Synthesis and Photovoltaic Effect of Electron-Withdrawing Units for Low Band Gap Conjugated Polymers Bearing Bi(thienylenevinylene) Side Chains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characteriazation
2.2. Preparation of Photovoltaic Devices
2.3. Synthesis of PBDT-TVT-ID and PBDT-TVT-DTNT
2.3.1. Synthesis of PBDT-TVT-ID
2.3.2. Synthesis of PBDT-TVT-DTNT
3. Results
3.1. Synthesis and Characterization
3.2. Optical and Electrochemical Performance
3.3. Hole Mobility
3.4. Theoretical Calculations
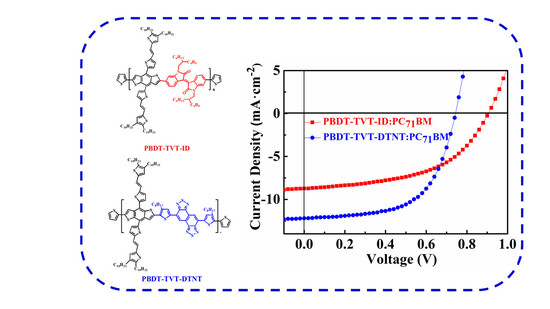
3.5. Photovoltaic Performance
3.6. Morphology Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, K.; Li, L.; Lai, T.; Xiao, L.; Huang, Y.; Huang, F.; Peng, J.; Cao, Y.; Liu, F.; Russell, T.P.; et al. Deep Absorbing Porphyrin Small Molecule for High-Performance Organic Solar Cells with Very Low Energy Losses. J. Am. Chem. Soc. 2015, 137, 7282–7285. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, B.; Xu, Y.; Xu, B.; Hou, J. Printable MoOx Anode Interlayers for Organic Solar Cells. Adv. Mater. 2018, 30, 1801718. [Google Scholar] [CrossRef]
- Kang, Q.; Ye, L.; Xu, B.; An, C.; Stuard, S.J.; Zhang, S.; Yao, H.; Ade, H.; Hou, J. A Printable Organic Cathode Interlayer Enables over 13% Efficiencyfor 1-cm2 Organic Solar Cells. Joule 2019, 3, 227–239. [Google Scholar] [CrossRef]
- Han, L.; Uranbileg, N.; Jiang, S.; Xie, Y.; Jiang, H.; Lan, Z.; Yu, D.; Bao, X.; Yang, R. An Extraordinary Cyclohexylmethyl Side Chain Dominating Polymeric Donor Packing Patterns and Energy Levels for Efficient Non-Fullerene Polymer Solar Cells. J. Mater. Chem. A 2019, 7, 10505–10513. [Google Scholar] [CrossRef]
- Gao, K.; Miao, J.; Xiao, L.; Deng, W.; Kan, Y.; Liang, T.; Wang, C.; Huang, F.; Peng, J.; Cao, Y.; et al. Multi-Length-Scale Morphologies Driven by Mixed Additives in Porphyrin-Based Organic Photovoltaics. Adv. Mater. 2016, 28, 4727–4733. [Google Scholar] [CrossRef]
- Liu, T.; Luo, Z.; Fan, Q.; Zhang, G.; Gao, W.; Guo, X.; Ma, W.; Zhang, M.; Yang, C.; Li, Y.; et al. Use of two structurally similar small molecular acceptors enabling ternary organic solar cells with high efficiencies and fill factors. Energy Environ. Sci. 2018, 11, 3275–3282. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, Z.; Xu, B.; Jo, S.B.; Kan, Y.; Peng, X.; Jen, A.K.Y. Highly Effcient Porphyrin-Based OPV/Perovskite Hybrid Solar Cells with Extended Photoresponse and High Fill Factor. Adv. Mater. 2017, 29, 1703980. [Google Scholar] [CrossRef]
- Li, X.; Huang, G.; Zheng, N.; Li, Y.; Kang, X.; Qiao, S.; Jiang, H.; Chen, W.; Yang, R. High-Efficiency Polymer Solar Cells Over 13.9% With a High VOC Beyond 1.0 V by Synergistic Effect of Fluorine and Sulfur. Sol. RRL 2019, 3, 1900005. [Google Scholar] [CrossRef]
- Liu, T.; Huo, L.; Chandrabose, S.; Chen, K.; Han, G.; Qi, F.; Meng, X.; Xie, D.; Ma, W.; Yi, Y.; et al. Optimized Fibril Network Morphology by Precise Side-Chain Engineering to Achieve High-Performance Bulk-Heterojunction Organic Solar Cells. Adv. Mater. 2018, 30, 1707353. [Google Scholar] [CrossRef]
- Chen, W.; Huang, G.; Li, X.; Li, Y.; Wang, H.; Jiang, H.; Zhao, Z.; Yu, D.; Wang, E.; Yang, R. Revealing the Position Effect of an Alkylthio Side Chain in Phenyl Substituted Benzodithiophene-Based Donor-Polymers on Photovoltaic Performance of Non-Fullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Chen, W.; Shen, W.; Wang, H.; Liu, F.; Duan, L.; Xu, X.; Zhu, D.; Qiu, M.; Wang, E.; Yang, R. Enhanced Efficiency of Polymer Solar Cells by Improving Molecular Aggregation and Broadening the Absorption Spectra. Dyes Pigments 2019, 166, 42–48. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Wang, Y.; Li, H.; Tong, J.; Bao, X.; Xia, Y. Enhanced Efficiency of Polymer Solar Cellsthrough Synergistic Optimization of Mobilityand Tuning Donor Alloys by Adding High-Mobility Conjugated Polymers. J. Mater. Chem. C 2018, 6, 11015–11022. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Li, X.; Qin, J.; Ren, M.; Yang, C.; Bao, X.; Xia, Y.; Li, J. Self-Doping n-type Polymer as Cathode Interface Layer Enables Efficient Organic Solar Cells by Increasing Built-in Electric Field and Boosting Interface Contact. J. Mater. Chem. C 2019. [Google Scholar] [CrossRef]
- Gao, K.; Jo, S.B.; Shi, X.; Nian, L.; Zhang, M.; Kan, Y.; Lin, F.; Kan, B.; Xu, B.; Rong, Q. Over 12% Effciency Nonfullerene All-Small-MoleculeOrganic Solar Cells with Sequentially Evolved Multilength Scale Morphologies. Adv. Mater. 2019, 31, 1807842. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-Processed Organic Tandem Solar Cells with Power Conversion Efficiencies >12%. Nat. Photon. 2017, 11, 85–90. [Google Scholar] [CrossRef]
- Liang, Z.; Tong, J.; Li, H.; Wang, Y.; Wang, N.; Li, J.; Yang, C.; Xia, Y. The Comprehensive Utilization of the Synergistic Effect of Fullerene and Non-Fullerene Acceptors to Achieve Highly Efficient Polymer Solar Cells. J. Mater. Chem. A 2019, 7, 15841–15850. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, S.; Guo, X.; Xu, F.; Li, Y.; Hou, J. Replacing Alkoxy Groups with Alkylthienyl Groups: A Feasible Approach to Improve the Properties of Photovoltaic Polymers. Angew. Chem. Int. Ed. 2011, 50, 9697–9702. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, Y.; Guo, X.; Liu, F.; Zhang, S.; Huo, L.; Russell, T.P.; Hou, J. Efficient Polymer Solar Cells Based on Benzothiadiazole and Alkylphenyl Substituted Benzodithiophene with a Power Conversion Efficiency over 8%. Adv. Mater. 2013, 25, 4944–4949. [Google Scholar] [CrossRef]
- Dou, L.; Gao, J.; Richard, E.; You, J.; Chen, C.; Cha, K.C.; He, Y.; Li, G.; Yang, Y. Systematic Investigation of Benzodithiophene- and Diketopyrrolopyrrole-Based Low-Bandgap Polymers Designed for Single Junction and Tandem Polymer Solar Cells. J. Am. Chem. Soc. 2012, 134, 10071–10079. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.; Ma, W.; Zhang, S.; Huo, L.; Ade, H.; Hou, J. An Easy and Effective Method to Modulate Molecular Energy Level of the Polymer Based on Benzodithiophene for the Application in Polymer Solar Cells. Adv. Mater. 2014, 26, 2089–2095. [Google Scholar] [CrossRef]
- Zhu, D.; Bao, X.; Ouyang, D.; Wang, J.; Yuan, X.; Wang, Q.; Zhou, D.; Wen, S.; Yang, R. Single-Junction Fullerene Solar Cells with 10% Efficiency and High Open-Circuit Voltage Approaching 1 V. Nano Energy 2017, 40, 495–503. [Google Scholar] [CrossRef]
- Hwang, M.C.; Kang, H.; Yu, K.; Yun, H.; Kwon, S.K.; Lee, K.; Kim, Y.H. New Polybenzo[1,2-b: 4,5-b′] Dithiophene Derivative with an Alkoxyphenyl Side Chain: Applications in Organic Photovoltaic Cells and Organic Semiconductors. Sol. Energy Mater. Sol. Cells 2014, 125, 39–46. [Google Scholar] [CrossRef]
- Han, L.; Bao, X.; Hu, T.; Du, Z.; Chen, W.; Zhu, D.; Liu, Q.; Sun, M.; Yang, R. Novel Donor-Acceptor Polymer Containing 4,7-Bis(thiophen-2-yl)benzo[c][2–4]thiadiazole for Polymer Solar Cells with Power Conversion Efficiency of 6.21%. Macromol. Rapid Commun. 2014, 35, 1153–1157. [Google Scholar] [CrossRef]
- Sonar, P.; Zhuo, J.; Zhao, L.; Lim, K.M.; Chen, J.; Rondinone, A.J.; Singh, S.P.; Chua, L.L.; Ho, P.K.H.; Dodabalapur, A. Furan Substituted Diketopyrrolopyrrole and Thienylenevinylene Based Low Band Gap Copolymer for High Mobility Organic Thin Film Transistors. J. Mater. Chem. 2012, 22, 17284–17292. [Google Scholar] [CrossRef]
- Lim, B.; Yeo, J.S.; Khim, D.; Kim, D.Y. Synthesis and Photovoltaic Properties of a Thienylenevinylene and Diketopyrrolopyrrole Copolymer with High Mobility. Macromol. Rapid Commun. 2011, 32, 1551–1556. [Google Scholar] [CrossRef]
- Lim, B.; Baeg, K.J.; Jeong, H.G.; Jo, J.; Kim, H.; Park, J.W.; Noh, Y.Y.; Vak, D.; Park, J.H.; Park, J.W.; et al. A New Poly(thienylenevinylene) Derivative with High Mobility and Oxidative Stability for Organic Thin-Film Transistors and Solar Cells. Adv. Mater. 2009, 21, 2808–2814. [Google Scholar] [CrossRef]
- Kim, J.; Lim, B.; Baeg, K.; Baeg, K.J.; Noh, Y.Y.; Khim, D.; Jeong, H.G.; Yun, J.M.; Kim, D.Y. Highly Soluble Poly(thienylenevinylene) Derivatives with Charge-Carrier Mobility Exceeding 1 cm2 V−1 s−1. Chem. Mater. 2011, 23, 4663–4665. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, H.; Ye, L.; Zhao, W.; Zhang, S.; Hou, J. Molecular Design and Application of a Photovoltaic Polymer with Improve Optical Properties and molecular energy levels. Macromolecules 2015, 48, 3493–3499. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, W.H.; Song, C.E.; Shin, Y.; Kim, J.; Lee, S.K.; Shin, W.S.; Moon, S.J.; Kang, I.N. Highly Conjugated Side-Chain-Substituted Benzo[1,2-b:4,5-b’]dithiophene-Based Conjugated Polymers for Use in Polymer. Macromolecules 2014, 47, 97–105. [Google Scholar] [CrossRef]
- Guo, J.; Bin, H.; Wang, W.; Chen, B.; Guo, J.; Sun, R.; Zhang, Z.; Jiao, X.; Li, Y.; Min, J. All-Small Molecule Solar Cells Based on Donor Molecular Optimization with Highly Enhanced Efficiency and Stability. J. Mater. Chem. A 2018, 6, 15675–15683. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, E.; Jarvid, M.E.; Henriksson, P.; Inganas, O.; Zhang, F.; Andersson, M.R. Synthesis and Characterization of Benzodithiophene–Isoindigo Polymers for Solar Cells. J. Mater. Chem. 2012, 22, 2306–2314. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, W.; Himmelberger, S.; Vandewal, K.; Tang, Z.; Bergqvist, J.; Salleo, A.; Andreasen, J.W.; Inganas, O.; Andersson, M.R.; et al. Structure-Property Relationships of Oligothiophene–Isoindigo Polymers for Efficient Bulk-Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 361–369. [Google Scholar] [CrossRef]
- Yang, T.; Wang, M.; Duan, C.; Hu, X.; Huang, L.; Peng, J.; Huang, F.; Gong, X. Inverted Polymer Solar Cells with 8.4% Efficiency by Conjugated Polyelectrolyte. Energy Environ. Sci. 2012, 5, 8208–8214. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and Morphology Control Enables Multiple Cases of High-Efficiency Polymer Solar Cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef]
- Tong, J.; Li, J.; Zhang, P.; Ma, X.; Wang, M.; An, L.; Sun, J.; Guo, P.; Yang, G.; Xia, Y. Naphtho[1,2-c:5,6-c’]bis[2–4]thiadiazole-based Conjugated Polymers Consisting of Oligothiophenes for Efficient Polymer Solar Cells. Polymer 2017, 121, 183–195. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Osaka, I.; Shimawaki, M.; Mori, H.; Doi, I.; Miyazaki, E.; Koganezawa, T.; Takimiya, K. Synthesis, Characterization, and Transistor and Solar Cell Applications of a Naphthobisthiadiazole-Based Semiconducting Polymer. J. Am. Chem. Soc. 2012, 134, 3498–3507. [Google Scholar] [CrossRef]
- Wang, M.; Hu, X.; Liu, P.; Li, W.; Gong, X.; Huang, F.; Cao, Y. Donor-Acceptor Conjugated Polymer Based on Naphtho[1,2-c:5,6-c]bis[2–4]thiadiazole for High-Performance Polymer Solar Cells. J. Am. Chem. Soc. 2011, 133, 9638–9641. [Google Scholar] [CrossRef]
- Lei, T.; Wang, J.; Pei, J. Design, Synthesis, and Structure–Property Relationships of Isoindigo-Bed Conjugated Polymers. Acc. Chem. Res. 2014, 47, 1117–1126. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Liang, Z.; Wang, N.; Tong, J.; Yang, C.; Bao, X.; Xia, Y. Enhanced Organic Photovoltaic Performance through Modulating Vertical Composition Distribution and Promoting Crystallinity of the Photoactive Layer by Diphenyl Sulfide Additives. ACS Appl. Mater. Interfaces 2019, 11, 7022–7029. [Google Scholar] [CrossRef]
- Tong, J.; An, L.; Li, J.; Zhang, P.; Guo, P.; Yang, C.; Su, Q.; Wang, X.; Xia, Y. Large Branched Alkylthienyl Bridged Naphtho[1,2-c:5,6-c’]bis[2–4]thiadiazole-Containing Low Bandgap Copolymers: Synthesis and Photovoltaic Application. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 176–185. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Wang, X.; Wang, F.; Xia, Y. Synthesis and Photovoltaic Properties of Silole-Containing Conjugated Polymers. Acta Chim. Sin. 2015, 73, 1055–1060. [Google Scholar]
- Chen, W.; Jiang, H.; Huang, G.; Zhang, J.; Cai, M.; Wan, X.; Yang, R. High-Efficiency Ternary Polymer Solar Cells Based on Intense FRET Energy Transfer Process. Sol. RRL 2018, 2, 1800101. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Y.; Wang, J.; Zhu, D.; Yang, C.; Li, Y.; Yang, C.; Xu, J.; Yang, R. High Extinction Coefficient Thieno[3,4-b]thiophene-Based Copolymer for Efficient Fullerene-Free Solar Cells with Large Current Density. Chem. Mater. 2017, 29, 6766–6771. [Google Scholar] [CrossRef]
- Du, Z.; Bao, X.; Li, Y.; Liu, D.; Wang, J.; Yang, C.; Wimmer, R.; Stade, L.W.; Yang, R.; Yu, D. Balancing High Open Circuit Voltage over 1.0 V and High Short Circuit Current in Benzodithiophene-Based Polymer Solar Cells with Low Energy Loss: A Synergistic Effect of Fluorination and Alkylthiolation. Adv. Energy Mater. 2017, 8, 1701471. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef]
- Moellmann, J.; Grimme, S. DFT-D3 Study of Some Molecular Crystals. J. Phys. Chem. C. 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Chen, W.; Huang, G.; Li, X.; Wang, H.; Li, Y.; Jiang, H.; Zheng, N.; Yang, R. Side-Chain-Promoted Benzodithiophene-based Conjugated Polymers toward Striking Enhancement of Photovoltaic Properties for Polymer Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 42747–42755. [Google Scholar] [CrossRef]








| Polymer | λonset (nm) | Eg1 (eV) | Egelect | HOMO 2 (eV) | LUMO 3 (eV) | HOMO 4 (eV) | LUMO 4 (eV) |
|---|---|---|---|---|---|---|---|
| PBDT-TVT-ID | 760 | 1.63 | 1.74 | −5.42 | −3.68 | −4.81 | −2.86 |
| PBDT-TVT-DTNT | 817 | 1.52 | 1.76 | −5.35 | −3.59 | −4.68 | −2.95 |
| Active Layer | Additive | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) Best | PCE (%) Average a |
|---|---|---|---|---|---|---|
| PBDT-TVT-ID:PC71BM=1:1 | 0.5% DPS | 0.90 | 7.82 | 44.60 | 3.16 | 3.10 |
| PBDT-TVT-ID:PC71BM=1:1.5 | 0.5% DPS | 0.91 | 8.72 | 51.49 | 4.09 | 3.89 |
| PBDT-TVT-ID:PC71BM=1:2 | 0.5% DPS | 0.90 | 8.16 | 48.38 | 3.55 | 3.42 |
| PBDT-TVT-DTNT:PC71BM=1:1 | 0.5% DPS | 0.74 | 10.60 | 52.00 | 4.08 | 3.91 |
| PBDT-TVT-DTNT:PC71BM=1:1.5 | 0.5% DPS | 0.74 | 12.21 | 60.25 | 5.44 | 5.26 |
| PBDT-TVT-DTNT:PC71BM=1:2. | 0.5% DPS | 0.74 | 11.09 | 55.11 | 4.53 | 4.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Wang, N.; Liang, Z.; Wang, X.; Peng, Y.; Tong, J.; Yang, C.; Xia, Y. Synthesis and Photovoltaic Effect of Electron-Withdrawing Units for Low Band Gap Conjugated Polymers Bearing Bi(thienylenevinylene) Side Chains. Polymers 2019, 11, 1461. https://doi.org/10.3390/polym11091461
Li J, Wang Y, Wang N, Liang Z, Wang X, Peng Y, Tong J, Yang C, Xia Y. Synthesis and Photovoltaic Effect of Electron-Withdrawing Units for Low Band Gap Conjugated Polymers Bearing Bi(thienylenevinylene) Side Chains. Polymers. 2019; 11(9):1461. https://doi.org/10.3390/polym11091461
Chicago/Turabian StyleLi, Jianfeng, Yufei Wang, Ningning Wang, Zezhou Liang, Xu Wang, Yichun Peng, Junfeng Tong, Chunyan Yang, and Yangjun Xia. 2019. "Synthesis and Photovoltaic Effect of Electron-Withdrawing Units for Low Band Gap Conjugated Polymers Bearing Bi(thienylenevinylene) Side Chains" Polymers 11, no. 9: 1461. https://doi.org/10.3390/polym11091461
APA StyleLi, J., Wang, Y., Wang, N., Liang, Z., Wang, X., Peng, Y., Tong, J., Yang, C., & Xia, Y. (2019). Synthesis and Photovoltaic Effect of Electron-Withdrawing Units for Low Band Gap Conjugated Polymers Bearing Bi(thienylenevinylene) Side Chains. Polymers, 11(9), 1461. https://doi.org/10.3390/polym11091461