Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Homopolyimide and Copolyimide Films
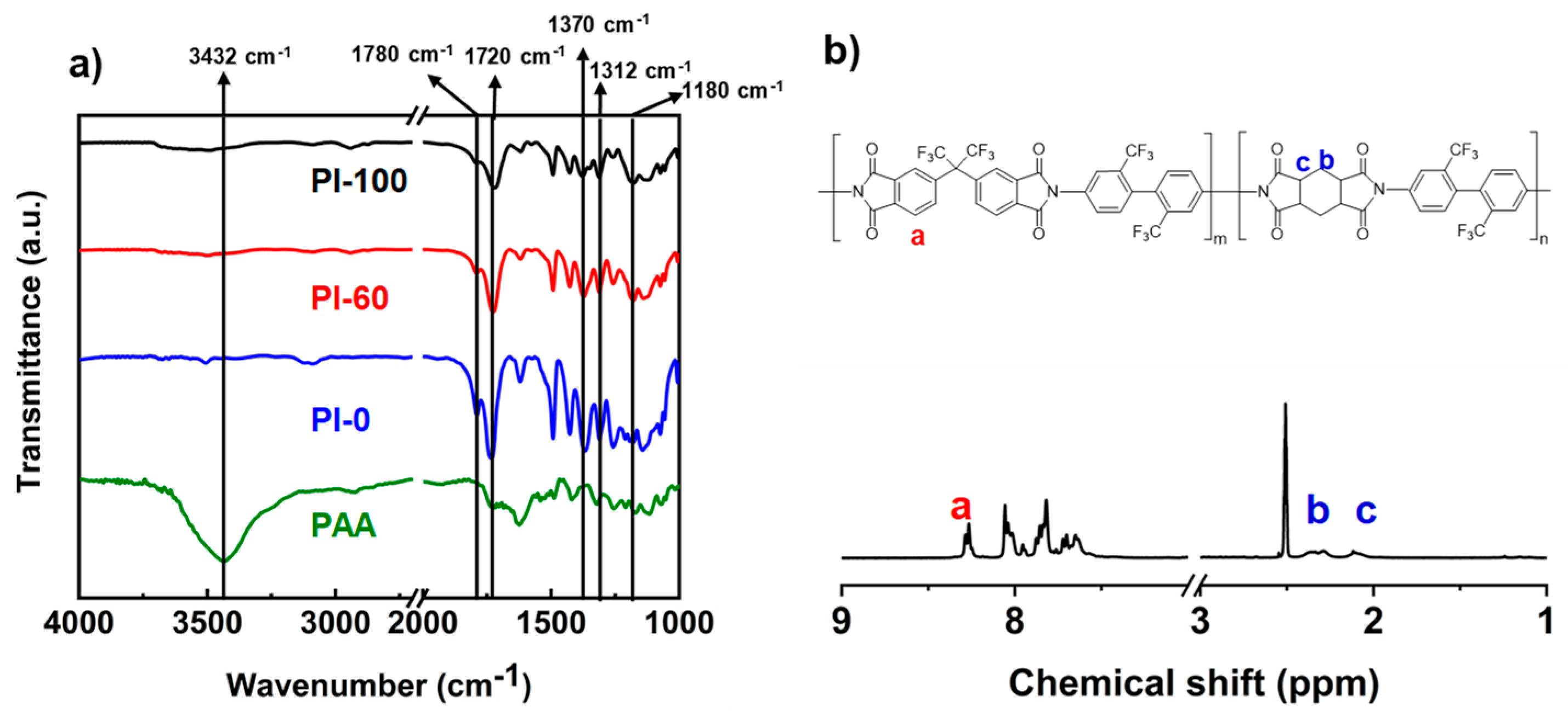
2.3. Characterization
3. Results and Discussion
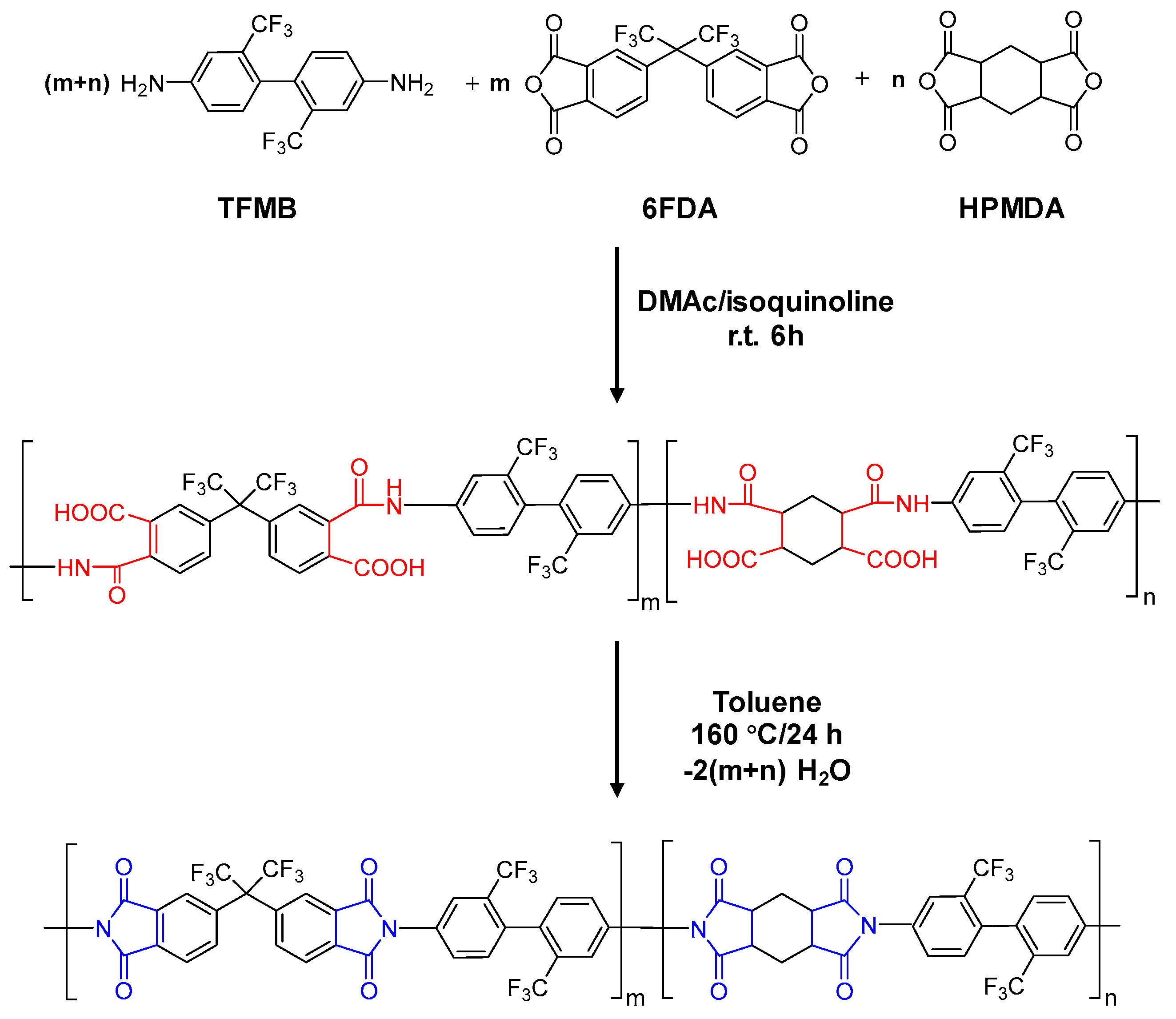
3.1. Synthesis of Homopolyimide and Copolyimide
3.2. Solubility
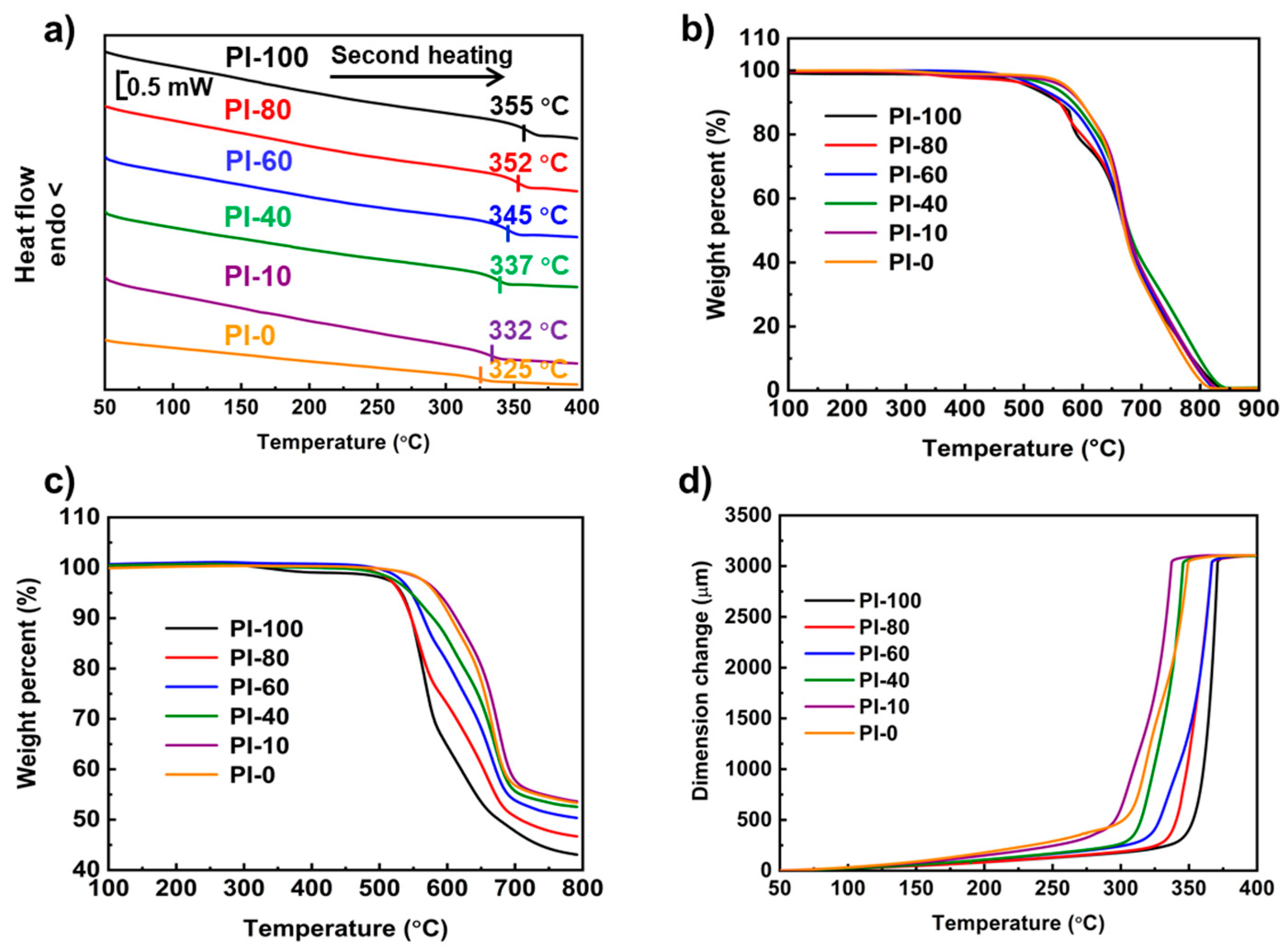
3.3. Thermal Behavior
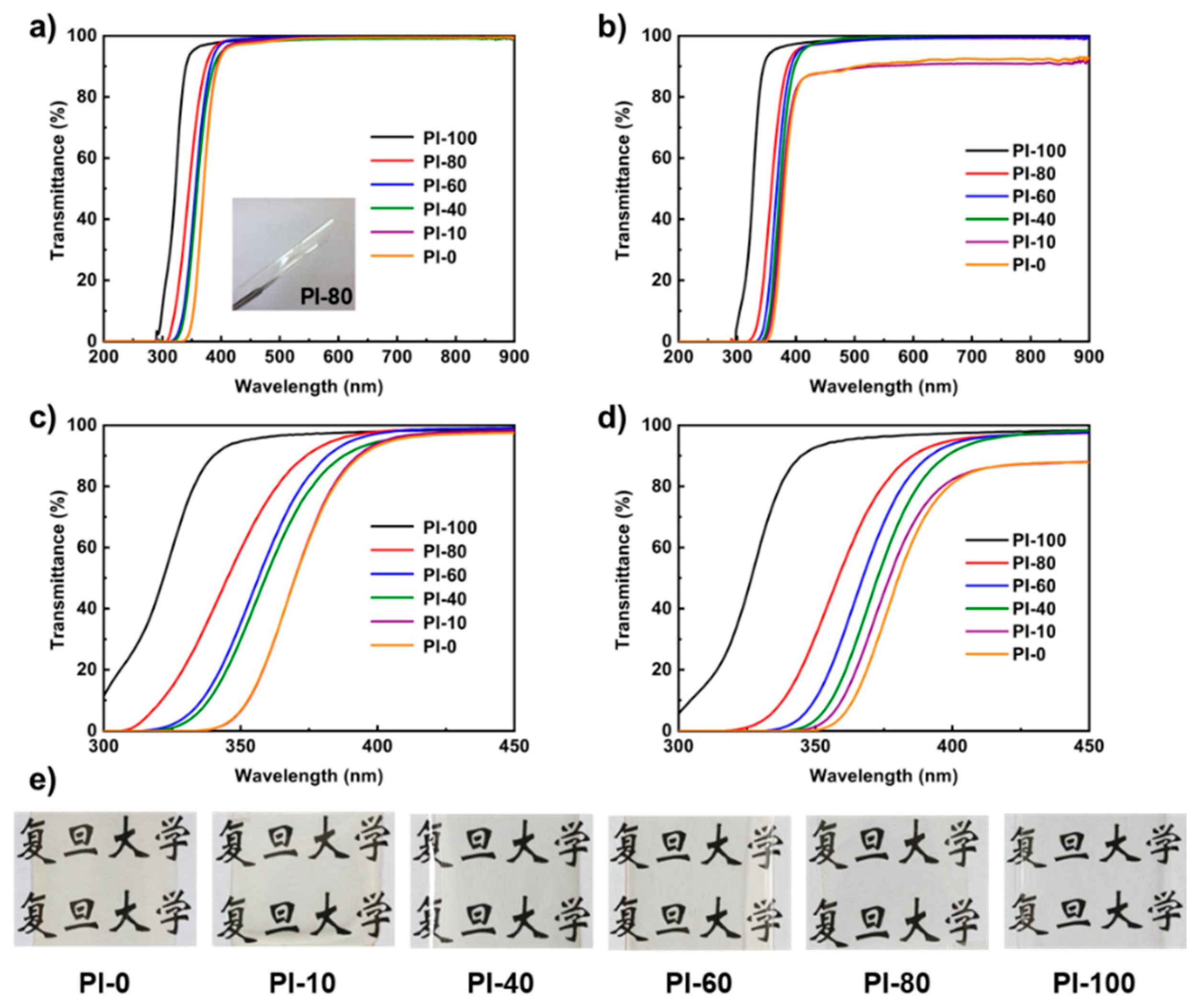
3.4. Optical Properties
3.5. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gouzman, I.; Crossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.Y.; Hu, W.P.; Fuchs, H. Recent Progress in Aromatic Polyimide Dielectrics for Organic Electronic Devices and Circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, S.Y.; Fan, L. Preparation and characterization of highly transparent and colorless semi-aromatic polyimide films derived from alicyclic dianhydride and aromatic diamines. Polymer 2012, 53, 3529–3539. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-Processable Colorless Polyimides Derived from Hydrogenated Pyromellitic Dianhydride with Controlled Steric Structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Yeo, H.; Goh, M.; Ku, B.C.; You, N.H. Synthesis and characterization of highly-fluorinated colorless polyimides derived from 4,4′-((perfluoro-[1,1′-biphenyl]-4,4′-diyl)bis(oxy))bis(2,6-dimethylaniline) and aromatic dianhydrides. Polymer 2015, 76, 280–286. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, S.Y.; Chung, I.S. Soluble and Transparent Polyimides from Unsymmetrical Diamine Containing Two Trifluoromethyl Groups. J. Polym. Sci. Pol. Chem. 2013, 51, 4413–4422. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, S.; Heo, J.; Kim, S.Y.; Chung, I.S. Soluble polyimides with trifluoromethyl pendent groups. Polymer 2013, 54, 5648–5654. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wang, H.M.; Chen, W.J.; Lee, T.M.; Leu, C.M. Synthesis and Properties of Novel Triptycene-Based Polyimides. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3109–3120. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficients of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Hasegawa, M. Semi-aromatic polyimides with low dielectric constant and low CTE. High Perform. Polym. 2001, 13, S93–S106. [Google Scholar] [CrossRef]
- Kumar, S.V.; Yu, H.C.; Choi, J.; Kudo, K.; Jang, Y.H.; Chung, C.M. Structure-property relationships for partially aliphatic polyimides. J. Polym. Res. 2011, 18, 1111–1117. [Google Scholar] [CrossRef]
- Ji, X.D.; Wang, Z.K.; Yan, J.L.; Wang, Z. Partially bio-based polyimides from isohexide-derived diamines. Polymer 2015, 74, 38–45. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.C.; Jung, Y.S.; Cho, H.J.; Seo, D.J.; Ha, C.S. Synthesis and Characterization of Fully Aliphatic Polyimides from an Aliphatic Dianhydride with Piperazine Spacer for Enhanced Solubility, Transparency, and Low Dielectric Constant. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2316–2328. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Ishii, J.; Yamaguchi, S.; Takezawa, E.; Kagayama, T.; Ishikawa, A. Colorless polyimides derived from 1S,2S,4R,5R-cyclohexanetetra carboxylic dianhydride, self-orientation behavior during solution casting, and their optoelectronic applications. Polymer 2014, 55, 4693–4708. [Google Scholar] [CrossRef]
- Hu, X.F.; Yan, J.L.; Wang, Y.X.; Mu, H.L.; Wang, Z.K.; Cheng, H.Y.; Zhao, F.Y.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Huang, W.; Yan, D.Y.; Lu, Q.H. Synthesis and Characterization of a Highly Soluble Aromatic Polyimide from 4,4′-Methylenebis(2-tertbutylaniline). Macromol. Rapid Commun. 2001, 22, 1481–1484. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.C.; Jeong, K.M.; Ando, S.; Ha, C.S. Transparent Aromatic Polyimides Derived from Thiophenyl-Substituted Benzidines with High Refractive Index and Small Birefringence. Macromolecules 2015, 48, 3462–3474. [Google Scholar] [CrossRef]
- Liaw, D.J.; Chang, F.C.; Leung, M.K.; Chou, M.Y.; Muellen, K. High Thermal Stability and Rigid Rod of Novel Organosoluble Polyimides and Polyamides Based on Bulky and Noncoplanar Naphthalene-Biphenyldiamine. Macromolecules 2005, 38, 4024–4029. [Google Scholar] [CrossRef]
- Yi, L.; Li, C.Y.; Huang, W.; Yan, D.Y. Soluble and Transparent Polyimides with High T-g from a New Diamine Containing tert-Butyl and Fluorene Units. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 976–984. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, L.; Zhu, Y.L.; Zheng, A.M.; Shen, Y.Z. Tristable data storage device of soluble polyimides based on novel asymmetrical diamines containing carbazole. Polym. Chem. 2016, 7, 1765–1772. [Google Scholar] [CrossRef]
- Han, K.S.; You, K.; Jang, W.H.; Rhee, T.H. Synthesis and properties of chlorinated polyimides. Macromol. Chem. Phys. 2000, 201, 747–751. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Yang, C.P.; Chen, S.H. Synthesis and Properties of Ortho-Linked Aromatic Polyimides Based on 1,2-Bis(4-aminophenoxy)-4-tertbutylbenzene. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1551–1559. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, L.; Zhu, Y.L.; Song, Y.J.; Wang, L.J.; Shen, Y.Z. Synthesis and memory characteristics of novel soluble polyimides based on asymmetrical diamines containing carbazole. Polymer 2016, 91, 118–127. [Google Scholar] [CrossRef]
- Liu, C.J.; Pei, X.L.; Huang, X.H.; Wei, C.; Sun, X.Y. Novel Non-Coplanar and Tertbutyl-Substituted Polyimides: Solubility, Optical, Thermal and Dielectric Properties. Chin. J. Chem. 2015, 33, 277–284. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Kim, Y.J.; Glass, T.E.; Lyle, G.D.; McGrath, J.E. Kinetic and mechanistic investigations of the formation of polyimides under homogeneous conditions. Macromolecules 1993, 26, 1344–1358. [Google Scholar] [CrossRef]
- Hu, X.F.; Mu, H.L.; Wang, Y.X.; Wang, Z.; Yan, J.L. Colorless polyimides derived from isomeric dicyclohexyl-tetracarboxylic dianhydrides for optoelectronic applications. Polymer 2018, 134, 8–19. [Google Scholar] [CrossRef]
- Yu, H.C.; Kumar, S.V.; Lee, J.H.; Oh, S.Y.; Chung, C.M. Preparation of Robust, Flexible, Transparent Films from Partially Aliphatic Copolyimides. Macromol. Res. 2015, 23, 566–573. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.G.; Jiang, G.L.; Zhang, Y.; Guo, C.Y.; Zhang, Y.J.; Qi, L.; Zhang, X.M. Highly transparent preimidized semi-alicyclic polyimide varnishes with low curing temperatures and desirable processing viscosities at high solid contents: Preparation and applications for LED chip passivation. J. Mater. Sci. Mater. Electron. 2019, 30, 549–560. [Google Scholar] [CrossRef]
- Yu, H.C.; Jung, J.W.; Choi, J.Y.; Chung, C.M. Kinetic Study of Low-Temperature Imidization of Poly(amic acid)s and Preparation of Colorless, Transparent Polyimide Films. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1593–1602. [Google Scholar] [CrossRef]
| Sample | HPMDA/6FDA (mmol/mmol) | TFMB (mmol) a | Mn (×104 g/mol) | Mw (×104 g/mol) | PDI |
|---|---|---|---|---|---|
| PI-0 | 0:10 | 10 | 4.51 | 6.63 | 1.47 |
| PI-10 | 1:9 | 10 | 4.51 | 7.36 | 1.63 |
| PI-40 | 4:6 | 10 | 3.43 | 6.05 | 1.76 |
| PI-60 | 6:4 | 10 | 3.68 | 6.10 | 1.66 |
| PI-80 | 8:2 | 10 | 3.14 | 5.74 | 1.82 |
| PI-100 | 10:0 | 10 | 2.71 | 4.78 | 1.76 |
| Sample | DMAc | DMF | DMSO | THF | EA | Acetone | DCM | m-Cresol | MeOH |
|---|---|---|---|---|---|---|---|---|---|
| PI-0 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +- | - |
| PI-10 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +- | - |
| PI-40 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +- | - |
| PI-60 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | - |
| PI-80 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | - |
| PI-100 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | - |
| Sample | Tg (°C) a | In Air | In Nitrogen | CTE (ppm/K) f | ||
|---|---|---|---|---|---|---|
| Td5 (°C) b | Td10 (°C) c | Td5 (°C) d | Td10 (°C) e | |||
| PI-0 | 325 | 573 | 597 | 582 | 606 | 148.9 |
| PI-10 | 332 | 567 | 596 | 586 | 613 | 123.7 |
| PI-40 | 337 | 547 | 584 | 546 | 580 | 97.0 |
| PI-60 | 345 | 523 | 569 | 547 | 565 | 87.3 |
| PI-80 | 352 | 517 | 560 | 531 | 547 | 75.6 |
| PI-100 | 355 | 491 | 551 | 532 | 547 | 68.7 |
| Sample | 15 μm Film | 45 μm Film | ||||
|---|---|---|---|---|---|---|
| λ0 (nm) a | T400 (%) b | T500 (%) c | λ0 (nm) | T400 (%) | T500 (%) | |
| PI-0 | 333 | 93.3 | 98.5 | 346 | 81.0 | 90.1 |
| PI-10 | 333 | 94.1 | 99.0 | 340 | 82.2 | 90.5 |
| PI-40 | 318 | 94.6 | 98.4 | 339 | 91.2 | 99.2 |
| PI-60 | 313 | 97.3 | 99.5 | 331 | 93.9 | 98.4 |
| PI-80 | 305 | 97.9 | 99.4 | 315 | 95.1 | 98.4 |
| PI-100 | 289 | 98.0 | 99.2 | 297 | 97.4 | 98.9 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| PI-0 | 91.4 ± 3.0 | 41.9 ± 2.7 | 501.1 ± 52.8 |
| PI-10 | 167.3 ± 5.4 | 55.9 ± 3.7 | 702.9 ± 25.9 |
| PI-40 | 155.1 ± 26.8 | 40.9 ± 2.8 | 787.7 ± 111.9 |
| PI-60 | 126.3 ± 5.4 | 48.5 ± 3.7 | 595.3 ± 42.1 |
| PI-80 | 119.6 ± 13.0 | 48.1 ± 3.0 | 546.2 ± 60.0 |
| PI-100 | 122.2 ± 10.8 | 54.2 ± 3.3 | 534.0 ± 18.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Li, C.; Yu, Y.; Wei, J. Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility. Polymers 2019, 11, 1319. https://doi.org/10.3390/polym11081319
Lan Z, Li C, Yu Y, Wei J. Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility. Polymers. 2019; 11(8):1319. https://doi.org/10.3390/polym11081319
Chicago/Turabian StyleLan, Zhongxu, Chunyu Li, Yanlei Yu, and Jia Wei. 2019. "Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility" Polymers 11, no. 8: 1319. https://doi.org/10.3390/polym11081319






