Development of a Simplified Radiation-Induced Emulsion Graft Polymerization Method and Its Application to the Fabrication of a Heavy Metal Adsorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
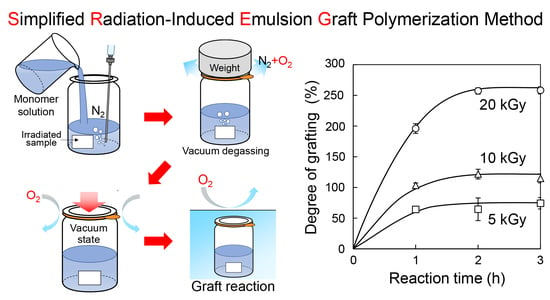
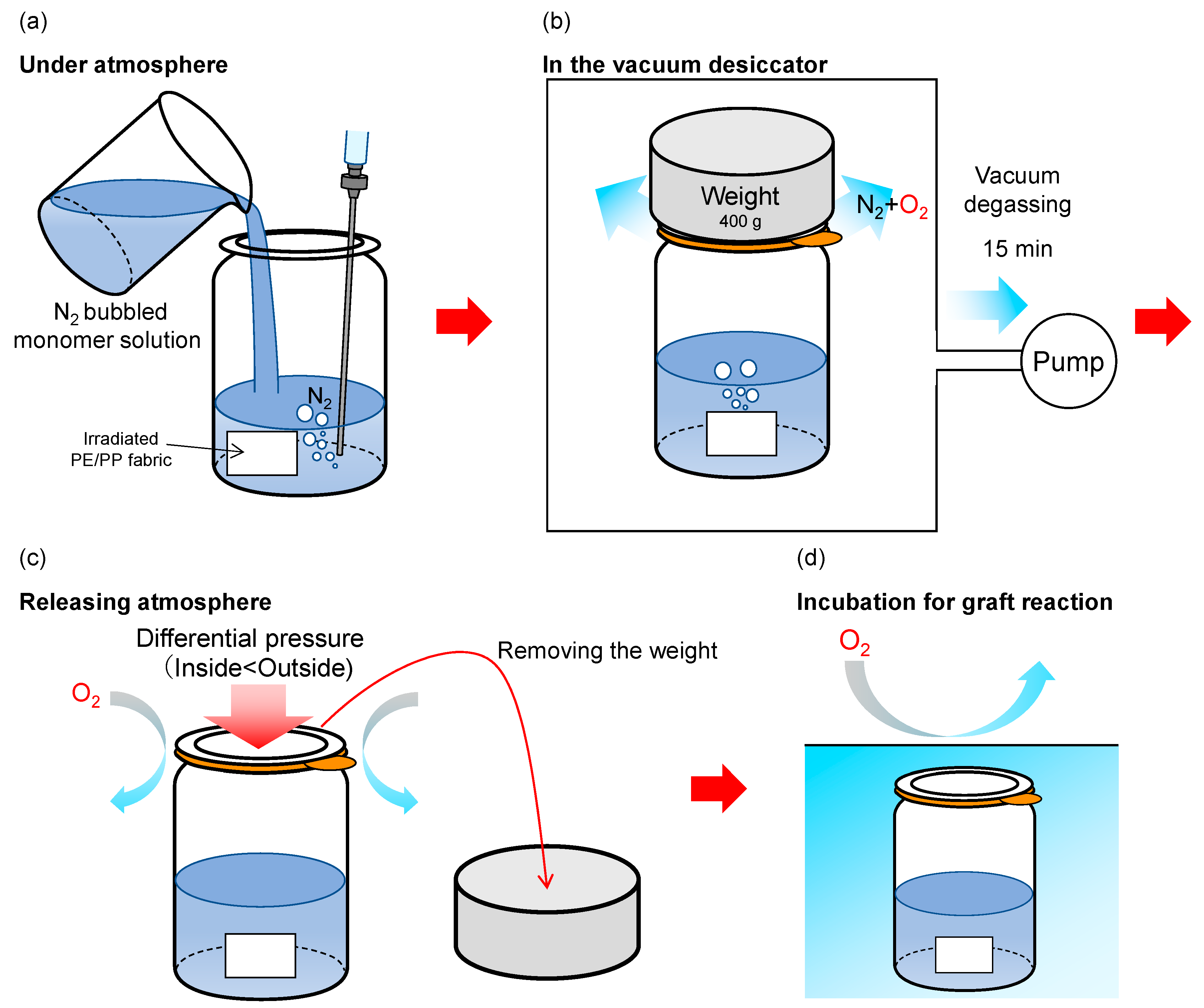
2.2. SREG Method
2.3. Introduction of Iminodiacetic Groups onto PE/PP-g-GMA Fabrics
2.4. Heavy Metal Adsorption Test
3. Results and Discussion
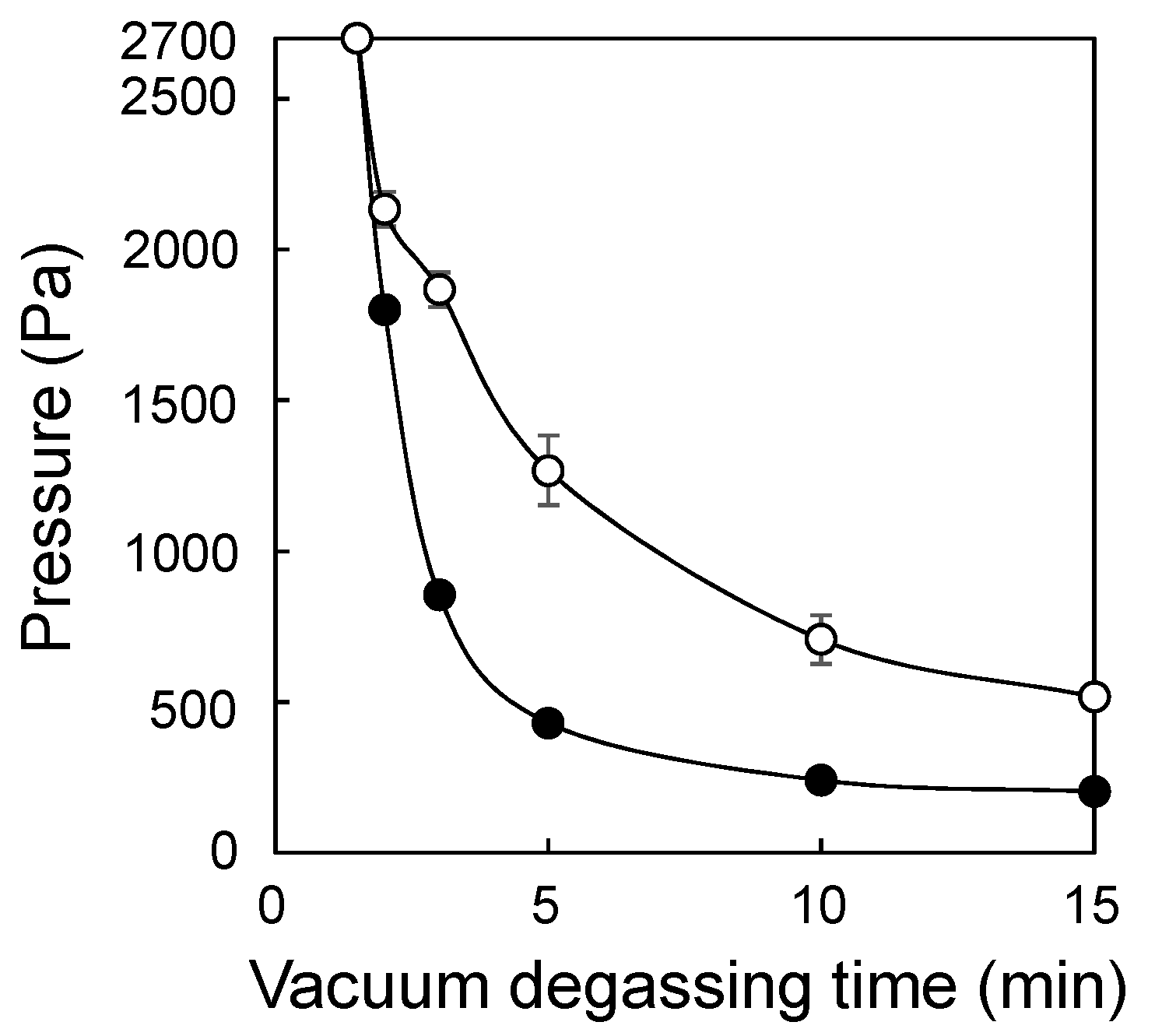
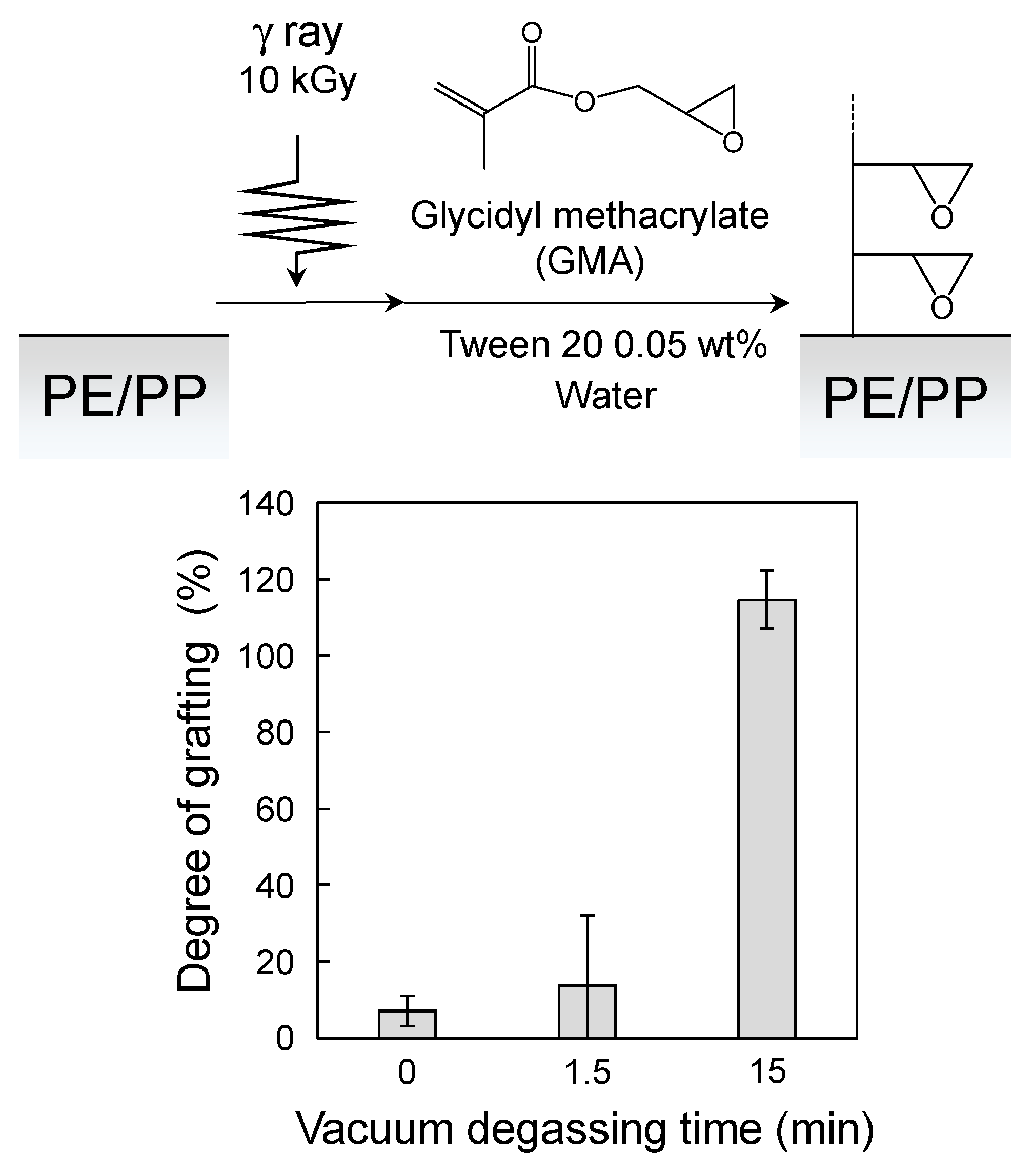
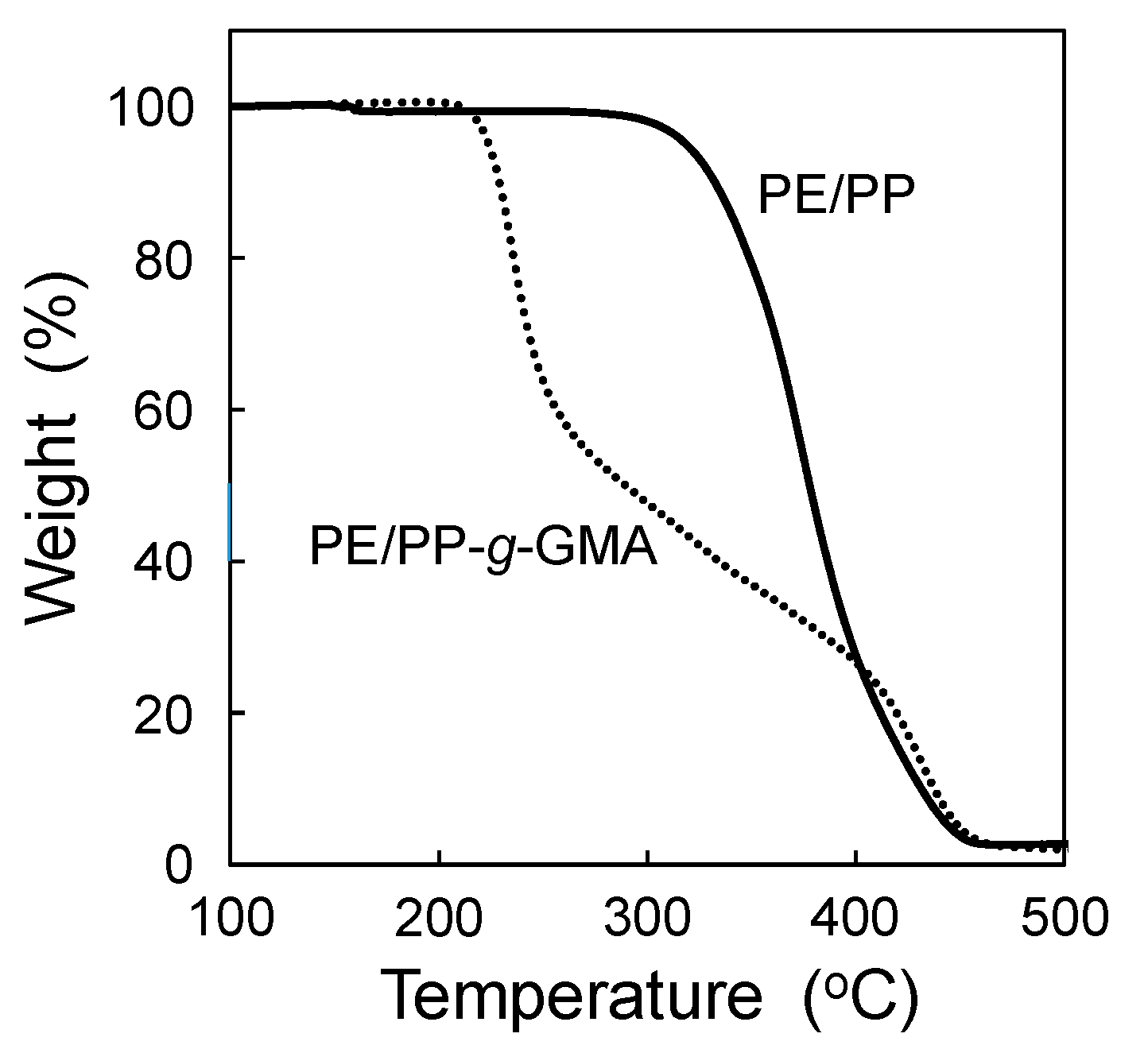
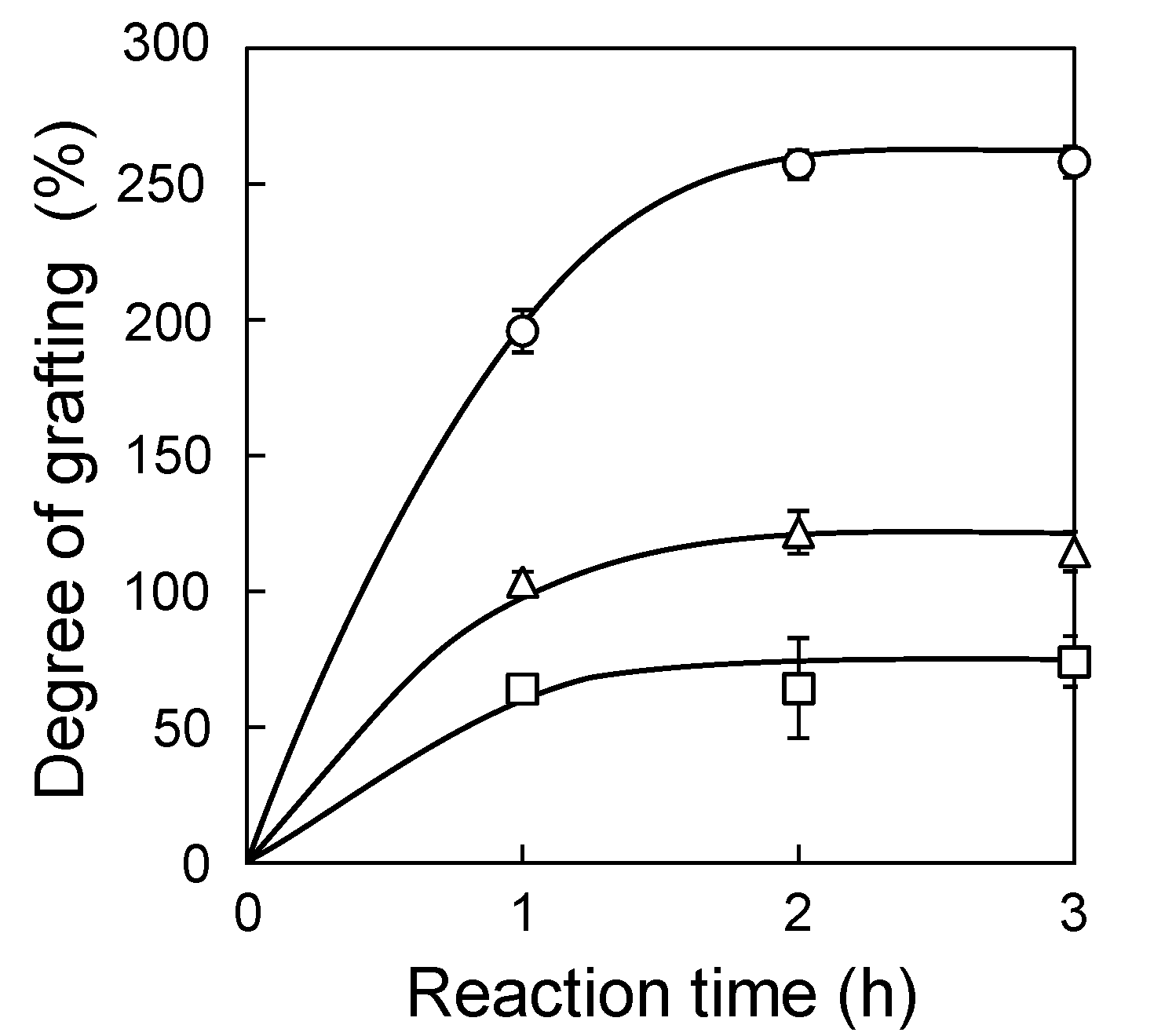
3.1. Application of the SREG Method to GMA Emulsion Graft Polymerization
3.2. Fabrication of Heavy Metal Adsorbent Using the SREG Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okamoto, J.; Sugo, T.; Fujiwara, K.; Sekiguchi, H. The synthesis of a new type adsorbent for the removal of toxic gas by radiation-induced graft-polymerization. Radiat. Phys. Chem. 1990, 35, 113–116. [Google Scholar] [CrossRef]
- Seko, N.; Katakai, A.; Hasegawa, S.; Tamada, M.; Kasai, N.; Takeda, H.; Sugo, T.; Saito, K. Aquaculture of uranium in seawater by a fabric-adsorbent submerged system. Nucl. Technol. 2003, 144, 274–278. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Li, J.Y.; Hou, Z.C.; Yao, S.D.; Shi, L.Q.; Liang, G.M.; Sheng, K.L. Microfiltration membranes prepared from polyethersulfone powder grafted with acrylic acid by simultaneous irradiation and their pH dependence. Radiat. Phys. Chem. 2008, 77, 898–906. [Google Scholar] [CrossRef]
- Nasef, M.M.; Guven, O. Radiation-grafted copolymers for separation and purification purposes: Status, challenges and future directions. Prog. Polym. Sci. 2012, 37, 1597–1656. [Google Scholar] [CrossRef]
- Iwanade, A.; Kasai, N.; Hoshina, H.; Ueki, Y.; Saiki, S.; Seko, N. Hybrid grafted ion exchanger for decontamination of radioactive cesium in Fukushima prefecture and other contaminated areas. J. Radioanal. Nucl. Chem. 2012, 293, 703–709. [Google Scholar] [CrossRef]
- Takeda, T.; Tamada, M.; Seko, N.; Ueki, Y. Ion exchange fabric synthesized by graft polymerization and its application to ultra-pure water production. Radiat. Phys. Chem. 2010, 79, 223–226. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; Lopez-Saucedo, F.; Lopez-Barriguete, J.E.; Varca, G.H.C.; Bucio, E. Radiation grafting for the functionalization and development of smart polymeric materials. Top. Curr. Chem. 2016, 374, 28. [Google Scholar] [CrossRef]
- Ballantine, D.; Glines, A.; Adler, G.; Metz, D.J. Graft copolymerization by pre-irradiation technique. J. Polym. Sci. 1959, 34, 419–438. [Google Scholar] [CrossRef]
- Takács, E.; Mirzadeh, H.; Wojnárovits, L.; Borsa, J.; Mirzataheri, M.; Benke, N. Comparison of simultaneous and pre-irradiation grafting of N-vinylpyrrolidone to cotton-cellulose. Nucl. Instrum. Meth. B 2007, 265, 217–220. [Google Scholar] [CrossRef]
- Yamagishi, H.; Saito, K.; Furusaki, S.; Sugo, T.; Ishigaki, I. Comparison of simultaneous and preirradiation grafting of methyl methacrylate onto a porous membrane. Chem. Mater. 1991, 3, 987–989. [Google Scholar] [CrossRef]
- Seko, N.; Bang, L.T.; Tamada, M. Syntheses of amine-type adsorbents with emulsion graft polymerization of glycidyl methacrylate. Nucl. Instrum. Meth. B 2007, 265, 146–149. [Google Scholar] [CrossRef]
- Wada, Y.; Tamada, M.; Seko, N.; Mitomo, H. Emulsion grafting of vinyl acetate onto preirradiated poly(3-hydroxybutyrate) film. J. Appl. Polym. Sci. 2008, 107, 2289–2294. [Google Scholar] [CrossRef]
- Seko, N.; Ninh, N.T.Y.; Tamada, M. Emulsion grafting of glycidyl methacrylate onto polyethylene fiber. Radiat. Phys. Chem. 2010, 79, 22–26. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, B.W.; Deng, B.; Yang, X.X.; Sheng, K.L.; Xie, L.D.; Lu, X.F.; Li, J.Y. Preirradiation-induced emulsion graft polymerization of glycidyl methacrylate onto poly (vinylidene fluoride) powder. J. Appl. Polym. Sci. 2010, 117, 3575–3581. [Google Scholar] [CrossRef]
- Liu, H.Z.; Yu, M.; Deng, B.; Li, L.F.; Jiang, H.Q.; Li, J.Y. Pre-irradiation induced emulsion graft polymerization of acrylonitrile onto polyethylene nonwoven fabric. Radiat. Phys. Chem. 2012, 81, 93–96. [Google Scholar] [CrossRef]
- Moawia, R.M.; Nasef, M.M.; Mohamed, N.H.; Ripin, A. Modification of flax fibres by radiation induced emulsion graft copolymerization of glycidyl methacrylate. Radiat. Phys. Chem. 2016, 122, 35–42. [Google Scholar] [CrossRef]
- Seko, N.; Hoshina, H.; Kasai, N.; Shibata, T.; Saiki, S.; Ueki, Y. Development of a water purifier for radioactive cesium removal from contaminated natural water by radiation-induced graft polymerization. Radiat. Phys. Chem. 2018, 143, 33–37. [Google Scholar] [CrossRef]
- Hoshina, H.; Kasai, N.; Shibata, T.; Aketagawa, Y.; Takahashi, M.; Yoshii, A.; Tsunoda, Y.; Seko, N. Synthesis of arsenic graft adsorbents in pilot scale. Radiat. Phys. Chem. 2012, 81, 1033–1035. [Google Scholar] [CrossRef]
- Dafader, N.C.; Rahman, N.; Majumdar, S.K.; Khan, M.M.R.; Rahman, M.M. Preparation and characterization of iminodiacetate group containing nonwoven polyethylene fabrics and its application in chromium adsorption. J. Polym. Environ. 2017, 26, 740–748. [Google Scholar] [CrossRef]
- Konishi, S.; Saito, K.; Furusaki, S.; Sugo, T. Sorption kinetics of cobalt in chelating porous membrane. Ind. Eng. Chem. Res. 1992, 31, 2722–2727. [Google Scholar] [CrossRef]
- Ogawa, H.; Sugita, K.; Saito, K.; Kim, M.; Tamada, M.; Katakai, A.; Sugo, T. Binding of ionic surfactants to charged polymer brushes grafted onto porous substrates. J. Chromatogr. A 2002, 954, 89–97. [Google Scholar] [CrossRef]
- Iwanade, A.; Umeno, D.; Saito, K.; Sugo, T. Protein binding to amphoteric polymer brushes grafted onto a porous hollow-fiber membrane. Biotechnol. Prog. 2007, 23, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Kavaklı, P.A.; Seko, N.; Tamada, M.; Güven, O. Radiation-induced graft polymerization of glycidyl methacrylate onto PE/PP nonwoven fabric and its modification toward enhanced amidoximation. J. Appl. Polym. Sci. 2007, 105, 1551–1558. [Google Scholar] [CrossRef]
- Nava-Ortiz, C.A.B.; Burillo, G.; Bucio, E.; Alvarez-Lorenzo, C. Modification of polyethylene films by radiation grafting of glycidyl methacrylate and immobilization of β-cyclodextrin. Radiat. Phys. Chem. 2009, 78, 19–24. [Google Scholar] [CrossRef]
- Goto, S.; Umino, S.; Amakai, W.; Fujiwara, K.; Sugo, T.; Kojima, T.; Kawai-Noma, S.; Umeno, D.; Saito, K. Impregnation structure of cobalt ferrocyanide microparticles by the polymer chain grafted onto nylon fiber. J. Nucl. Sci. Technol. 2016, 53, 1251–1255. [Google Scholar] [CrossRef]
- Liu, Y.; Munisso, M.C.; Mahara, A.; Kambe, Y.; Fukazawa, K.; Ishihara, K.; Yamaoka, T. A surface graft polymerization process on chemically stable medical ePTFE for suppressing platelet adhesion and activation. Biomater. Sci. 2018, 6, 1908–1915. [Google Scholar] [CrossRef]
- Vijayanand, P.S.; Kato, S.; Satokawa, S.; Kojima, T. Homopolymer and copolymers of 4-cyanophenyl acrylate with glycidyl methacrylate: Synthesis, characterization, thermal properties, and determination of monomer reactivity ratios. J. Appl. Polym. Sci. 2008, 108, 1523–1530. [Google Scholar] [CrossRef]
- Ueki, Y.; Chandra Dafader, N.; Hoshina, H.; Seko, N. Study and optimization on graft polymerization under normal pressure and air atmospheric conditions, and its application to metal adsorbent. Radiat. Phys. Chem. 2012, 81, 889–898. [Google Scholar] [CrossRef]
- Kavaklı, P.A.; Kavaklı, C.; Güven, O. Preparation and characterization of Fe (III)-loaded iminodiacetic acid modified GMA grafted nonwoven fabric adsorbent for anion adsorption. Radiat. Phys. Chem. 2014, 94, 105–110. [Google Scholar] [CrossRef]
- Yamagishi, H.; Saito, K.; Furusaki, S.; Sugo, T.; Ishigaki, I. Introduction of a high-density chelating group into a porous membrane without lowering the flux. Ind. Eng. Chem. Res. 1991, 30, 2234–2237. [Google Scholar] [CrossRef]
- Vassileva, E.; Furuta, N. Application of iminodiacetate chelating resin muromac A-1 in on-line preconcentration and inductively coupled plasma optical emission spectroscopy determination of trace elements in natural waters. Spectrochim. Acta. B 2003, 58, 1541–1552. [Google Scholar] [CrossRef]
- Valverde, J.L.; de Lucas, A.; Carmona, M.; González, M.; Rodríguez, J.F. Equilibrium data of the exchange of Cu2+, Cd2+ and Zn2+ ions for H+ on the cationic exchanger Lewatit TP-207. J. Chem. Technol. Biot. 2004, 79, 1371–1375. [Google Scholar] [CrossRef]
- Sumida, T.; Nakazato, T.; Tao, H.; Oshima, M.; Motomizu, S. On-line preconcentration system using mini-column packed with a chelating resin for the characterization of seasonal variations of trace elements in seawater by ICP-MS and ICP-AES. Anal. Sci. 2006, 22, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Rahmi, D.; Zhu, Y.; Fujimori, E.; Umemura, T.; Haraguchi, H. Multielement determination of trace metals in seawater by ICP-MS with aid of down-sized chelating resin-packed minicolumn for preconcentration. Talanta 2007, 72, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Atia, A.A.; Donia, A.M.; Yousif, A.M. Removal of some hazardous heavy metals from aqueous solution using magnetic chelating resin with iminodiacetate functionality. Sep. Purif. Technol. 2008, 61, 348–357. [Google Scholar] [CrossRef]
- Noureddine, C.; Lekhmici, A.; Mubarak, M.S. Sorption properties of the iminodiacetate ion exchange resin, amberlite IRC-718, toward divalent metal ions. J. Appl. Polym. Sci. 2008, 107, 1316–1319. [Google Scholar] [CrossRef]
- Zainol, Z.; Nicol, M.J. Comparative study of chelating ion exchange resins for the recovery of nickel and cobalt from laterite leach tailings. Hydrometallurgy 2009, 96, 283–287. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Senan, P. Adsorption of phosphate ions from water using a novel cellulose-based adsorbent. Chem. Ecol. 2011, 27, 147–164. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chiang, C.-L. Preparation of cotton fibers with antibacterial silver nanoparticles. Mater. Lett. 2008, 62, 3607–3609. [Google Scholar] [CrossRef]
- Iwata, H.; Saito, K.; Furusaki, S.; Sugo, T.; Okamoto, J. Adsorption characteristics of an immobilized metal affinity membrane. Biotechnol. Prog. 1991, 7, 412–418. [Google Scholar] [CrossRef]
- Tsuneda, S.; Saito, K.; Furusaki, S.; Sugo, T.; Okamoto, J. Metal collection using chelating hollow fiber membrane. J. Memb. Sci. 1991, 58, 221–234. [Google Scholar] [CrossRef]
- Shibata, T.; Seko, N.; Kasai, N.; Hoshina, H.; Ueki, Y. Evaluation of antibacterial effect by using a fibrous grafted material loaded Ag ligand. Int. J. Org. Chem. 2015, 5, 100–107. [Google Scholar] [CrossRef][Green Version]
- Khan, F. Characterization of methyl methacrylate grafting onto preirradiated biodegradable lignocellulose fiber by γ-radiation. Macromol. Biosci. 2005, 5, 78–89. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Borsa, J.; Papp, J.; Hargittai, P.; Korecz, L. Modification of cotton-cellulose by preirradiation grafting. Nucl. Instrum. Methods Phys. Res. B 2005, 236, 259–265. [Google Scholar] [CrossRef]
- Sharif, J.; Mohamad, S.F.; Fatimah Othman, N.A.; Bakaruddin, N.A.; Osman, H.N.; Güven, O. Graft copolymerization of glycidyl methacrylate onto delignified kenaf fibers through pre-irradiation technique. Radiat. Phys. Chem. 2013, 91, 125–131. [Google Scholar] [CrossRef]
- Kodama, Y.; Barsbay, M.; Güven, O. Radiation-induced and RAFT-mediated grafting of poly(hydroxyethyl methacrylate) (PHEMA) from cellulose surfaces. Radiat. Phys. Chem. 2014, 94, 98–104. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Viretto, A.; Apolinario, G.; Ienny, P. Improving the flame retardancy of flax fabrics by radiation grafting of phosphorus compounds. Eur. Polym. J. 2015, 68, 313–325. [Google Scholar] [CrossRef]
- Nicolas, L.M.; Rodolphe, S.; Roland, E.H.; Sophie, R. Radiation-induced modifications in natural fibres and their biocomposites: Opportunities for controlled physico-chemical modification pathways? Ind. Crops. Prod. 2017, 109, 199–213. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omichi, M.; Ueki, Y.; Seko, N.; Maekawa, Y. Development of a Simplified Radiation-Induced Emulsion Graft Polymerization Method and Its Application to the Fabrication of a Heavy Metal Adsorbent. Polymers 2019, 11, 1373. https://doi.org/10.3390/polym11081373
Omichi M, Ueki Y, Seko N, Maekawa Y. Development of a Simplified Radiation-Induced Emulsion Graft Polymerization Method and Its Application to the Fabrication of a Heavy Metal Adsorbent. Polymers. 2019; 11(8):1373. https://doi.org/10.3390/polym11081373
Chicago/Turabian StyleOmichi, Masaaki, Yuji Ueki, Noriaki Seko, and Yasunari Maekawa. 2019. "Development of a Simplified Radiation-Induced Emulsion Graft Polymerization Method and Its Application to the Fabrication of a Heavy Metal Adsorbent" Polymers 11, no. 8: 1373. https://doi.org/10.3390/polym11081373
APA StyleOmichi, M., Ueki, Y., Seko, N., & Maekawa, Y. (2019). Development of a Simplified Radiation-Induced Emulsion Graft Polymerization Method and Its Application to the Fabrication of a Heavy Metal Adsorbent. Polymers, 11(8), 1373. https://doi.org/10.3390/polym11081373