bead foaming behaviour of PLA-PBAT and PLA-PBSA blends with different morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Li, M.-C.; Song, K.; Qing, Y.; Wu, Q.; Lee, S.; Wu, Y. Cellulose nanoparticles: Structure–morphology–rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yeh, S.; Yang, J.; Zhang, Y.; Chen, F.; Fan, P.; Zhong, M. A new promising nucleating agent for polymer foaming: Applications of ordered mesoporous silica particles in polymethyl methacrylate supercritical carbon dioxide microcellular foaming. Ind. Eng. Chem. Res. 2013, 52, 14169–14178. [Google Scholar]
- Heng-Huey, Y.; Chang, D. The effect of nucleating agents on the foam extrusion characteristics. J. Appl. Polym. Sci. 1984, 29, 4465–4470. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface acetylation of cellulose nanocrystal and its reinforcing function in poly (lactic acid). Carbohydr. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
- Pracella, M.; Haque, M.M.-U.; Puglia, D. Morphology and properties tuning of PLA/cellulose nanocrystals bio-nanocomposites by means of reactive functionalization and blending with PVAc. Polymers 2014, 55, 3720–3728. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Polybutylene succinate/cellulose nanocrystals: Role of phthalic anhydride in squeeze oriented bionanocomposites. Carbohydr. Polym. 2018, 196, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R.; Farrag, Y. PHBV/CNC bionanocomposites processed by extrusion: Structural characterization and properties. Polym. Compos. 2017, 40, E275–E284. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Zhang, Y. Structure and properties of surface-acetylated cellulose nanocrystal/poly (butylene adipate-co-terephthalate) composites. Polym. Bull. 2016, 73, 2073–2085. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, M.; Zhao, Y.; Jin, T.; Fu, Q. Effect of stretching on the mechanical properties in melt-spun poly (butylene succinate)/microfibrillated cellulose (MFC) nanocomposites. Carbohydr. Polym. 2016, 140, 383–392. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Khan, W. Biodegradable polymers-an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B.; And, A.A. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers—A review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Part C 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Visakh, P.M.; Thomas, S. Preparation of bionanomaterials and their polymer nanocomposites from waste and biomass. Waste Biomass Valoriz. 2010, 1, 121–134. [Google Scholar] [CrossRef]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of cellulose by ring-opening polymerization—A review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef]
- Ray, D.; Sain, S. In situ processing of cellulose nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 83, 19–37. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Graft modification of cellulose: Methods, properties and applications. Polymers 2015, 70, A1–A16. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting polymers from cellulose nanocrystals: Synthesis, properties, and applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Jain, R.K.; Agnish, S.L.; Lal, K.; Bhatnagar, H.L. Reactivity of hydroxyl groups in cellulose towards chlorotri (p-tolyl) methane. Makromol. Chem. 1985, 186, 2501–2512. [Google Scholar] [CrossRef]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Pagnocelli, M.; Baldasso, C.; Zattera, A.J. Producing aerogels from silanized cellulose nanofiber suspension. Cellulose 2017, 24, 769–779. [Google Scholar] [CrossRef]
- Morelli, C.L.; Belgacem, M.N.; Branciforti, M.C.; Salon, M.C.B.; Bras, J.; Bretas, R.E.S. Nanocomposites of PBAT and cellulose nanocrystals modified byin situpolymerization and melt extrusion. Polym. Eng. Sci. 2016, 56, 1339–1348. [Google Scholar] [CrossRef]
- Lönnberg, H.; Fogelström, L.; Samir, M.A.S.A.; Berglund, L.; Malmström, E.; Hult, A. Surface grafting of microfibrillated cellulose with poly (ε-caprolactone)-synthesis and characterization. Eur. Polym. J. 2008, 44, 2991–2997. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated cellulose surface modification: A review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Peltzer, M.; Pei, A.; Zhou, Q.; Berglund, L.; Jiménez, A. Surface modification of cellulose nanocrystals by grafting with poly (lactic acid). Polym. Int. 2014, 63, 1056–1062. [Google Scholar] [CrossRef]
- Wang, T.; Drzal, L.T. Cellulose-nanofiber-reinforced poly (lactic acid) composites prepared by a water-based approach. ACS Appl. Mater. Interfaces 2012, 4, 5079–5085. [Google Scholar] [CrossRef]
- Morouço, P.; Biscaia, S.; Viana, T.; Franco, M.; Malça, C.; Mateus, A.; Moura, C.; Ferreira, F.C.; Mitchell, G.; Alves, N.M. Fabrication of poly (ε-caprolactone) scaffolds reinforced with cellulose nanofibers, with and without the addition of hydroxyapatite nanoparticles. Biomed. Res. Int. 2016, 2016, 1596157. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Piorkowska, E.; Kulpinski, P.; Pracella, M. Mechanical and thermal properties of PLA composites with cellulose nanofibers and standard size fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1509–1514. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nanocomposite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Dasan, Y.; Bhat, A.; Ahmad, F. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Benini, K.C.; Cioffi, M.O.H.; Voorwald, H.J.C. PHBV/cellulose nanofibrils composites obtained by solution casting and electrospinning process. Matéria 2017, 22, e11837. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel nanocomposites based on fatty acid modified cellulose nanofibers/poly (lactic acid): Morphological and physical properties. Food Packag. Shelf Life 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crop. Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Lv, Q.; Xu, C.; Wu, D.; Wang, Z.; Lan, R.; Wu, L. The role of nanocrystalline cellulose during crystallization of poly(ε-caprolactone) composites: Nucleation agent or not? Compos. Part A Appl. Sci. Manuf. 2017, 92, 17–26. [Google Scholar] [CrossRef]
- Lönnberg, H.; Fogelström, L.; Zhou, Q.; Hult, A.; Berglund, L.; Malmström, E. Investigation of the graft length impact on the interfacial toughness in a cellulose/poly (ε-caprolactone) bilayer laminate. Compos. Sci. Technol. 2011, 71, 9–12. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.-S. Incorporation of poly (ethylene glycol) grafted cellulose nanocrystals in poly (lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, I.; Ferreira, F.; Souza, D.; Gouveia, R.; Lona, L.; Morales, A.; Mei, L. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017, 97, 356–365. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Reinforcement effect of poly (butylene succinate) (PBS)-grafted cellulose nanocrystal on toughened PBS/polylactic acid blends. Carbohydr. Polym. 2016, 140, 374–382. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Poly (butylene succinate -co- butylene adipate)/cellulose nanocrystal composites modified with phthalic anhydride. Carbohydr. Polym. 2015, 134, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.R.; Khoshkava, V. Effect of cellulose nanocrystals (CNC) on rheological and mechanical properties and crystallization behavior of PLA/CNC nanocomposites. Carbohydr. Polym. 2015, 123, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, D.; Tam, K.C.; Pan, K.; Zheng, Z.; Tam, M.K. Effect of surface modification of cellulose nanocrystal on nonisothermal crystallization of poly (β-hydroxybutyrate) composites. Carbohydr. Polym. 2017, 157, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Goffin, A.-L.; Habibi, Y.; Raquez, J.-M.; Dubois, P. Polyester-grafted cellulose nanowhiskers: A new approach for tuning the microstructure of immiscible polyester blends. ACS Appl. Mater. Interfaces 2012, 4, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.-M.; Murena, Y.; Goffin, A.-L.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-modification of cellulose nanowhiskers and their use as nanoreinforcers into polylactide: A sustainably-integrated approach. Compos. Sci. Technol. 2012, 72, 544–549. [Google Scholar] [CrossRef]
- Boujemaoui, A.; Sanchez, C.C.; Engström, J.; Bruce, C.; Fogelström, L.; Carlmark, A.; Malmström, E. Polycaprolactone nanocomposites reinforced with cellulose nanocrystals surface-modified via covalent grafting or physisorption—A comparative study. ACS Appl. Mater. Interfaces 2017, 9, 35305–35318. [Google Scholar] [CrossRef] [PubMed]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Alstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Ruiz, J.A.R.; Vincent, M.; Agassant, J.F.; Sadik, T.; Pillon, C.; Carrot, C. Polymer foaming with chemical blowing agents: Experiment and modelling. Polym. Eng. Sci. 2015, 55, 2018–2029. [Google Scholar] [CrossRef]
- Wong, A.; Wijnands, S.F.L.; Kuboki, T.; Park, C.B. Mechanisms of nanoclay-enhanced plastic foaming processes: Effects of nanoclay intercalation and exfoliation. J. Nanopart. Res. 2013, 15, 1815. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Wang, C.; Leung, S.N.; Bussmann, M.; Zhai, W.T.; Park, C.B. Numerical investigation of nucleating-agent-enhanced heterogeneous nucleation. Ind. Eng. Chem. Res. 2010, 49, 12783–12792. [Google Scholar] [CrossRef]
- Leung, S.N.; Wong, A.; Wang, L.C.; Park, C.B. Mechanism of extensional stress-induced cell formation in polymeric foaming processes with the presence of nucleating agents. J. Supercrit. Fluids 2012, 63, 187–198. [Google Scholar] [CrossRef]
- Wong, A.; Park, C.B. The effects of extensional stresses on the foamability of polystyrene-talc composites blown with carbon dioxide. Chem. Eng. Sci. 2012, 75, 49–62. [Google Scholar] [CrossRef]
- Feng, J.J.; Bertelo, C.A. Prediction of bubble growth and size distribution in polymer foaming based on a new heterogeneous nucleation model. J. Rheol. 2004, 48, 439. [Google Scholar] [CrossRef]
- Leung, S.N.; Wong, A.; Guo, Q.; Park, C.B.; Zong, J.H. Change in the critical nucleation radius and its impact on cell stability during polymeric foaming processes. Chem. Eng. Sci. 2009, 64, 4899–4907. [Google Scholar] [CrossRef]
- Basso, M.; Giovando, S.; Pizzi, A.; Celzard, A.; Fierro, V. Tannin/furanic foams without blowing agents and formaldehyde. Ind. Crop. Prod. 2013, 49, 17–22. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Panayiotou, C. Foaming of chitin hydrogels processed by supercritical carbon dioxide. J. Supercrit. Fluids 2008, 47, 302–308. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Cangemi, M.; Fierro, V.; Celzard, A. Flexible natural tannin-based and protein-based biosourced foams. Ind. Crop. Prod. 2012, 37, 389–393. [Google Scholar] [CrossRef]
- Bergel, B.F.; Da Luz, L.M.; Santana, R.M.C. Comparative study of the influence of chitosan as coating of thermoplastic starch foam from potato, cassava and corn starch. Prog. Org. Coat. 2017, 106, 27–32. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Kerdchoechuen, O.; Laohakunjit, N. Characterization of cassava starch based foam blended with plant proteins, kraft fiber, and palm oil. Carbohydr. Polym. 2014, 110, 70–77. [Google Scholar] [CrossRef]
- Demitri, C.; Raucci, M.G.; Giuri, A.; Benedictis, V.M.D.; Giugliano, D.; Calcagnile, P.; Sannino, A.; Ambrosio, L. Cellulose-based porous scaffold for bone tissue engineering applications: Assessment of hMSC proliferation and differentiation. J. Biomed. Res. Part A 2016, 104, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Le Moigne, N.; Sauceau, M.; Benyakhlef, M.; Jemai, R.; Benezet, J.-C.; Rodier, E.; Lopez-Cuesta, J.-M.; Fages, J. Foaming of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/organo-clays nano-biocomposites by a continuous supercritical CO2 assisted extrusion process. Eur. Polym. J. 2014, 61, 157–171. [Google Scholar] [CrossRef]
- Ventura, H.; Sorrentino, L.; Laguna-Gutierrez, E.; Rodriguez-Perez, M.A.; Ardanuy, M. Gas dissolution foaming as a novel approach for the production of lightweight biocomposites of PHB/natural fibre fabrics. Polymers 2018, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Jahani, D.; Chang, E.; Alemdar, A.; Park, C.B.; Sain, M. Development of PLA/cellulosic fiber composite foams using injection molding: Crystallization and foaming behaviors. Compos. Part A Appl. Sci. Manuf. 2016, 83, 130–139. [Google Scholar] [CrossRef]
- Pantani, R.; Volpe, V.; Titomanlio, G. Foam injection molding of poly (lactic acid) with environmentally friendly physical blowing agents. J. Mater. Process. Technol. 2014, 214, 3098–3107. [Google Scholar] [CrossRef]
- Bocz, K. Characterisation of natural fibre reinforced PLA foams prepared by supercritical CO2 assisted extrusion. Express Polym. Lett. 2016, 10, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, S.; Yang, X.; Xi, Z.; Zhao, L.; Cen, L.; Lu, E.; Yang, Y. Novel fabricating process for porous polyglycolic acid scaffolds by melt-foaming using supercritical carbon dioxide. ACS Biomater. Sci. Eng. 2018, 4, 694–706. [Google Scholar] [CrossRef]
- Pradeep, S.A.; Kharbas, H.; Turng, L.-S.; Avalos, A.; Lawrence, J.G.; Pilla, S. Investigation of thermal and thermomechanical properties of biodegradable PLA/PBSA composites processed via supercritical fluid-assisted foam injection molding. Polymers 2017, 9, 22. [Google Scholar] [CrossRef]
- Karimi, M.; Heuchel, M.; Weigel, T.; Schossig, M.; Hofmann, D.; Lendlein, A. Formation and size distribution of pores in poly (ε-caprolactone) foams prepared by pressure quenching using supercritical CO2. J. Supercrit. Fluids 2012, 61, 175–190. [Google Scholar] [CrossRef]
- Salerno, A.; Di Maio, E.; Iannace, S.; Netti, P.; Netti, P. Solid-state supercritical CO2 foaming of PCL and PCL-HA nano-composite: Effect of composition, thermal history and foaming process on foam pore structure. J. Supercrit. Fluids 2011, 58, 158–167. [Google Scholar] [CrossRef]
- Stagner, J.; Narayan, R. Preparation and properties of biodegradable foams. J. Polym. Environ. 2011, 19, 598–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, B.; Lv, F.; Guo, W.; Ji, J.; Chu, P.K.; Zhang, C. Effect of processing conditions on poly (butylene succinate) foam materials. J. Appl. Polym. Sci. 2012, 126, 756–761. [Google Scholar] [CrossRef]
- Li, G.; Qi, R.; Lu, J.; Hu, X.; Luo, Y.; Jiang, P. Rheological properties and foam preparation of biodegradable poly (butylene succinate). J. Appl. Polym. Sci. 2013, 127, 3586–3594. [Google Scholar] [CrossRef]
- Sampath, U.G.T.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Mane, S. Effect of porogens (type and amount) on polymer porosity: A review. Can. Chem. Trans. 2016, 4, 210–225. [Google Scholar] [CrossRef]
- Borkotoky, S.S.; Dhar, P.; Katiyar, V. Biodegradable poly (lactic acid)/cellulose nanocrystals (CNCs) composite microcellular foam: Effect of nanofillers on foam cellular morphology, thermal and wettability behavior. Int. J. Boil. Macromol. 2018, 106, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Dlouhá, J.; Suryanegara, L.; Yano, H. The role of cellulose nanofibres in supercritical foaming of polylactic acid and their effect on the foam morphology. Soft Matter 2012, 8, 8704–8713. [Google Scholar] [CrossRef]
- Qiu, Y.; Lv, Q.; Wu, D.; Xie, W.; Peng, S.; Lan, R.; Xie, H. Cyclic tensile properties of the polylactide nanocomposite foams containing cellulose nanocrystals. Cellulose 2018, 25, 1795–1807. [Google Scholar] [CrossRef]
- Lin, N.; Chen, Y.; Hu, F.; Huang, J. Mechanical reinforcement of cellulose nanocrystals on biodegradable microcellular foams with melt-compounding process. Cellulose 2015, 22, 2629–2639. [Google Scholar] [CrossRef]
- Ries, S.; Spoerrer, A.; Altstaedt, V. Foam injection molding of thermoplastic elastomers: Blowing agents, foaming process and characterization of structural foams. AIP Conf. Proc. 2014, 1593, 401–410. [Google Scholar] [CrossRef]
- Yusa, A.; Yamamoto, S.; Goto, H.; Uezono, H.; Asaoka, F.; Wang, L.; Ando, M.; Ishihara, S.; Ohshima, M. A new microcellular foam injection-molding technology using non-supercritical fluid physical blowing agents. Polym. Eng. Sci. 2016, 57, 105–113. [Google Scholar] [CrossRef]
- Wang, L.; Hikima, Y.; Ohshima, M.; Yusa, A.; Yamamoto, S.; Goto, H. Development of a simplified foam injection molding technique and its application to the production of high void fraction polypropylene foams. Ind. Eng. Chem. Res. 2017, 56, 13734–13742. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Peng, J.; Salick, M.R.; Peng, X.-F.; Turng, L.-S. Poly (ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion assisted by supercritical CO2: A review on its application to biopolymers. J. Supercrit. Fluids 2017, 120, 408–420. [Google Scholar] [CrossRef]
- Park, C.B.; Baldwin, D.F.; Suh, N.P. Effect of the pressure drop rate on cell nucleation in continuous processing of microcellular polymers. Polym. Eng. Sci. 1995, 35, 432–440. [Google Scholar] [CrossRef]
- Zhao, N.; Mark, L.H.; Zhu, C.; Park, C.B.; Li, Q.; Glenn, R.; Thompson, T.R. Foaming poly (vinyl alcohol)/microfibrillated cellulose composites with CO2 and water as co-blowing agents. Ind. Eng. Chem. Res. 2014, 53, 11962–11972. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Baillon, F.; Fages, J. Foaming PLA with thermoplastic starch by extrusion assisted by supercritical CO2. In Proceedings of the 16th European Meeting on Supercritical Fluids, Lisbon, Portugal, 25–28 April 2017; Available online: http://eventos.fct.unl.pt/emsf2017/home (accessed on 1 May 2018).
- Ji, G.; Zhai, W.; Lin, D.; Ren, Q.; Zheng, W.; Jung, D.W. Microcellular foaming of poly (lactic acid)/silica nanocomposites in compressed CO2: Critical influence of crystallite size on cell morphology and foam expansion. Ind. Eng. Chem. Res. 2013, 52, 6390–6398. [Google Scholar] [CrossRef]
- Miyamoto, R.; Utano, T.; Yasuhara, S.; Ishihara, S.; Ohshima, M. Effect of crystals and fibrous network polymer additives on cellular morphology of microcellular foams. AIP Conf. Proc. 2015, 1664, 40001. [Google Scholar]
- Srithep, Y.; Ellingham, T.; Peng, J.; Sabo, R.; Clemons, C.; Turng, L.-S.; Pilla, S. Melt compounding of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/nanofibrillated cellulose nanocomposites. Polym. Degrad. Stab. 2013, 98, 1439–1449. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Ma, W. Promoting effect of crystallization on the foaming behavior in polypropylene homopolymer/polypropylene block copolymer blends. Polym. Eng. Sci. 2016, 56, 1175–1181. [Google Scholar] [CrossRef]
- Ching, Y.C.; Ali, M.E.; Abdullah, L.C.; Choo, K.W.; Kuan, Y.C.; Julaihi, S.J.; Chuah, C.H.; Liou, N.-S. Rheological properties of cellulose nanocrystal-embedded polymer composites: A review. Cellulose 2016, 23, 1011–1030. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, S.; Zhang, R.; Cai, C. Enhancing the melt strength of poly (Lactic Acid) via micro-crosslinking and blending with poly (Butylene adipate-co-butylene terephthalate) for the preparation of foams. J. Polym. Environ. 2016, 25, 1335–1341. [Google Scholar]
- Zhou, J.; Yao, Z.; Zhou, C.; Wei, D.; Li, S. Mechanical properties of PLA/PBS foamed composites reinforced by organophilic montmorillonite. J. Appl. Polym. Sci. 2014, 131, 40773. [Google Scholar] [CrossRef]
- Nofar, M.; Ameli, A.; Park, C.B. A novel technology to manufacture biodegradable polylactide bead foam products. Mater. Des. 2015, 83, 413–421. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, H.H.; Yun, Y.S.; Jin, H.J. Influence of cellulose nanofibers on the morphology and physical properties of poly (lactic acid) foaming by supercritical carbon dioxide. Macromol. Res. 2013, 21, 529–533. [Google Scholar] [CrossRef]
- Song, T.; Tanpichai, S.; Oksman, K. Cross-linked polyvinyl alcohol (PVA) foams reinforced with cellulose nanocrystals (CNCs). Cellulose 2016, 23, 1925–1938. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Z.; Tian, H.; Gu, M.; Liu, D. Biodegradable poly (vinyl alcohol) foams supported by cellulose nanofibrils: Processing, structure, and properties. Langmuir 2014, 30, 9544–9550. [Google Scholar]
- Dlouhá, J.; Suryanegara, L.; Yano, H. Cellulose nanofibre–poly (lactic acid) microcellular foams exhibiting high tensile toughness. React. Funct. Polym. 2014, 85, 201–207. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M. A review on dynamic mechanical properties of natural fibre reinforced polymer composites. Constr. Build. Mater. 2016, 106, 149–159. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Pistor, V.; Ornaghi, F.G.; Ornaghi, H.L.; Zattera, A.J. Dynamic mechanical characterization of epoxy/epoxycyclohexyl–POSS nanocomposites. Mater. Sci. Eng. A 2012, 532, 339–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Heath, R.J.; Hourston, D.J. Morphology, mechanical properties, and thermal stability of polyurethane–epoxide resin interpenetrating polymer network rigid foams. J. Appl. Polym. Sci. 2000, 75, 406–416. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Pagacz, J.; Hebda, E.; Michałowski, S.; Ozimek, J.; Sternik, D.; Pielichowski, K. Polyurethane foams chemically reinforced with POSS—Thermal degradation studies. Thermochim. Acta 2016, 642, 95–104. [Google Scholar] [CrossRef]
- Gepu, G.; Qingyu, M.; Fang, W.; Bo, Z.; Dong, Z. Kinetic evaluation of the size-dependent decomposition performance of solvent-free microcellular polylactic acid foams. Chin. Sci. Bull. 2012, 57, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Gedler, G.; Antunes, M.; Realinho, V.; Velasco, J. Thermal stability of polycarbonate-graphene nanocomposite foams. Polym. Degrad. Stab. 2012, 97, 1297–1304. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Besco, S.; Hrelja, D.; Semenzato, S.; Bertani, R.; Michelin, R. Synergism between flame retardant and modified layered silicate on thermal stability and fire behaviour of polyurethane nanocomposite foams. Polym. Degrad. Stab. 2008, 93, 2166–2171. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Velasco, J. Low density polycarbonate–graphene nanocomposite foams produced by supercritical carbon dioxide two-step foaming. Thermal stability. Compos. Part B Eng. 2016, 92, 299–306. [Google Scholar] [CrossRef]
- Vadas, D.; Igricz, T.; Sarazin, J.; Bourbigot, S.; Marosi, G.; Bocz, K. Flame retardancy of microcellular poly (lactic acid) foams prepared by supercritical CO2-assisted extrusion. Polym. Degrad. Stab. 2018, 153, 100–108. [Google Scholar] [CrossRef]
- Wang, P.; Aliheidari, N.; Zhang, X.; Ameli, A. Strong ultralight foams based on nanocrystalline cellulose for high-performance insulation. Carbohydr. Polym. 2019, 218, 103–111. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on flammability of biofibres and biocomposites. Carbohydr. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Ghanadpour, M.; Larsson, P.T.; Carosio, F.; Wågberg, L. Phosphorylated cellulose nanofibrils: A renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.; Laoutid, F.; Khelifa, F.; Rose, G.; Brohez, S.; Delvosalle, C.; Dubois, P. Cellulose/phosphorus combinations for sustainable fire retarded polylactide. Eur. Polym. J. 2016, 74, 218–228. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Zhang, P.; Liu, J.; Song, L.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Xu, Y.; Essawy, H.; Wu, Z.; Du, G. Tannin-furanic resin foam reinforced with cellulose nanofibers (CNF). Ind. Crop. Prod. 2019, 134, 107–112. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Gautam, R.; Bassi, A.S.; Yanful, E.K. A review of biodegradation of synthetic plastic and foams. Appl. Biochem. Biotechnol. 2007, 141, 85–108. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]








| Origin | Family | Common Examples | Refs. |
|---|---|---|---|
| Biomass | Polysaccharides | Cellulose, Starch, Chitin, etc. | [2,30] |
| Proteins | Collagen, Gelatin, Albumin, Soya, Glutan | ||
| Microorganisms | Polyhydroxyalkanoates (PHA) | Polyhydroxybutyrate (PHB) | [2,29,30] |
| Poly (γ-glutamic acid) | |||
| Poly (hydrobutyrate-co-hydroxyvalerate) (PHBV) | |||
| Petroleum oil | Polyesters | Poly (ԑ-caprolactone) (PCL) | [29,30,33] |
| Poly (butylene succinate) (PBS) | |||
| Poly (butylene succinate-co-adipate) (PBSA) | |||
| Poly (butylene adipate-co-terephthalate) (PBAT) | |||
| Bio-derived | Polyesters | Poly (lactic acid) (PLA) | [30] |
| CN-Based Nanocomposite | CN Modification | Processing Strategy | Solvent | Temp./°C | Time | Screw Speed/rpm | Refs. |
|---|---|---|---|---|---|---|---|
| PLA/CNF | - | Solvent evaporation | Water | - | - | [49] | |
| PCL/CNF | - | Solvent evaporation | DMF # | - | - | [50] | |
| PLA/CNF | - | Solvent evaporation | dichloromethane | - | - | - | [51] |
| Melt mixing/batch | - | 170 | 10 min | 60 | |||
| PLA/CNF | acetylation | Solvent casting | chloroform | - | 60 min | - | [52] |
| PLA/PHBV/CNC | TEMPO-mediated oxidation | Solvent casting | chloroform | - | [53] | ||
| PHBV/CNF | Solvent casting | chloroform/DMF | 50 | 30 h | - | [54] | |
| PLA/CNF | Esterification by oleic acid | Solvent casting | chloroform | - | [55] | ||
| PLA/PBS/CNC | Surfactant-modified | Solvent casting | chloroform | - | - | [56] | |
| PCL/CNC | acetylation | Solvent casting | chloroform | - | - | [57] | |
| PLA/CNC | Acetylation | Solvent casting | dichloromethane | - | - | [23] | |
| PCL-MFC | Grafting polymerisation | - | Toluene | 100 | - | [58] | |
| PLA/CNC | PEG $-grafted-CNC | Solution-based (electrospun) | DMF/chloroform | ambient | - | [59] | |
| PBS/MFC | CNC-modified by acetylchloride with ball milling | Solvent-based (MB §) | DMF | 60 | - | [28] | |
| Melt-mixing/batch | - | 140 | 5 min | 60 | |||
| PBAT/CNC | Octadecyl isocyanate | Melt mixing (batch) | - | 130 | 3 min | 30 | [60] |
| PBS/PLA/CNC | CNC-grafted-PBS | Melt mixing/batch | - | 190 | 10 min | 60 | [61] |
| PHBV/CNC | - | Melt mixing/small-extrusion | - | 165 | [26] | ||
| PBAT/CNC | acetylation | Melt mixing/batch | - | 120 | 10 min | 50 | [27] |
| PBSA/CNC | - | Melt mixing/batch | - | 120 | 10 min | 50 | [62] |
| PBAT/CNC | PBG *-grafted-CNC | Melt extrusion | - | 150 | 5 min | 100 | [44] |
| PLA/CNC | Silanisation | Melt extrusion/batch | - | 165 | 5 min | 100 | [63] |
| PHB/CNC | PLA-grafted-CNC | Melt extrusion/batch | - | 180 | 5 min | 50 | [64] |
| PLA/PCL/CNC | CNC-grafting-PCL & PLA | Melt extrusion/batch | - | 165 | 5 min | 100 | [65] |
| PLA/CNC | - | Melt mixing/batch | - | 190 | 10 | 60 | [66] |
| PCL/CNC | CNC-grafted-PBMA ¥; Micelles; Latex | Melt mixing | - | 110 | 6 min | 100 | [67] |
| Origin | Biopolymer-Based Foam | Processing Strategy | Blow Agent | Refs. |
|---|---|---|---|---|
| Biomass | Tannin/furfuryl alcohol | Solvent-based | Self-blowing | [77] |
| Chitin hydrogels | High-pressure cell | ScCO2 | [78] | |
| Albumin protein | Solvent based & Microwave drying | Self-blowing | [79] | |
| Cassava starch containing additives | Baking process | - | [80] | |
| Thermoplastic starch coated with chitosan | Baking process | Water | [81] | |
| CMCNa/PEGDA700 1 | Solvent-based | Surfactant (Pluronic) | [82] | |
| Microorganisms | PHBV/Clay | Extrusion foaming | ScCO2 | [83] |
| PHBV | Extrusion foaming | Sodium bicarbonate & citric acid | [84] | |
| PHB/Natural Fibre | Batch Process (pressure quench) | CO2 | [85] | |
| Bio-derived | PLA/cellulosic fiber | Injection foaming | N2 (0.5 wt.%) | [86] |
| PLA | Injection foaming | N2 | [87] | |
| PLA | Extrusion foaming | ScCO2 | [88] | |
| PGA | Batch process | ScCO2 | [89] | |
| PLA/PBSA | Injection foaming | N2 | [15] | |
| PLA/PBAT | Extrusion foaming | ScCO2 | [90] | |
| Petroleum-oil | PCL | Batch-process | ScCO2 | [91] |
| PCL/HA 2 | Batch process | ScCO2 | [92] | |
| PBAT/MTPS 3 | Single-screw extruder | SAFTEC® UBA-60 5 (3, 5, 7 wt.%) | [93] | |
| PBS | Mold method | Ammonium bicarbonate (1–10 wt.%) | [94] | |
| PBS/TMPTMA/DCP 4 | Compression molding | Azodicarbonamide | [95] |
| Sample | Average Cell Diameter (μm) | Cell Density × 105 (mm−3) |
|---|---|---|
| Neat PLA | 2.8 ± 1.3 | 2.1 |
| PLA/CNC-1% | 1.04 ± 0.43 | 3.0 |
| PLA/CNC-2% | 1.5 ± 0.54 | 2.7 |
| PLA/CNC-3% | 1.81 ± 0.61 | 2.9 |
| CN-Based Foam | CN-Concentration | Mechanical Property | % Improvement | Refs. |
|---|---|---|---|---|
| PVOH/CNC-crosslinked with formaldehyde for 10 and 120 s | 1.5 wt.% | Compressive strength at 70% strain (KPa) | 769% at 10 s 76% at 120 s | [118] |
| Compressive modulus (KPa) | 476% at 10 s 9% at 120 s 60% at 120 s * | |||
| PVOH/CNC | 30 wt.% | Compressive stress at 50% strain (KPa) | 62% | [119] |
| Young’s modulus (MPa) | 75% | |||
| Energy absorbed (KJ/m3) | 35% | |||
| PLA/CNF | 9 wt.% | Specific modulus | 44% | [120] |
| Specific strength | 46% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motloung, M.P.; Ojijo, V.; Bandyopadhyay, J.; Ray, S.S. Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives. Polymers 2019, 11, 1270. https://doi.org/10.3390/polym11081270
Motloung MP, Ojijo V, Bandyopadhyay J, Ray SS. Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives. Polymers. 2019; 11(8):1270. https://doi.org/10.3390/polym11081270
Chicago/Turabian StyleMotloung, Mpho Phillip, Vincent Ojijo, Jayita Bandyopadhyay, and Suprakas Sinha Ray. 2019. "Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives" Polymers 11, no. 8: 1270. https://doi.org/10.3390/polym11081270
APA StyleMotloung, M. P., Ojijo, V., Bandyopadhyay, J., & Ray, S. S. (2019). Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives. Polymers, 11(8), 1270. https://doi.org/10.3390/polym11081270






