Graphene/Carbon Paper Combined with Redox Active Electrolyte for Supercapacitors with High Performance
Abstract
1. Introduction
2. Experimental
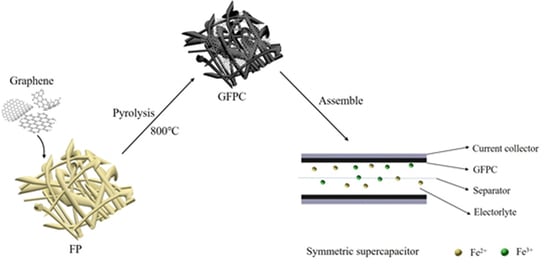
2.1. Preparation of Filter Paper-Derived Carbon (FPC) Electrode and Graphene Modified Filter Paper-Derived Carbon (GFPC)
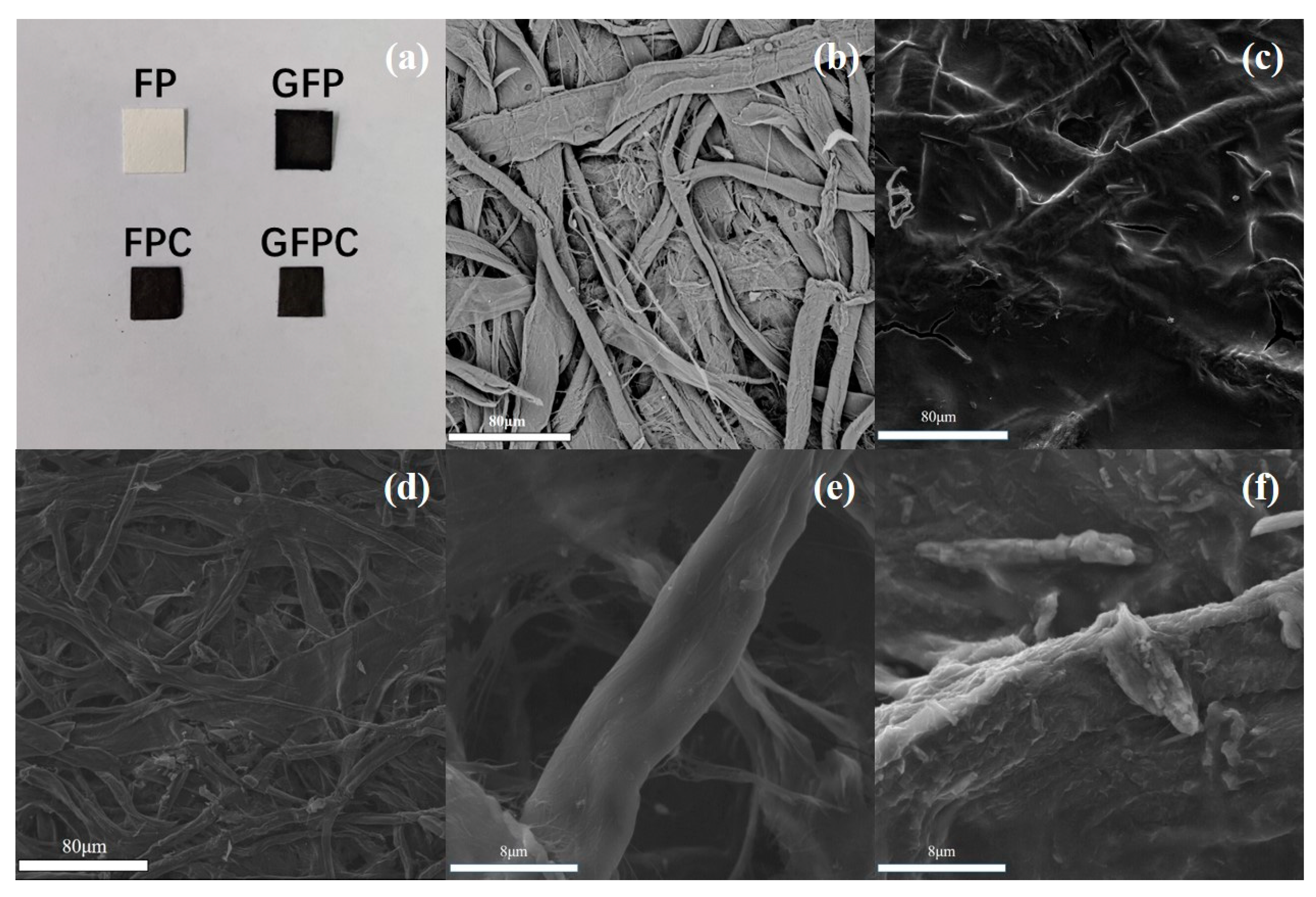
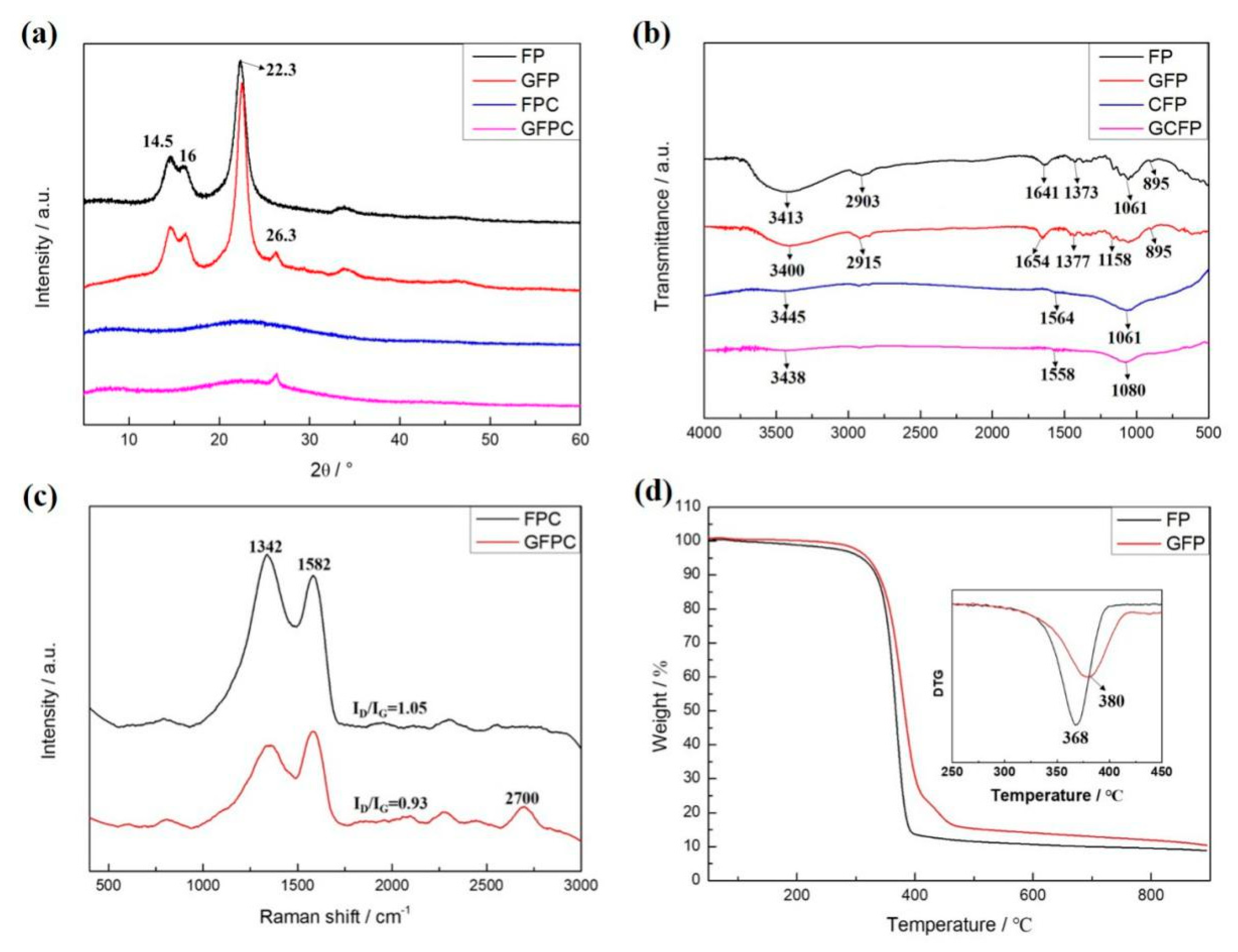
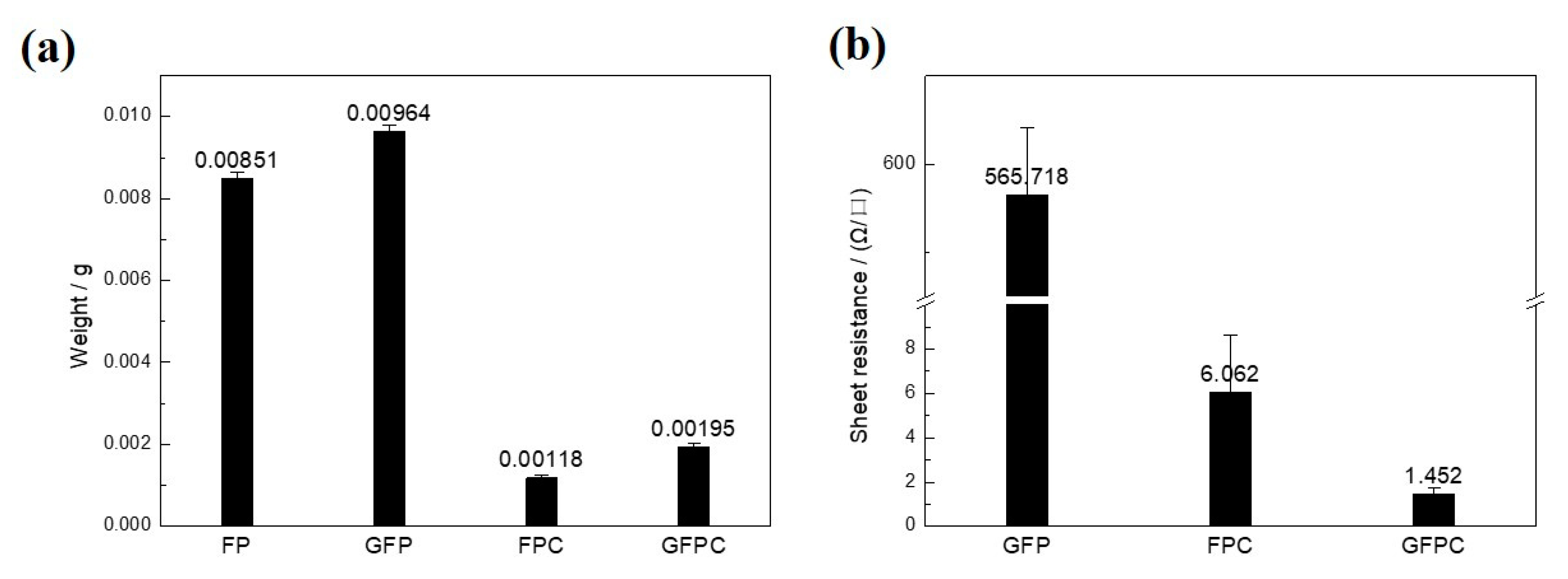
2.2. Characterization
2.3. Electrochemical Measurement
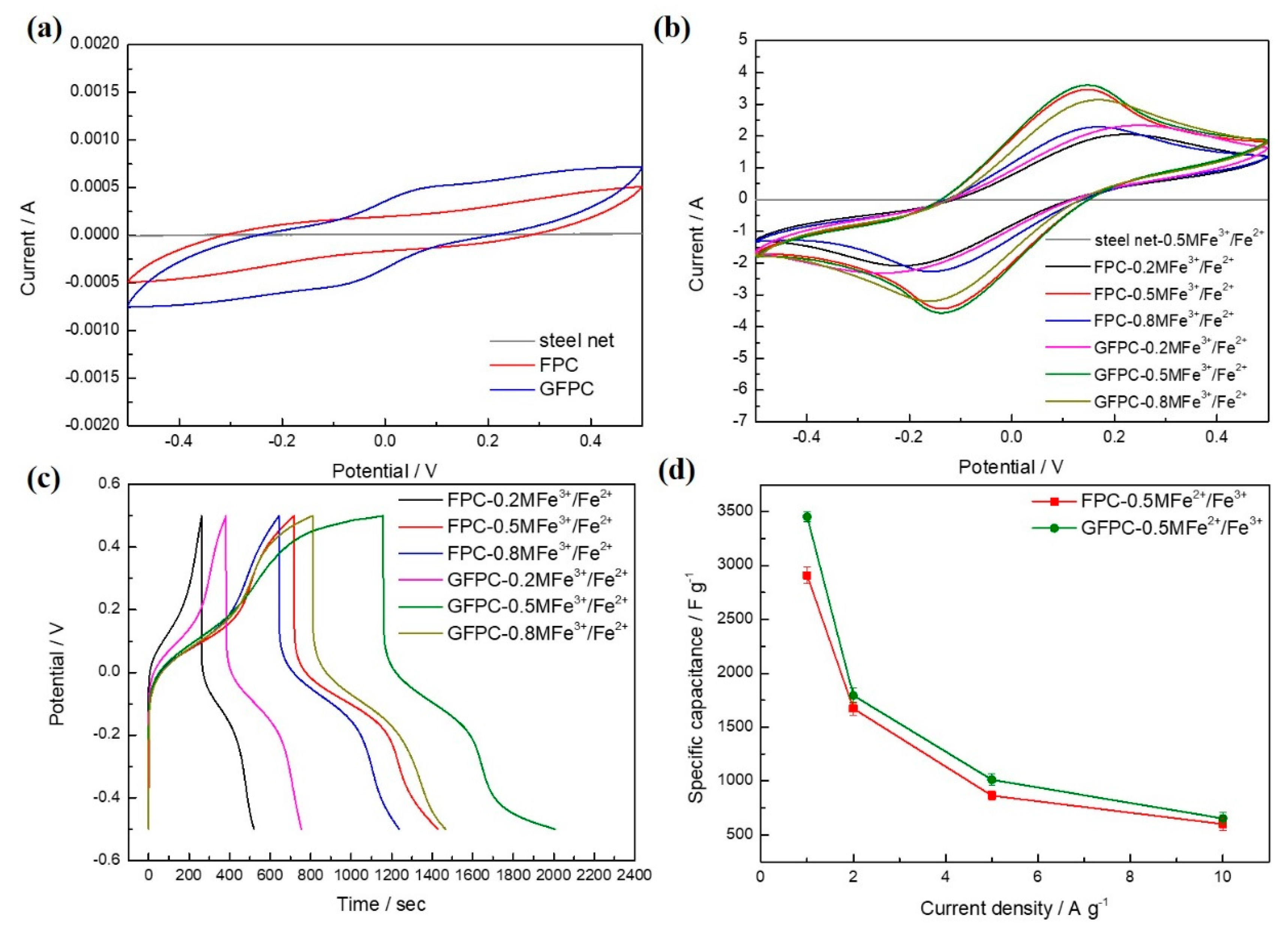
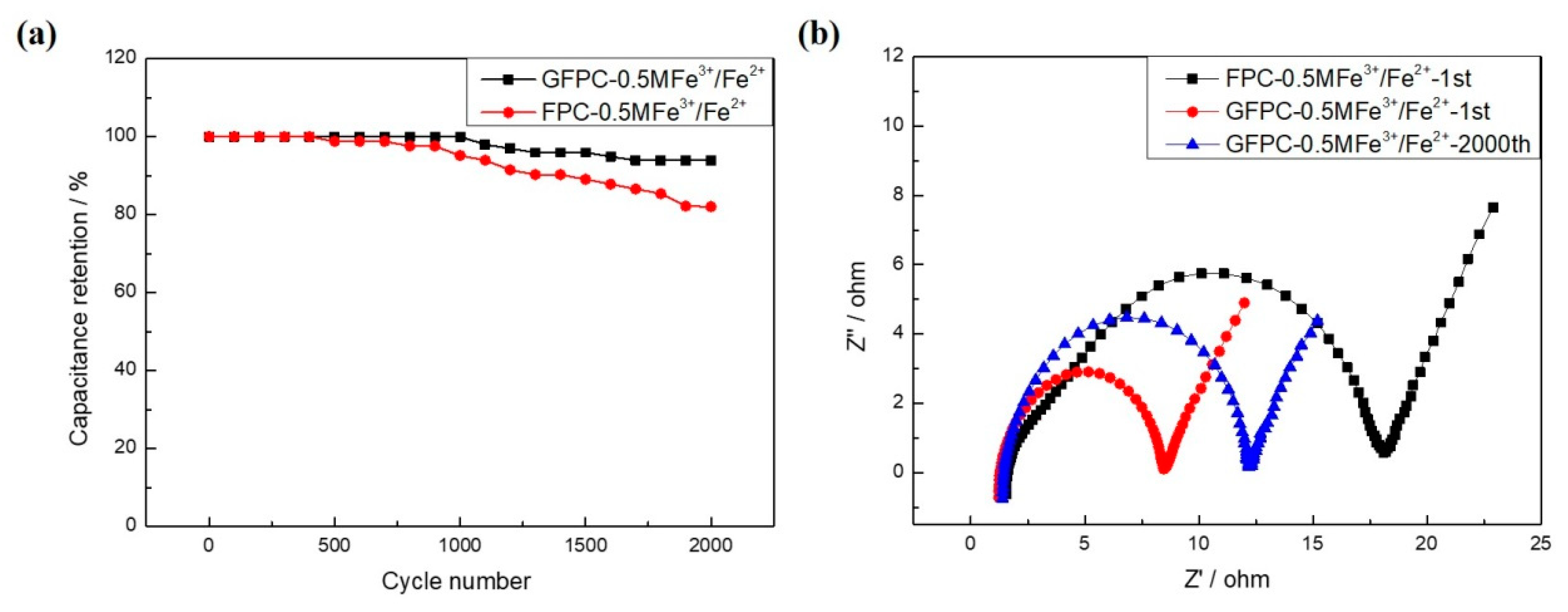
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Wen, Z.; Kim, H.; Lu, G.; Yu, K.; Chen, J. One-step fabrication and capacitive behavior of electrochemical double layer capacitor electrodes using vertically-oriented graphene directly grown on metal. Carbon 2012, 50, 4379–4387. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, J.; Wang, B.; Ni, W.; Gu, G.; Li, X.; Huang, L.; Yang, G.; Nie, F. Needle-like Co3O4 anchored on the graphene with enhanced electrochemical performance for aqueous supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7626–7632. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.V.; Selvan, R.K. Improved electrochemical performances of reduced graphene oxide based supercapacitor using redox additive electrolyte. Carbon 2015, 90, 260–273. [Google Scholar] [CrossRef]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Gu, B.; Su, H.; Chu, X.; Wang, Q.; Huang, H.; He, J.; Wu, T.; Deng, W.; Zhang, H.; Yang, W. Rationally assembled porous carbon superstructures for advanced supercapacitors. Chem. Eng. J. 2019, 361, 1296–1303. [Google Scholar] [CrossRef]
- Zhang, L.C.; Hu, Z.; Wang, L.; Teng, F.; Yu, Y.; Chen, C.H. Rice paper-derived 3D-porous carbon films for lithium-ion batteries. Electrochim. Acta 2013, 89, 310–316. [Google Scholar] [CrossRef]
- Davidsen, R.S.; Hemanth, S.; Keller, S.S.; Bek, T.; Hansen, O. Evaluation of the capacitive behavior of 3D carbon electrodes for sub-retinal photovoltaic prosthesis. Micro Nano Eng. 2019, 2, 110–116. [Google Scholar] [CrossRef]
- Quang, L.N.; Halder, A.; Rezaei, B.; Larsen, P.E.; Sun, Y.; Boisen, A.; Keller, S.S. Electrochemical pyrolytic carbon resonators for mass sensing on electrodeposited polymers. Micro Nano Eng. 2019, 2, 64–69. [Google Scholar] [CrossRef]
- Zhang, C.; Cha, R.; Yang, L.; Mou, K.; Jiang, X. Fabrication of cellulose/graphene paper as a stable-cycling anode material without collector. Carbohydr. Polym. 2018, 184, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review, current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 5583. [Google Scholar] [CrossRef]
- Kafy, A.; Akther, A.; Zhai, L.; Kim, H.C.; Kim, J. Porous cellulose/graphene oxide nanocomposite as flexible and renewable electrode material for supercapacitor. Synth. Met. 2017, 223, 94–100. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.-Y.; Mai, Y.-W. Ultrafast Synthesis of Multifunctional N-Doped Graphene Foam in an Ethanol Flame. ACS Nano 2016, 10, 453–462. [Google Scholar] [CrossRef]
- Hu, J.; Kang, Z.; Li, F.; Huang, X. Graphene with three-dimensional architecture for high performance supercapacitor. Carbon 2014, 67, 221–229. [Google Scholar] [CrossRef]
- Shi, G.; Araby, S.; Gibson, C.T.; Meng, Q.S.; Zhu, S.M.; Ma, J. Graphene Platelets and Their Polymer Composites, Fabrication; Structure; Properties; and Applications. Adv. Funct. Mater. 2018, 28, 1706705. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors Based on Flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes, a novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Roldán, S.; Blanco, C.; Granda, M.; Menéndez, R.; Santamaría, R. Towards a further generation of high-energy carbon-based capacitors by using redox-active electrolytes. Angew. Chem. Int. Ed. 2011, 50, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.T.; Selvan, R.K.; Lee, Y.S.; Melo, J.S. Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J. Mater. Chem. A 2013, 1, 1086–1095. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Chaudhary, M.; Lin, T.-W. Enhancing the stability and capacitance of vanadium oxide nanoribbons/3D-graphene binder-free electrode by using VOSO4 as redox-active electrolyte. Chem. Eng. J. 2019, 355, 830–839. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lu, L.; Wang, T.; Xu, H.; Yu, C.; Li, H.; Tian, W. Improving the electrochemical performances of active carbon-based supercapacitors through the combination of introducing functional groups and using redox additive electrolyte. J. Saudi Chem. Soc. 2018, 22, 908–918. [Google Scholar] [CrossRef]
- Jain, D.; Kanungo, J.; Tripathi, S.K. Performance enhancement approach for supercapacitor by using mango kernels derived activated carbon electrode with p-hydroxyaniline based redox additive electrolyte. Mater. Chem. Phys. 2019, 229, 66–77. [Google Scholar] [CrossRef]
- Xia, C.; Leng, M.; Tao, W.; Wang, Q.; Gao, Y.; Zhang, Q. Polyaniline/carbon nanotube core–shell hybrid and redox active electrolyte for high-performance flexible supercapacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 4427–4436. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.H. High capacitive property for supercapacitor using Fe3+/Fe2+ redox couple additive electrolyte. Electrochim. Acta 2017, 231, 705–712. [Google Scholar] [CrossRef]
- Nieva, L.M.L.; Sieben, J.M.; Comignani, V.; Duarte, M.; Volpe, M.A.; Moyano, E.L. Biochar from pyrolysis of cellulose, An alternative catalyst support for the electro-oxidation of methanol. Int. J. Hydrogen Energy 2016, 41, 10695–10706. [Google Scholar] [CrossRef]
- Wang, L.; Schutz, C.; Salazar-Alvarez, G.; Titirici, M.-M. Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv. 2014, 4, 17549–17554. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Fan, Z.; Wei, T.; Zhang, L. High-performance supercapacitor electrodes based on highly corrugated graphene sheets. Carbon 2012, 50, 2179–2188. [Google Scholar] [CrossRef]
- Hwang, H.-C.; Woo, J.S.; Park, S.-Y. Flexible carbonized cellulose/single-walled carbon nanotube films with high conductivity. Carbohydr. Polym. 2018, 196, 168–175. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Abd Mutalib, M.; Jamil, S.M. Feasibility of recycled newspaper as cellulose source for regenerated cellulose membrane fabrication. J. Appl. Polym. Sci. 2015, 132, 42684. [Google Scholar] [CrossRef]
- Boruah, B.D.; Misra, A. Nickel hydroxide coated carbon nanoparticles mediated hybrid three-dimensional graphene foam assembly for supercapacitor. RSC Adv. 2016, 6, 36307–36313. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, Z.; Hu, Q.; Zheng, Q.; Jiang, N.; Xie, F.; Jie, W.; Xu, C.; Lin, D. Hierarchical nitrogen-doped porous carbon/carbon nanotube composites for high-performance supercapacitor. Superlattices Microstruct. 2019, 130, 50–60. [Google Scholar] [CrossRef]
- Wan, C.; Li, J. Graphene oxide/cellulose aerogels nanocomposite, Preparation; pyrolysis; and application for electromagnetic interference shielding. Carbohydr. Polym. 2016, 150, 172–179. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Fan, L.; Xu, K.; Zhong, X.; Lin, Y.; Lin, J. Improvement of the performance for quasi-solid-state supercapacitor by using PVA–KOH–KI polymer gel electrolyte. Electrochim. Acta 2011, 56, 6881–6886. [Google Scholar] [CrossRef]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Xu, G.; Yu, M.; Wu, M. Synthesis of microporous carbon/graphene composites for high-performance supercapacitors. Diam. Relat. Mater. 2016, 66, 119–125. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Fan, L.; Lin, Y.; Xu, K.; Tang, Z.; Cheng, C.; Tang, S.; Lin, J.; Huang, M.; et al. A novel redox-mediated gel polymer electrolyte for high-performance supercapacitor. J. Power Sour. 2012, 198, 402–407. [Google Scholar] [CrossRef]
- Frackowiak, E.; Fic, K.; Meller, M.; Lota, G. Electrochemistry Serving People and Nature, High-Energy Ecocapacitors based on Redox-Active Electrolytes. Chemsuschem 2012, 5, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zu, L.; Lian, H.; Hu, Z.; Jiang, Y.; Liu, Y.; Wang, X.; Cui, X. An ultrahigh performance supercapacitor based on simultaneous redox in both electrode and electrolyte. J. Alloys Compd. 2017, 694, 136–144. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Z.; Qian, M.; Zhang, Z.; Lin, J.; Huang, F. Enhanced specific capacitance by a new dual redox-active electrolyte in activated carbon-based supercapacitors. Carbon 2019, 143, 300–308. [Google Scholar] [CrossRef]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, S.; Fan, W.; Miao, Y.-E.; Liu, T. Filter paper-derived carbon fiber/polyaniline composite paper for high energy storage applications. Compos. Sci. Technol. 2014, 101, 152–158. [Google Scholar] [CrossRef]



| Electrode Materials | Redox Active Electrolyte | Cs (F·g−1) | Reference |
|---|---|---|---|
| Active carbon (from mango kernels) | 1 M H2SO4 + 50 mM p-hydroxyaniline (PHA) | 587.1 F·g−1 at 1 A·g−1 | [26] |
| Active carbon | H2SO4 + p-benzenediol | 474.29 F·g−1 at 0.83 A·g−1 | [41] |
| Active carbon | 1 M H2SO4 + 0.38 M hydroquinone | 901 F·g−1 at 2.65 mA·cm−1 | [22] |
| Nitrogen-doped porous carbon | 1 M H2SO4 + 0.08 M NaMoO4 | 1416F·g−1 at 15 A·g−1 | [42] |
| MWCNTs/PANI | 1 M H2SO4 + 0.05 M KI | 3331 F·g−1 at 1 A·g−1 | [43] |
| Active carbon | 1 M H2SO4 + 0.05 M FeBr3 | 885 F·g−1 at 2 A·g−1 | [44] |
| Porous carbon microspheres | 1 M HNO3 + 0.12 M CuCl2 | 4700 F·g−1 at 5 mV·s−1 | [45] |
| CNTs/PANI | 1 M H2SO4 + 0.02 M Fe3+/Fe2+ | 1128 F·g−1 at 5 A·g−1 | [27] |
| Graphene/Carbon (from filter paper) | 1 M H2SO4 + 0.5 M Fe3+/Fe2+ | 3396 F·g−1 at 1 A·g−1 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Mo, Y.; Meng, W.; Du, X.; Ma, C. Graphene/Carbon Paper Combined with Redox Active Electrolyte for Supercapacitors with High Performance. Polymers 2019, 11, 1355. https://doi.org/10.3390/polym11081355
Xia Y, Mo Y, Meng W, Du X, Ma C. Graphene/Carbon Paper Combined with Redox Active Electrolyte for Supercapacitors with High Performance. Polymers. 2019; 11(8):1355. https://doi.org/10.3390/polym11081355
Chicago/Turabian StyleXia, Yanlin, Youtian Mo, Wei Meng, Xusheng Du, and Chuanguo Ma. 2019. "Graphene/Carbon Paper Combined with Redox Active Electrolyte for Supercapacitors with High Performance" Polymers 11, no. 8: 1355. https://doi.org/10.3390/polym11081355
APA StyleXia, Y., Mo, Y., Meng, W., Du, X., & Ma, C. (2019). Graphene/Carbon Paper Combined with Redox Active Electrolyte for Supercapacitors with High Performance. Polymers, 11(8), 1355. https://doi.org/10.3390/polym11081355