Fluorescent Nanodiamonds Enable Long-Term Detection of Human Adipose-Derived Stem/Stromal Cells in an In Vivo Chondrogenesis Model Using Decellularized Extracellular Matrices and Fibrin Glue Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. FNDs Production and Characterization
2.2. Culture of Human Adipose-Derived Stem/Stromal Cells (hASCs)
2.3. Cell Labelling
2.4. MTT Assay
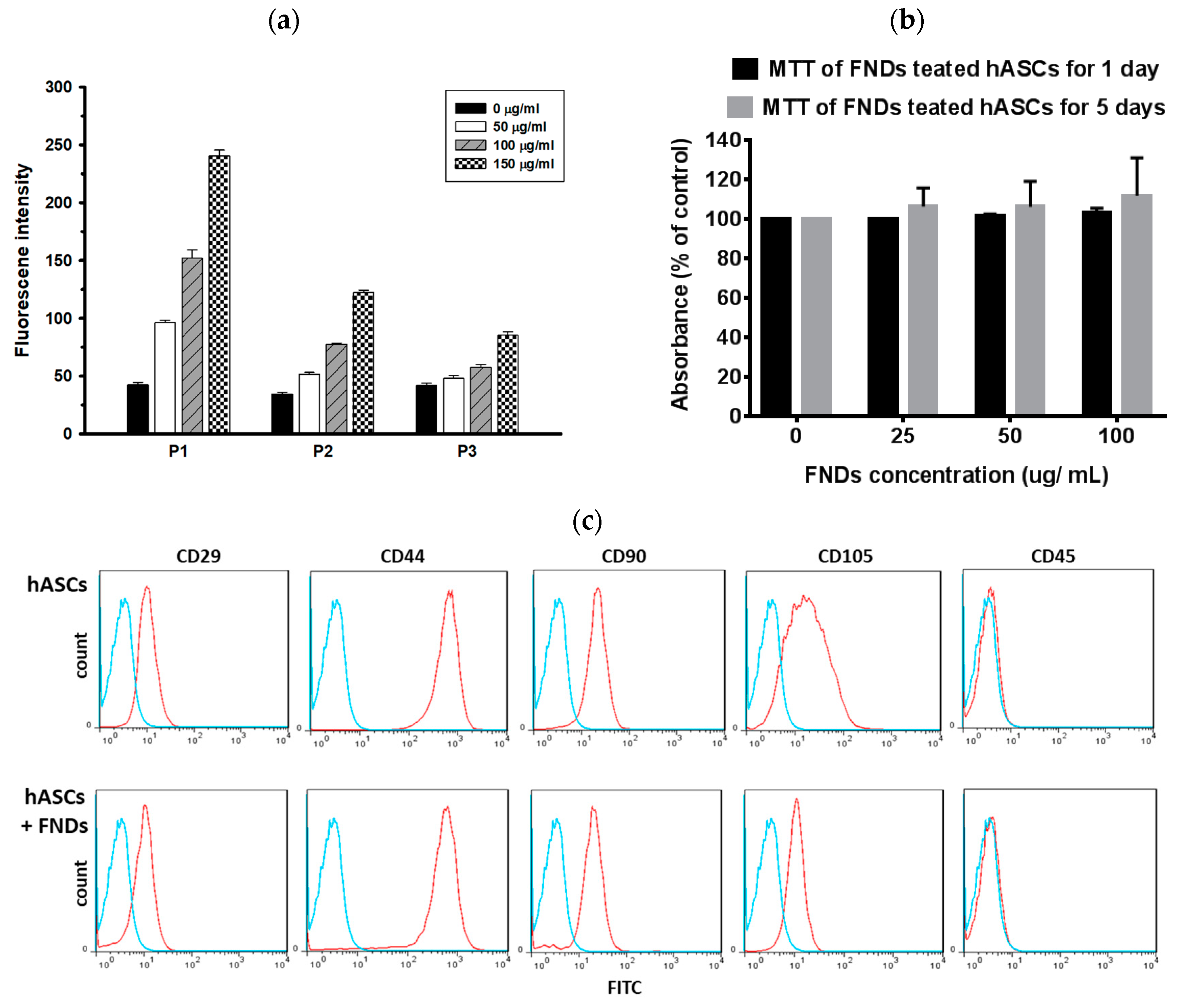
2.5. Flow Cytometry Analysis
2.6. In Vitro Multipotency Differentiation Assay
2.7. Animals and In Vivo Implantation
2.8. Xenograft Construct Preparation and Measurement
2.9. Histological Analysis
2.10. Quantification of Cell Number
2.11. Alcian Blue Staining
2.12. Masson’s Trichrome Staining
2.13. Immunofluorescence
2.14. Magnetically Modulated Fluorescence (MMF)
2.15. Statistical Analysis
3. Results and Discussion
3.1. FNDs Preparation
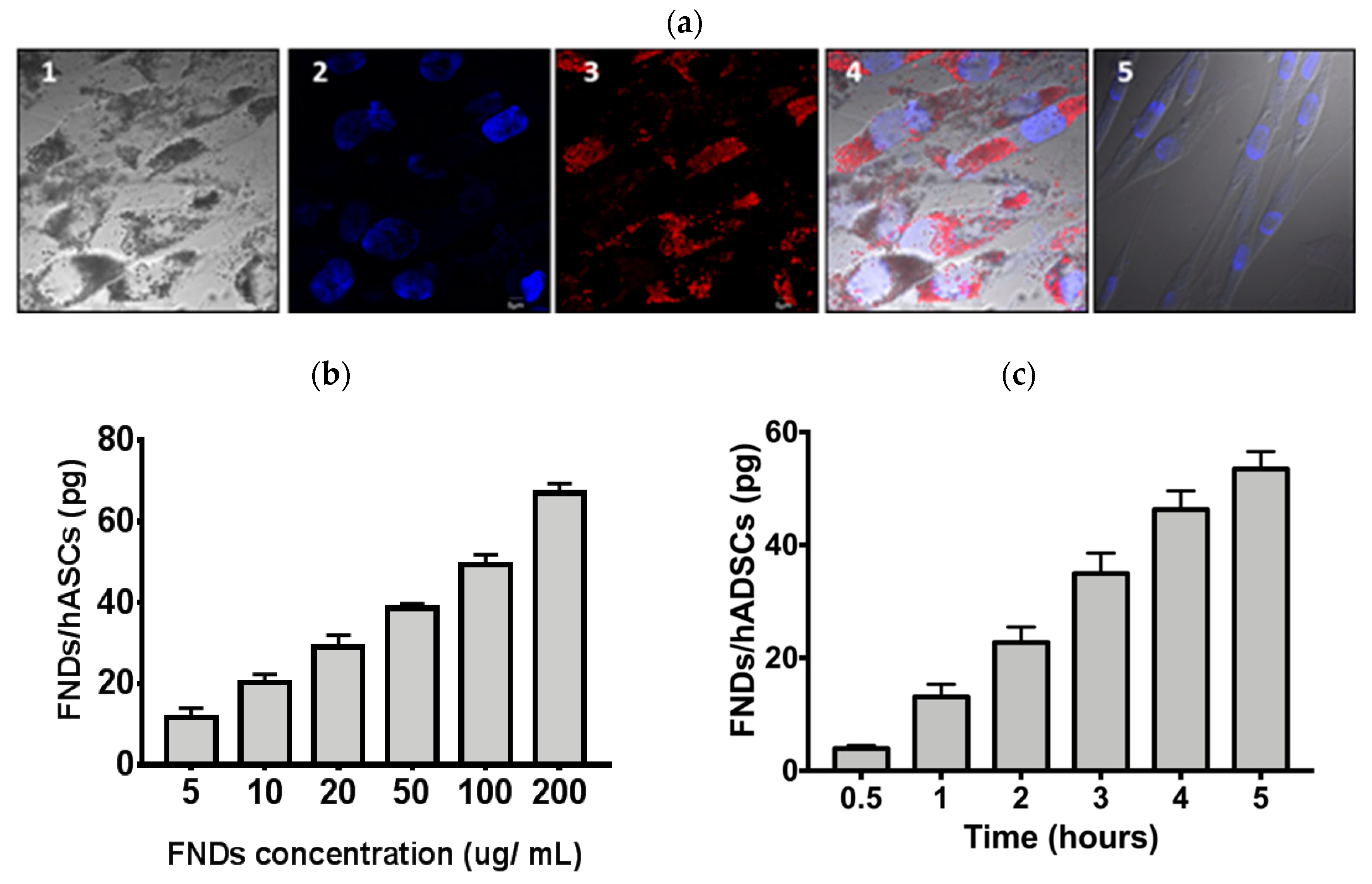
3.2. Characterization of FND—Labeled hASCs
3.3. Proliferations and Differentiation of hASCs Not Affected by FNDs
3.4. hASCs Maintained the Volume of Xenografts Containing Decellularized Cartilage and Fibrin Glue Polymer
3.5. Long-Term Detection of FND-Labeled hASCs with an In Vivo Chondrogenesis Model
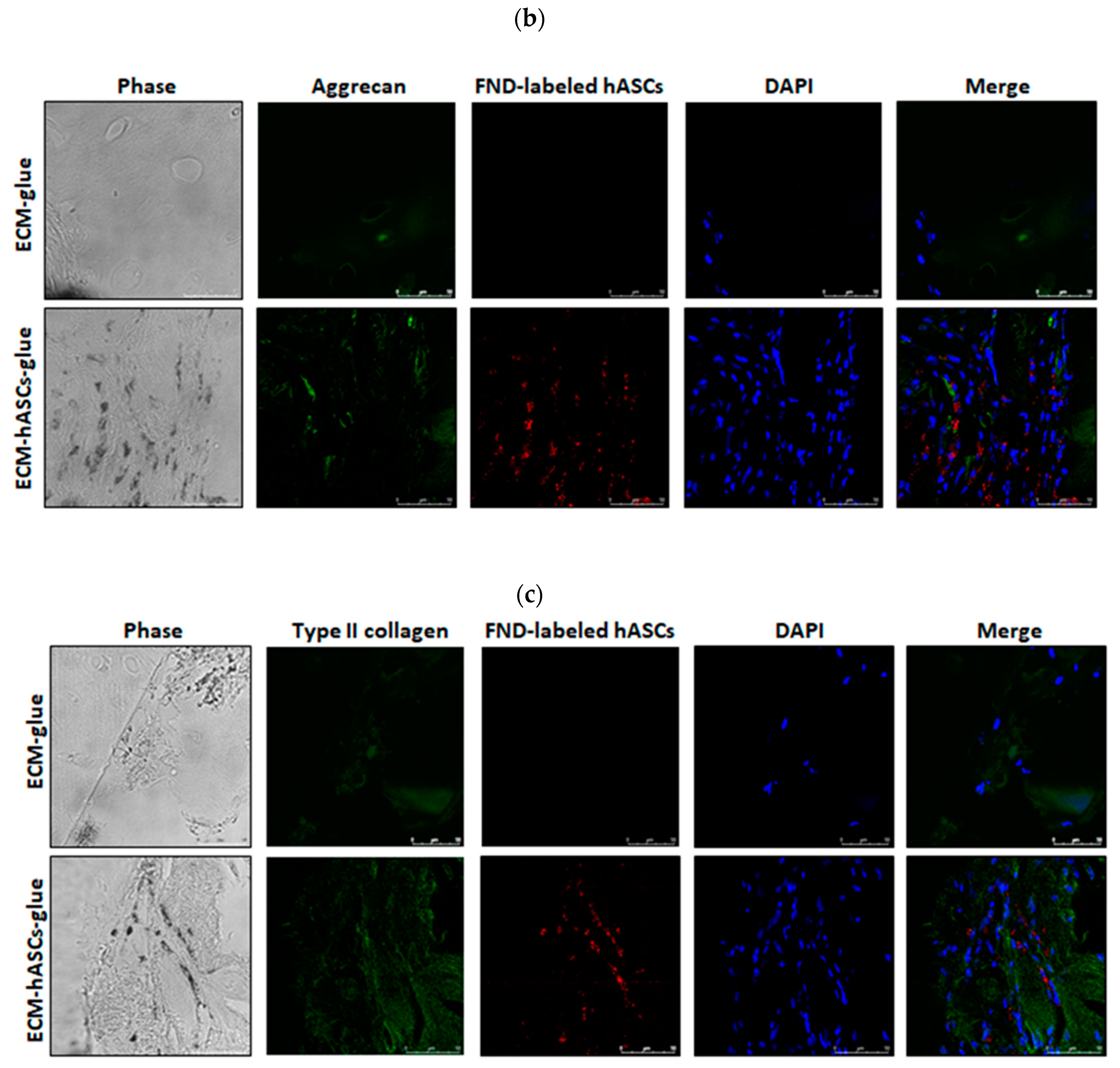
3.6. Functional In Vivo Chondrogenesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunziker, E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: precepts & practice – myths & misconceptions – progress & prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Moseley, J.B.; O’Malley, K.; Petersen, N.J.; Menke, T.J.; Brody, B.A.; Kuykendall, D.H.; Hollingsworth, J.C.; Ashton, C.M.; Wray, N.P. A Controlled Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. N. Engl. J. Med. 2002, 347, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.K.; Yoon, K.H.; Song, S.J. Cartilage Healing After Microfracture in Osteoarthritic Knees. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Steinwachs, M.R.; Erggelet, C.; Krause, S.J.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minas, T.; Von Keudell, A.; Bryant, T.; Gomoll, A.H. The John Insall Award: A Minimum 10-year Outcome Study of Autologous Chondrocyte Implantation. Clin. Orthop. Relat. Res. 2014, 472, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behery, O.; Harris, J.; Karnes, J.; Siston, R.; Flanigan, D. Factors Influencing the Outcome of Autologous Chondrocyte Implantation: A Systematic Review. J. Knee Surg. 2012, 26, 203–212. [Google Scholar] [CrossRef]
- Bentley, G.; Biant, L.C.; Vijayan, S.; Macmull, S.; Skinner, J.A.; Carrington, R.W.J. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J. Bone Joint Surg. Br. 2012, 94-B, 504–509. [Google Scholar] [CrossRef]
- De Caro, F.; Bisicchia, S.; Amendola, A.; Ding, L. Large Fresh Osteochondral Allografts of the Knee: A Systematic Clinical and Basic Science Review of the Literature. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 757–765. [Google Scholar] [CrossRef]
- Gossec, L.; Paternotte, S.; Maillefert, J.F.; Combescure, C.; Conaghan, P.G.; Davis, A.M.; Gunther, K.-P.; Hawker, G.; Hochberg, M.; Katz, J.N.; et al. The role of pain and functional impairment in the decision to recommend total joint replacement in hip and knee osteoarthritis: An international cross-sectional study of 1909 patients. Report of the OARSI-OMERACT Task Force on total joint replacement. Osteoarthr. Cartil. 2011, 19, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.; Bhamra, J.S.; Gikas, P.D.; Skinner, J.A.; Carrington, R.; Briggs, T.W. Repair of osteochondral defects in joints—How to achieve success. Injury 2013, 44, S3–S10. [Google Scholar] [CrossRef]
- Marlovits, S.; Aldrian, S.; Wondrasch, B.; Zak, L.; Albrecht, C.; Welsch, G.; Trattnig, S. Clinical and Radiological Outcomes 5 Years After Matrix-Induced Autologous Chondrocyte Implantation in Patients With Symptomatic, Traumatic Chondral Defects. Am. J. Sports Med. 2012, 40, 2273–2280. [Google Scholar] [CrossRef]
- Wood, J.J.; Malek, M.A.; Frassica, F.J.; Polder, J.A.; Mohan, A.K.; Bloom, E.T.; Braun, M.M.; Coté, T.R. Autologous Cultured Chondrocytes: Adverse Events Reported to the United States Food and Drug Administration. J. Bone Jt. Surg. 2006, 88, 503. [Google Scholar] [CrossRef]
- Peterson, L.; Minas, T.; Brittberg, M.; Nilsson, A.; Sjögren-Jansson, E.; Lindahl, A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 2000, 212–234. [Google Scholar] [CrossRef] [PubMed]
- Adkisson, H.D.; Martin, J.A.; Amendola, R.L.; Milliman, C.; Mauch, K.A.; Katwal, A.B.; Seyedin, M.; Amendola, A.; Streeter, P.R.; Buckwalter, J.A. The Potential of Human Allogeneic Juvenile Chondrocytes for Restoration of Articular Cartilage. Am. J. Sports Med. 2010, 38, 1324–1333. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; Milwid, J.M. Mesenchymal Stem Cells as Therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilak, F.; Awad, H.A.; Fermor, B.; Leddy, H.A.; Gimble, J.M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 2004, 41, 389–399. [Google Scholar] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blau, H.M.; Daley, G.Q. Stem Cells in the Treatment of Disease. N. Engl. J. Med. 2019, 380, 1748–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Celso, C.; Fleming, H.E.; Wu, J.W.; Zhao, C.X.; Miake-Lye, S.; Fujisaki, J.; Côté, D.; Rowe, D.W.; Lin, C.P.; Scadden, D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 2009, 457, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, S.H.; Jung, Y. Enhanced Cartilaginous Tissue Formation with a Cell Aggregate-Fibrin-Polymer Scaffold Complex. Polymers (Basel) 2017, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Sheykhhasan, M.; Qomi, R.T.; Ghiasi, M. Fibrin Scaffolds Designing in order to Human Adipose-derived Mesenchymal Stem Cells Differentiation to Chondrocytes in the Presence of TGF-β3. Int. J. Stem Cells 2015, 8, 219–222. [Google Scholar] [CrossRef]
- Jockenhoevel, S.; Zund, G.; Hoerstrup, S.P.; Chalabi, K.; Sachweh, J.S.; Demircan, L.; Messmer, B.J.; Turina, M. Fibrin gel—Advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2001, 19, 424–430. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- William, D. S. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive—A Laboratory and Clinical Perspective. ISRN Surg. 2014, 2014, 203943. [Google Scholar]
- Buchta, C.; Hedrich, H.C.; Macher, M.; Höcker, P.; Redl, H. Biochemical characterization of autologous fibrin sealants produced by CryoSeals and Vivostats in comparison to the homologous fibrin sealant product Tissucol/Tisseels. Biomaterials 2005, 26, 6233–6241. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.-W.; Hui, Y.Y.; Tsai, P.-C.; Chang, H.-C. Fluorescent Nanodiamond: A Versatile Tool for Long-Term Cell Tracking, Super-Resolution Imaging, and Nanoscale Temperature Sensing. Acc. Chem. Res. 2016, 49, 400–407. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Vaijayanthimala, V.; Tzeng, Y.-K.; Chang, H.-C.; Li, C.-L. The biocompatibility of fluorescent nanodiamonds and their mechanism of cellular uptake. Nanotechnology 2009, 20, 425103. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-K.; Wang, C.-C.; Cheng, C.-L.; Chao, J.-I. Endocytic carboxylated nanodiamond for the labeling and tracking of cell division and differentiation in cancer and stem cells. Biomaterials 2009, 30, 4249–4259. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Vaijayanthimala, V.; Cheng, C.-A.; Yeh, S.-H.; Chang, C.-F.; Li, C.-L.; Chang, H.-C. The Exocytosis of Fluorescent Nanodiamond and Its Use as a Long-Term Cell Tracker. Small 2011, 7, 3363–3370. [Google Scholar] [CrossRef]
- Su, L.-J.; Wu, M.-S.; Hui, Y.Y.; Chang, B.-M.; Pan, L.; Hsu, P.-C.; Chen, Y.-T.; Ho, H.-N.; Huang, Y.-H.; Ling, T.-Y.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci. Rep. 2017, 7, 45607. [Google Scholar] [CrossRef]
- Chang, Y.-R.; Lee, H.-Y.; Chen, K.; Chang, C.-C.; Tsai, D.-S.; Fu, C.-C.; Lim, T.-S.; Tzeng, Y.-K.; Fang, C.-Y.; Han, C.-C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. Nanotechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.-M.; Lin, H.-H.; Su, L.-J.; Lin, W.-D.; Lin, R.-J.; Tzeng, Y.-K.; Lee, R.-T.; Lee, Y.-C.; Yu, A.-L.; Chang, H.-C. Highly fluorescent nanodiamonds protein-functionalized for cell labeling and targeting. Adv. Funct. Mater. 2013, 23, 5737–5745. [Google Scholar]
- Kong, X.-L.; Huang, L.-C. L.; Hsu, C.-M.; Chen, W.-H.; Han, C.-C.; Chang, H.-C. High-Affinity Capture of Proteins by Diamond Nanoparticles for Mass Spectrometric Analysis. Anal. Chem. 2005, 77, 259–265. [Google Scholar]
- Wu, T.-J.; Tzeng, Y.-K.; Chang, W.-W.; Cheng, C.-A.; Kuo, Y.; Chien, C.-H.; Chang, H.-C.; Yu, J. Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat. Nanotechnol. 2013, 8, 682–689. [Google Scholar] [CrossRef]
- Lin, H.-H.; Lee, H.-W.; Lin, R.-J.; Huang, C.-W.; Liao, Y.-C.; Chen, Y.-T.; Fang, J.-M.; Lee, T.-C.; Yu, A.L.; Chang, H.-C. Tracking and finding slow-proliferating/quiescent cancer stem cells with fluorescent nanodiamonds. Small 2015, 11, 4394–4402. [Google Scholar] [CrossRef]
- Billinton, N.; Knight, A.W. Seeing the Wood through the Trees: A Review of Techniques for Distinguishing Green Fluorescent Protein from Endogenous Autofluorescence. Anal. Biochem. 2001, 291, 175–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Wang, Y.-C.; Wang, W.-T.; Wang, H.-M.D.; Lin, H.-H.; Su, L.-J.; Kuo, Y.-R.; Lai, C.-S.; Ho, M.-L.; Yu, J. Fluorescent Nanodiamonds Enable Long-Term Detection of Human Adipose-Derived Stem/Stromal Cells in an In Vivo Chondrogenesis Model Using Decellularized Extracellular Matrices and Fibrin Glue Polymer. Polymers 2019, 11, 1391. https://doi.org/10.3390/polym11091391
Wu Y-C, Wang Y-C, Wang W-T, Wang H-MD, Lin H-H, Su L-J, Kuo Y-R, Lai C-S, Ho M-L, Yu J. Fluorescent Nanodiamonds Enable Long-Term Detection of Human Adipose-Derived Stem/Stromal Cells in an In Vivo Chondrogenesis Model Using Decellularized Extracellular Matrices and Fibrin Glue Polymer. Polymers. 2019; 11(9):1391. https://doi.org/10.3390/polym11091391
Chicago/Turabian StyleWu, Yi-Chia, Ya-Chin Wang, Wei-Ting Wang, Hui-Min David Wang, Hsin-Hung Lin, Long-Jyun Su, Yur-Ren Kuo, Chung-Sheng Lai, Mei-Ling Ho, and John Yu. 2019. "Fluorescent Nanodiamonds Enable Long-Term Detection of Human Adipose-Derived Stem/Stromal Cells in an In Vivo Chondrogenesis Model Using Decellularized Extracellular Matrices and Fibrin Glue Polymer" Polymers 11, no. 9: 1391. https://doi.org/10.3390/polym11091391





