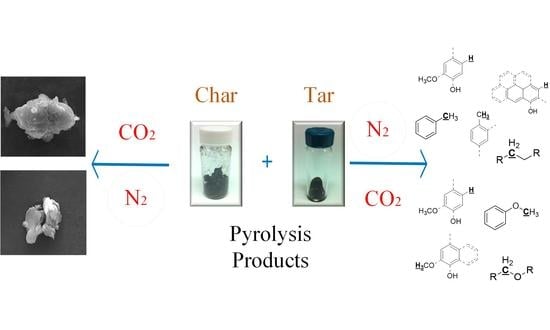
Impact of CO2 on Pyrolysis Products of Bituminous Coal and Platanus Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Tube Furnace Pyrolysis
2.3. Physicochemical Analysis
2.4. Infrared Analysis of Char
2.5. NMR Analysis of Tar
3. Results and Discussion
3.1. Product Yield of Coal and Biomass Pyrolysis
3.2. Effect of CO2 on Char Properties
3.1.1. Functional Groups of Char Structure
3.1.2. Characterization of Char Surface
SEM Analysis
BET Analysis
Elemental Composition of Char
3.3. Impact of CO2 on Tar Components
3.3.1. Analysis of 1H NMR Spectra
3.3.2. Analysis of 13C NMR Spectra
3.3.3. Elemental Composition of Tar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Ma, X.; He, Y. Co-pyrolysis characteristics of microalgae chlorella vulgaris and coal through TGA. Bioresour. Technol. 2012, 117, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Cao, F. Fine particulate matter (PM2.5) in China at a city level. Sci. Rep. 2015, 5, 14884. [Google Scholar] [CrossRef] [PubMed]
- Jamil, K.; Hayashi, J.; Li, C.-Z. Pyrolysis of a Victorian brown coal and gasification of nascent char in CO2 atmosphere in a wire-mesh reactor. Fuel 2004, 83, 833–843. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Interaction between biomass and different rank coals during co-pyrolysis. Renew. Energy 2010, 35, 288–292. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, V.; Ail, S.S.; Patuzzi, F.; Baratieri, M. Valorization of char from biomass gasification as catalyst support in dry reforming of methane. Front. Chem. 2019, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Bharathy, S.; Gnanasikamani, B.; Radhakrishnan Lawrence, K. Investigation on the use of plastic pyrolysis oil as alternate fuel in a direct injection diesel engine with titanium oxide nanoadditive. Environ. Sci. Pollut. Res. 2019, 26, 10319–10332. [Google Scholar] [CrossRef] [PubMed]
- Miura, K. Mild conversion of coal for producing valuable chemicals. Fuel Process. Technol. 2000, 62, 119–135. [Google Scholar] [CrossRef]
- Xu, W.-C.; Tomita, A. The effects of temperature and residence time on the secondary reactions of volatiles from coal pyrolysis. Fuel Process. Technol. 1989, 21, 25–37. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Encinar, J.M.; Beltrán, F.J.; Bernalte, A.; Ramiro, A.; González, J.F. Pyrolysis of two agricultural residues: Olive and grape bagasse. Influence of particle size and temperature. Biomass Bioenerg. 1996, 11, 397–409. [Google Scholar] [CrossRef]
- Guizani, C.; Escudero Sanz, F.J.; Salvador, S. Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties. Fuel 2014, 116, 310–320. [Google Scholar] [CrossRef]
- Yang, M.; Luo, B.; Shao, J.; Zeng, K.; Zhang, X.; Yang, H.; Chen, H. The influence of CO2 on biomass fast pyrolysis at medium temperatures. J. Renewable Sustainable Energy 2018, 10, 013108. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, D.; Cho, J.; He, G.; Shao, S.; Zhang, J. Hydrodynamics of a novel biomass autothermal fast pyrolysis reactor: Solid circulation rate and gas bypassing. Chem. Eng. J. 2012, 181, 685–693. [Google Scholar] [CrossRef]
- Kwon, E.E.; Jeon, Y.J.; Yi, H. New candidate for biofuel feedstock beyond terrestrial biomass for thermo-chemical process (pyrolysis/gasification) enhanced by carbon dioxide (CO2). Bioresour. Technol. 2012, 123, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.E.; Yi, H.; Castaldi, M.J. Utilizing carbon dioxide as a reaction medium to mitigate production of polycyclic aromatic hydrocarbons from the thermal decomposition of styrene butadiene rubber. Environ. Sci. Technol. 2012, 46, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhao, C.; Zhou, W.; Qu, C.; Chen, X. Investigation on coal pyrolysis in CO2 atmosphere. Energy Fuels 2009, 23, 3826–3830. [Google Scholar] [CrossRef]
- Farrow, T.S.; Sun, C.; Snape, C.E. Impact of CO2 on biomass pyrolysis, nitrogen partitioning, and char combustion in a drop tube furnace. J. Anal Appl. Pyrolysis 2015, 113, 323–331. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J.-I.; Ok, Y.S.; Kwon, E.E. Study on susceptibility of CO2-assisted pyrolysis of various biomass to CO2. Energy 2017, 137, 510–517. [Google Scholar] [CrossRef]
- Hanaoka, T.; Sakanishi, K.; Okumura, Y. The effect of N2/CO2/O2 content and pressure on characteristics and CO2 gasification behavior of biomass-derived char. Fuel Process. Technol. 2012, 104, 287–294. [Google Scholar] [CrossRef]
- Choi, D.; Kim, H.; Lee, S.S.; Nam, I.-H.; Lee, J.; Kim, K.-H.; Kwon, E.E. Enhanced accessibility of carbon in pyrolysis of brown coal using carbon dioxide. J. CO2 Utiliz. 2018, 27, 433–440. [Google Scholar] [CrossRef]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. Pyrolytic behavior of major biomass components in waste biomass. Polymers 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. In-situ evaluation for upgrading of biomass over noble metal catalysts by isotopic tracing and NMR monitoring. J. Anal. Appl. Pyrolysis 2019, 137, 253–258. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. NMR Characterization of Pyrolysis Oils from Kraft Lignin. Energy Fuels 2011, 25, 2322–2332. [Google Scholar] [CrossRef]
- Kosa, M.; Ben, H.; Theliander, H.; Ragauskas, A.J. Pyrolysis oils from CO2 precipitated Kraft lignin. Green Chem. 2011, 13, 3196–3202. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Rao, B.V.S.K.; Dalai, A.K. Slow pyrolysis of deoiled canola meal: product yields and characterization. Energy Fuels 2013, 27, 5268–5279. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Uchimiya, M.; Orlov, A.; Ramakrishnan, G.; Sistani, K. In situ and ex situ spectroscopic monitoring of biochar’s surface functional groups. J. Anal. Appl. Pyrolysis 2013, 102, 53–59. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Synergy in devolatilization characteristics of lignite and hazelnut shell during co-pyrolysis. Fuel 2007, 86, 373–380. [Google Scholar] [CrossRef]
- Haenel, M.W. Recent progress in coal structure research. Fuel 1992, 71, 1211–1223. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Luan, J.Y.; Wu, X.M.; Wu, G.F.; Shao, D.W. Analysis of Char Specific Surface Area and Porosity from the Fast Pyrolysis of Biomass and Pulverized Coal. Adv. Mater. Res. 2012, 608–609, 383–387. [Google Scholar] [CrossRef]
- Tarves, P.C.; Mullen, C.A.; Boateng, A.A. Effects of various reactive gas atmospheres on the properties of bio-oils produced using microwave pyrolysis. ACS Sustain. Chem. Eng. 2016, 4, 930–936. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, R.; Kumar, J.; Singh, R.; Gupta, P.; Krishna, B.B.; Bhaskar, T. Pyrolysis behavior of rice straw under carbon dioxide for production of bio-oil. Renew. Energy 2018, 129, 686–694. [Google Scholar] [CrossRef]




| Sample | Proximate Analysis wd/% | Ultimate Analysis wd/% | ||||||
|---|---|---|---|---|---|---|---|---|
| Ash | Volatile | Fixed Carbon | C | H | O * | N | S | |
| NSC | 17.06 | 34.99 | 47.95 | 65.69 | 4.35 | 25.84 | 1.17 | 2.95 |
| PS | 1.46 | 80.57 | 17.97 | 51.08 | 6.43 | 42.42 | 0.07 | -- |
| Sample Type | BET Surface Area A/(m2·g−1) | Average Pore Diameter d/nm | Total Pore Volume v/(cm3·g−1) |
|---|---|---|---|
| NSC(N2) | 123.56 | 2.24 | 0.069 |
| NSC(CO2) | 152.41 | 2.15 | 0.082 |
| PS(N2) | 288.18 | 2.11 | 0.152 |
| PS(CO2) | 334.45 | 1.97 | 0.165 |
| Element | NSC(N2) | NSC(CO2) | PS(N2) | PS(CO2) |
|---|---|---|---|---|
| C w/% | 64.86 | 66.85 | 80.23 | 82.04 |
| H w/% | 3.24 | 3.22 | 3.86 | 3.81 |
| O w/% | 28.44 | 26.27 | 15.72 | 13.98 |
| N w/% | 1.11 | 1.19 | 0.19 | 0.17 |
| S w/% | 2.35 | 2.47 | -- | -- |
| Assignments | Chemical Shift Ranges (ppm) | Hydrogen Percentages | |||
|---|---|---|---|---|---|
| NSC(N2) | NSC(CO2) | PS(N2) | PS(CO2) | ||
| –CHO, –COOH | 9.6–10.0 | 0.27 | 0.14 | 1.21 | 0.04 |
| (H–PAH) | 7.5–9.0 | 11.66 | 1.87 | 1.69 | 2.96 |
| (H-single ring aromatic) | 6.0–7.5 | 8.49 | 3.35 | 0.11 | 0.31 |
| (aromatic–OH, water) | ~4.0–5.0 | 5.86 | 14.36 | 4.28 | 12.62 |
| (CH3–O–aromatic, water) | ~3.8 | 15.52 | 30.05 | 33.52 | 36.75 |
| (CH3–O–aliphatic) | ~3.3 | 10.41 | 1.95 | 11.80 | 8.77 |
| (CH3–aromatic) | ~2.2 | 5.24 | 1.17 | 3.85 | 2.83 |
| (CH2/3–aliphatic) | 0.0–2.0 | 42.55 | 47.11 | 43.54 | 35.72 |
| Functional Group | Integration Region (ppm) | Carbon Percentages | ||||
|---|---|---|---|---|---|---|
| NSC(N2) | NSC(CO2) | PS(N2) | PS(CO2) | |||
| Carbonyl or Carboxyl bond | 215.0–166.5 | 3.48 | 8.73 | 2.36 | 6.31 | |
| Aromatic C–O bond | 166.5–142.0 | 0.92 | 1.29 | 1.72 | 4.95 | |
| Aromatic C–C bond | 142.0–125.0 | 5.97 | 14.22 | 27.92 | 17.94 | |
| Aromatic C–H bond | 125.0–95.8 | 17.63 | 12.36 | 17.25 | 9.79 | |
| Levoglucosan | C1 102.3, C2 72.0 | 3.99 | 3.17 | 0.16 | 0.20 | |
| C3 73.7, C4 71.7 | ||||||
| C5 76.5, C6 64.9 | ||||||
| Aliphatic C–O bond | 95.8–60.8 | 28.48 | 28.78 | 3.29 | 4.00 | |
| Methoxyl-Aromatic bond | 60.8–55.2 | 11.56 | 12.51 | 14.70 | 16.46 | |
| Aliphatic C–C bond | General | 55.2–0.0 | 31.98 | 22.11 | 32.74 | 40.56 |
| Methyl-Aromatic | 21.6–19.1 | 3.09 | 3.04 | 4.25 | 2.29 | |
| Methyl-Aromatic at ortho position of a hydroxyl or methoxyl group | 16.1–15.4 | 0.87 | 1.05 | 1.17 | 0.96 | |
| Element | NSC (N2) | NSC (CO2) | PS (N2) | PS (CO2) |
|---|---|---|---|---|
| C w/% | 55.84 | 58.43 | 71.14 | 67.02 |
| H w/% | 8.47 | 7.23 | 9.32 | 8.97 |
| O w/% | 35.40 | 33.96 | 17.98 | 22.64 |
| N w/% | 0.29 | 0.38 | 1.56 | 1.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Ben, H.; Wu, Z.; Nie, K.; Han, G.; Jiang, W. Impact of CO2 on Pyrolysis Products of Bituminous Coal and Platanus Sawdust. Polymers 2019, 11, 1370. https://doi.org/10.3390/polym11081370
Luo Y, Ben H, Wu Z, Nie K, Han G, Jiang W. Impact of CO2 on Pyrolysis Products of Bituminous Coal and Platanus Sawdust. Polymers. 2019; 11(8):1370. https://doi.org/10.3390/polym11081370
Chicago/Turabian StyleLuo, Ying, Haoxi Ben, Zhihong Wu, Kai Nie, Guangting Han, and Wei Jiang. 2019. "Impact of CO2 on Pyrolysis Products of Bituminous Coal and Platanus Sawdust" Polymers 11, no. 8: 1370. https://doi.org/10.3390/polym11081370
APA StyleLuo, Y., Ben, H., Wu, Z., Nie, K., Han, G., & Jiang, W. (2019). Impact of CO2 on Pyrolysis Products of Bituminous Coal and Platanus Sawdust. Polymers, 11(8), 1370. https://doi.org/10.3390/polym11081370