Ion-Conducting Redox-Active Polymer Gels Based on Stable Nitroxide Radicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of the 1,2,3-Triazole Cross-Linker
2.2.2. Quaternization of 1,2,3-Triazole Cross-Linker
- Triazolium+/TFSI− 1,2,3-triazole cross-linker (600 mg, 2.05 mmol) and N-methyl-bis[(trifluoromethyl) sulfonyl]imide (841 mg, 2.85 mmol) were mixed in acetonitrile (1 mL) and heated for 72 h at 41 °C. The crude compound was precipitated in diethyl ether and filtered on 0.22 µm poly(tetrafluoroethylene) (PTFE) filter. The obtained yellow solid was dried in vacuum at 40 °C (1.08 g, 90%).
- Triazolium+/I− 1,2,3-triazole cross-linkers (260 mg, 0.887 mmol) and methyl iodide (1.26 mg, 8.87 mmol) were mixed in acetonitrile (1 mL) and heated for 72 h at 41 °C to ensure complete solubility. The crude compound was precipitated in diethyl ether and filtered on 0.22 µm PTFE filter. The yellow solid obtained was dried in vacuum at 40 °C (360 mg, 93%).
2.2.3. Synthesis of PTMA Networks
2.2.4. Anion Exchange of Triazolium+/I− PTMA Network into Triazolium+/PF6− PTMA Network
2.2.5. Sample Preparation for Electrochemical Impedance Spectroscopy (EIS) Measurements
2.3. Instrumentation
2.3.1. Nuclear Magnetic Resonance (NMR)
2.3.2. Electrochemical Impedance Spectroscopy (EIS)
2.3.3. Transference Number Measurements
3. Results and Discussion
3.1. Synthesis of the PTMA Gels Incorporating the 1,2,3 Triazolium Groups
3.2. Influence of the Chemical Nature of the Crosslinking Nodes on the Ionic Conductivity
3.3. Influence of the Crosslinking Degree on the Ionic Conductivity
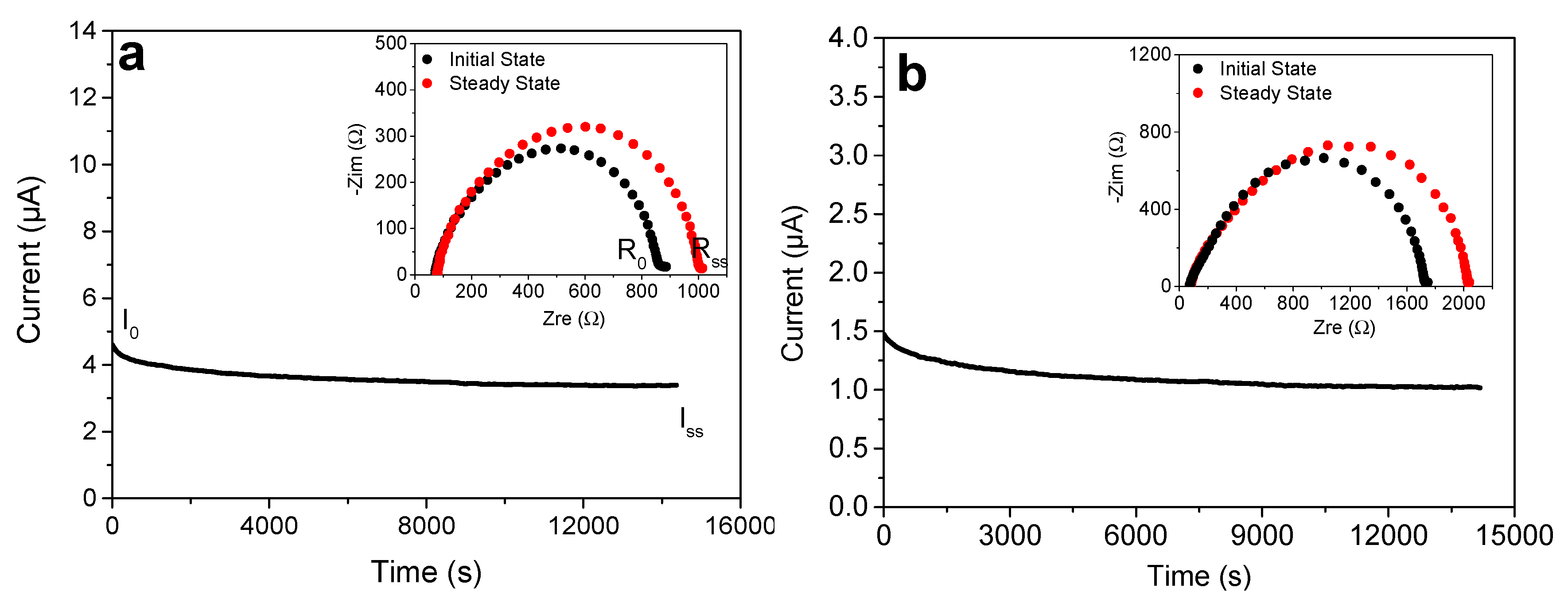
3.4. Loading of the Gels with Lithium Salts and Determination of the Lithium Ions Transference Number
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, Y.; Tao, Z.; Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
- Jähnert, T.; Häupler, B.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Synthesis and Charge-Discharge Studies of Poly(ethynylphenyl)galvinoxyles and Their Use in Organic Radical Batteries with Aqueous Electrolytes. Macromol. Chem. Phys. 2013, 214, 2616–2623. [Google Scholar] [CrossRef]
- Jähnert, T.; Häupler, B.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Polymers Based on Stable Phenoxyl Radicals for the Use in Organic Radical Batteries. Macromol. Rapid Commun. 2014, 35, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.-A.; Blinco, J.P. Nitroxide radical polymers—A versatile material class for high-tech applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- Nishide, H.; Iwasa, S.; Pu, Y.-J.; Suga, T.; Nakahara, K.; Satoh, M. Organic radical battery: Nitroxide polymers as a cathode-active material. Electrochim. Acta 2004, 50, 827–831. [Google Scholar] [CrossRef]
- Nishide, H.; Koshika, K.; Oyaizu, K. Environmentally benign batteries based on organic radical polymers. Pure Appl. Chem. 2009, 81, 1961–1970. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. High-rate capable organic radical cathodes for lithium rechargeable batteries. J. Power Sources 2007, 165, 870–873. [Google Scholar] [CrossRef]
- Suga, T.; Konishi, H.; Nishide, H. Photocrosslinked nitroxide polymer cathode-active materials for application in an organic-based paper battery. Chem. Commun. 2007, 43, 1730–1732. [Google Scholar] [CrossRef]
- Koshika, K.; Sano, N.; Oyaizu, K.; Nishide, H. An ultrafast chargeable polymer electrode based on the combination of nitroxide radical and aqueous electrolyte. Chem. Commun. 2009, 45, 836–838. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, Y.; Wang, L.; Fan, J.; Monteiro, M.J.; Jia, Z. The impact of the molecular weight on the electrochemical properties of poly(TEMPO methacrylate). Polym. Chem. 2017, 8, 1815–1823. [Google Scholar] [CrossRef]
- Boujioui, F.; Bertrand, O.; Ernould, B.; Brassinne, J.; Janoschka, T.; Schubert, U.S.; Vlad, A.; Gohy, J.-F. One-pot synthesis of electro-active polymer gels via Cu(0)-mediated radical polymerization and click chemistry. Polym. Chem. 2017, 8, 441–450. [Google Scholar] [CrossRef]
- Ernould, B.; Bertrand, O.; Minoia, A.; Lazzaroni, R.; Vlad, A.; Gohy, J.-F. Electroactive polymer/carbon nanotube hybrid materials for energy storage synthesized via a “grafting to” approach. RSC Adv. 2017, 7, 17301–17310. [Google Scholar] [CrossRef]
- Ernould, B.; Devos, M.; Bourgeois, J.-P.; Rolland, J.; Vlad, A.; Gohy, J.-F. Grafting of a redox polymer onto carbon nanotubes for high capacity battery materials. J. Mater. Chem. A 2015, 3, 8832–8839. [Google Scholar] [CrossRef]
- Hergué, N.; Ernould, B.; Minoia, A.; Lazzaroni, R.; Gohy, J.-F.; Dubois, P.; Coulembier, O. Improving the Performance of Batteries by using Multi-Pyrene PTMA Structures. Batter. Supercaps 2018, 1, 102–109. [Google Scholar] [CrossRef]
- Ernould, B.; Boujioui, F.; Vlad, A.; Bertrand, O.; Gohy, J.-F. Synthesis of polymer precursors of electroactive materials by SET-LRP. Polym. Chem. 2015, 6, 6067–6072. [Google Scholar]
- Geng, J.; Mantovani, G.; Tao, L.; Nicolas, J.; Chen, G.; Wallis, R.; Mitchell, D.A.; Johnson, B.R.G.; Evans, S.D.; Haddleton, D.M. Site-Directed Conjugation of “Clicked” Glycopolymers to Form Glycoprotein Mimics: Binding to Mammalian Lectin and Induction of Immunological Function. J. Am. Chem. Soc. 2007, 129, 15156–15163. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 3, 1977–1986. [Google Scholar] [CrossRef]
- Rostro, L.; Baradwaj, A.G.; Boudouris, B.W. Controlled Radical Polymerization and Quantification of Solid State Electrical Conductivities of Macromolecules Bearing Pendant Stable Radical Groups. ACS Appl. Mater. Interfaces 2013, 5, 9896–9901. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S. Electrolytes for Low-Temperature Lithium Batteries Based on Ternary Mixtures of Aliphatic Carbonates. J. Electrochem. Soc. 1999, 146, 486–492. [Google Scholar] [CrossRef]
- Fisher, A.S.; Khalid, M.B.; Widstrom, M.; Kofinas, P. Anion Effects on Solid Polymer Electrolytes Containing Sulfur Based Ionic Liquid for Lithium Batteries. J. Electrochem. Soc. 2012, 159, A592–A597. [Google Scholar] [CrossRef] [Green Version]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Phan, T.N.T.; Gigmes, D.; Drockenmuller, E. Enhancing Properties of Anionic Poly(ionic liquid)s with 1,2,3-Triazolium Counter Cations. ACS Macro Lett. 2014, 3, 658–662. [Google Scholar] [CrossRef]
- López-Peña, H.A.; Hernández-Muñoz, L.S.; Frontana-Uribe, B.A.; González, F.J.; Cardoso, J.; González, I.; Frontana, C. Tacticity Influence on the Electrochemical Reactivity of Group Transfer Polymerization-Synthesized PTMA. J. Phys. Chem. B 2012, 116, 5542–5550. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]










| Crosslinking Type | [Crosslinker]/[Monomer] | Mol % Crosslinking Agent |
|---|---|---|
| A | 0.006 | 0.6 |
| B | 0.025 | 2.4 |
| C | 0.1 | 9 |
| Gel Sample | SC 1 (wt.%) | Anion Volume 2 (Å3) |
|---|---|---|
| 4 | 83 | - |
| 5 | 86 | 34.31 |
| 6 | 93 | 72.61 |
| 7 | 95 | 147.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boujioui, F.; Gohy, J.-F. Ion-Conducting Redox-Active Polymer Gels Based on Stable Nitroxide Radicals. Polymers 2019, 11, 1322. https://doi.org/10.3390/polym11081322
Boujioui F, Gohy J-F. Ion-Conducting Redox-Active Polymer Gels Based on Stable Nitroxide Radicals. Polymers. 2019; 11(8):1322. https://doi.org/10.3390/polym11081322
Chicago/Turabian StyleBoujioui, Fadoi, and Jean-François Gohy. 2019. "Ion-Conducting Redox-Active Polymer Gels Based on Stable Nitroxide Radicals" Polymers 11, no. 8: 1322. https://doi.org/10.3390/polym11081322






