Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. BG Particles
2.2. Cu-TCP Particles
2.3. Modified PMMA-Based Bone Cements
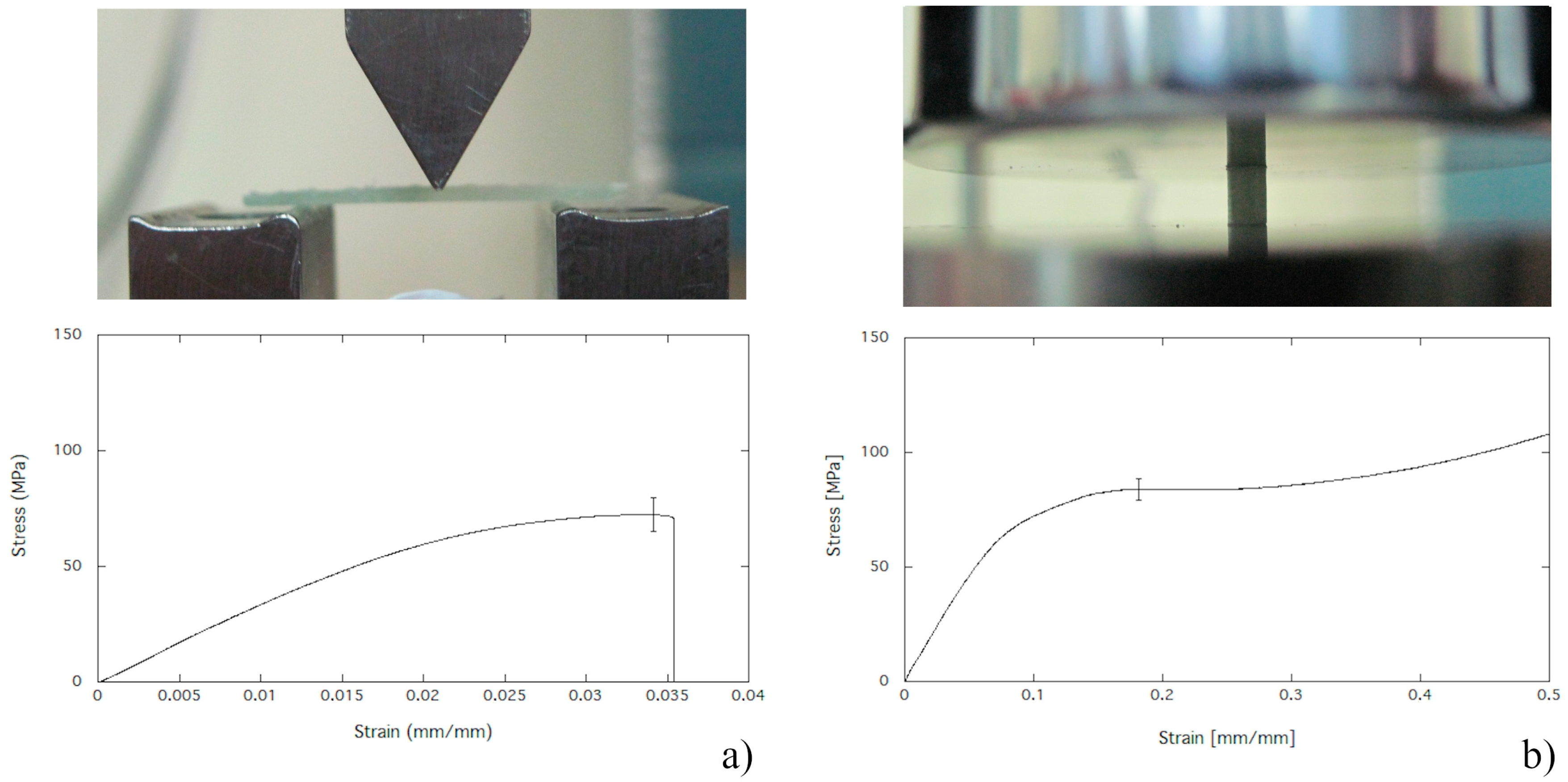
2.4. Mechanical Properties in Bending
2.5. Compression Strength
2.6. Cell Viability Assay
2.7. Cell Differentiation Assay
2.8. Viability of Bacterial Strains
2.9. Statistical Analysis
3. Results
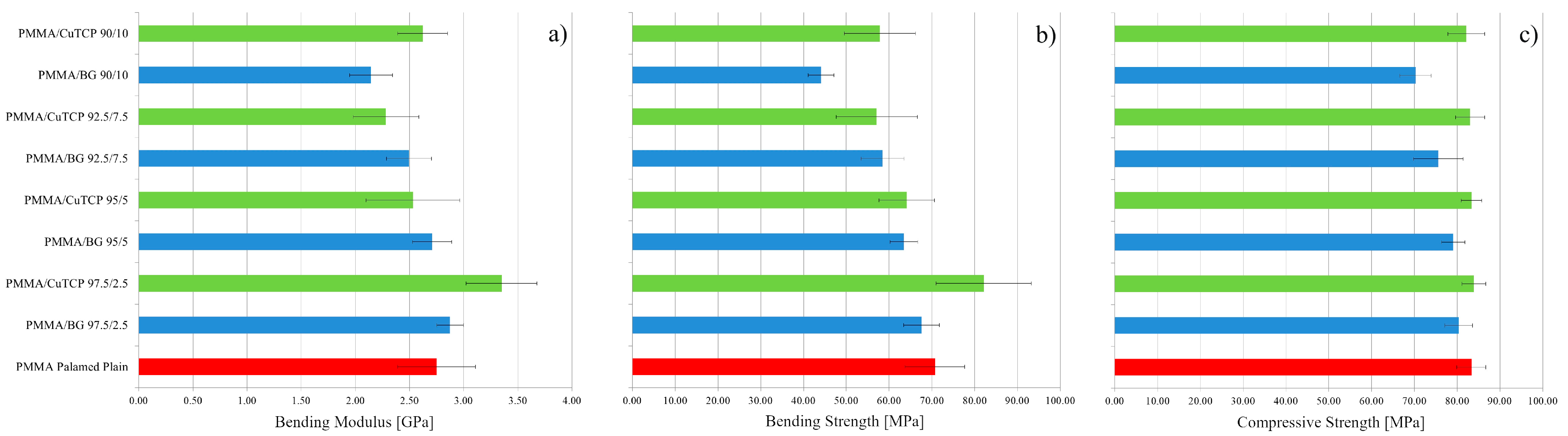
3.1. Mechanical Properties in Bending
3.2. Compression Strength
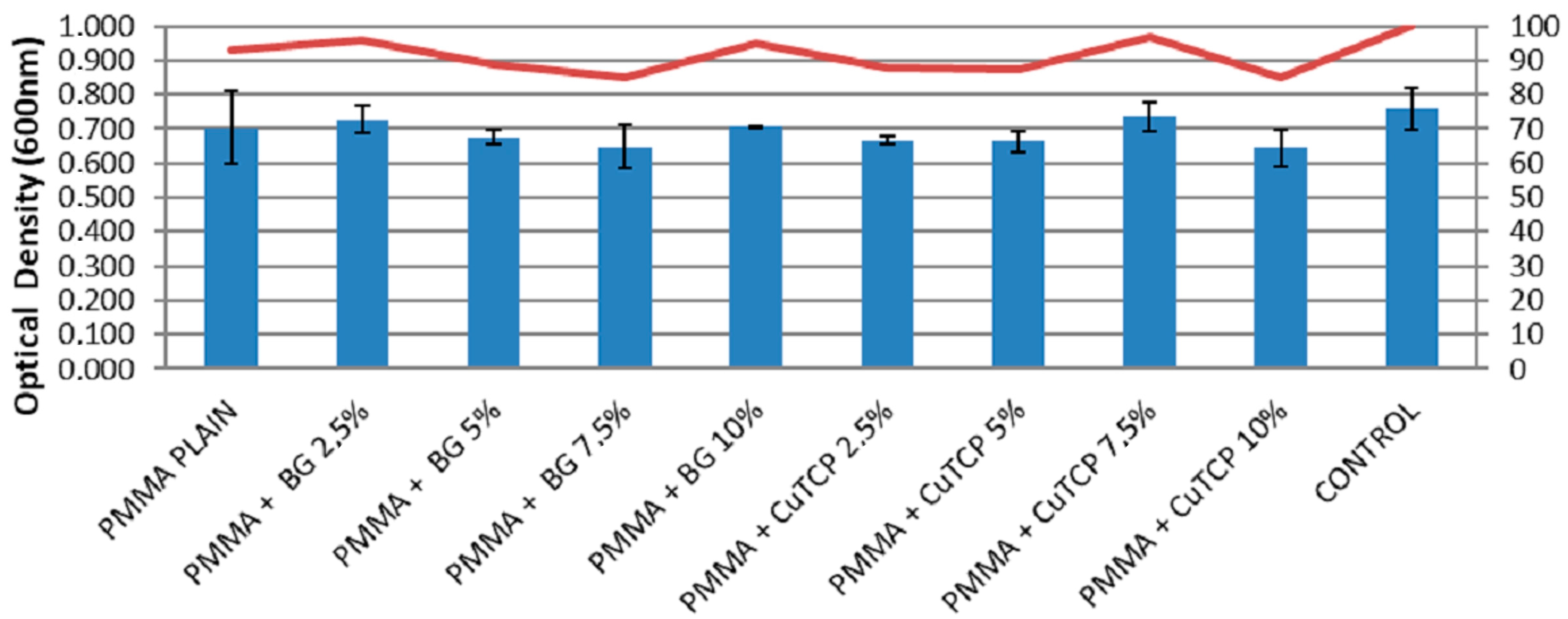
3.3. Cell Viability Assay
3.4. Cell Differentiation Assay
3.5. Viability of Bacterial Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unterhofer, C.; Wipplinger, C.; Verius, M.; Recheis, W.; Thomé, C.; Ortler, M. Reconstruction of large cranial defects with poly-methyl-methacrylate (PMMA) using a rapid prototyping model and a new technique for intraoperative implant modeling. Neurol. Neurochir. Pol. 2017, 51, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Piitulainen, J.M.; Kauko, T.; Aitasalo, K.M.; Vuorinen, V.; Vallittu, P.K.; Posti, J.P. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg. 2015, 83, 708–714. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloria, A.; Ambrosio, L. Materials and technologies for craniofacial tissue repair and regeneration. Top. Med. 2010, 16, 1–6. [Google Scholar]
- Cavalu, S.; Simon, V. Microstructure and bioactivity of acrylic bone cements for prosthetic surgery. J. Optoelectron. Adv. Mater. 2006, 8, 1520–1523. [Google Scholar]
- Moreira-Gonzalez, A.; Jackson, I.T.; Miyawaki, T.; Barakat, K.; DiNick, V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J. Craniofac. Surg. 2003, 14, 144–153. [Google Scholar] [CrossRef]
- Matsuno, A.; Tanaka, H.; Iwamuro, H.; Takanashi, S.; Miyawaki, S.; Nakashima, M.; Nakaguchi, H.; Nagashima, T. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006, 148, 535–540. [Google Scholar] [CrossRef]
- Spence, W.T. Form-fitting plastic cranioplasty. J. Neurosurg. 1954, 11, 219–225. [Google Scholar] [CrossRef]
- Lee, S.C.; Wu, C.T.; Lee, S.T.; Chen, P.J. Cranioplasty using polymethyl methacrylate prostheses. J. Clin. Neurosci. 2009, 16, 56–63. [Google Scholar] [CrossRef]
- Costantino, P.D.; Friedman, C.D.; Lane, A. Synthetic biomaterials in facial plastic and reconstructive surgery. Facial Plast. Surg. 1993, 9, 1–5. [Google Scholar] [CrossRef]
- Caro-Osorio, E.; De la Garza-Ramos, R.; Martínez-Sánchez, S.R.; Olazarán-Salinas, F. Cranioplasty with polymethylmethacrylate prostheses fabricated by hand using original bone flaps: Technical note and surgical outcomes. Surg. Neurol. Int. 2013, 4, 136. [Google Scholar] [CrossRef]
- Jaberi, J.; Gambrell, K.; Tiwana, P.; Madden, C.; Finn, R. Long-term clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J. Oral Maxillofac. Surg. 2013, 71, e81–e88. [Google Scholar] [CrossRef] [PubMed]
- Saringer, W.; Nöbauer-Huhmann, I.; Knosp, E. Cranioplasty with individual carbon fibre reinforced polymere (CFRP) medical grade implants based on CAD/CAM technique. Acta Neurochir. 2002, 144, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Msallem, B.; Beiglboeck, F.; Honigmann, P.; Jaquiéry, C.; Thieringer, F. Craniofacial reconstruction by a cost-efficient template-based process using 3d printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltola, M.J.; Vallittu, P.K.; Vuorinen, V.; Aho, A.A.; Puntala, A.; Aitasalo, K.M. Novel composite implant in craniofacial bone reconstruction. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espalin, D.; Arcaute, K.; Rodriguez, D.; Medina, F.; Posner, M.; Wicker, R. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010, 16, 164–173. [Google Scholar] [CrossRef]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S. An In Vitro Study of Osteoblast Response on Fused-Filament Fabrication 3D Printed PEEK for Dental and Cranio-Maxillofacial Implants. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.K.; Jammalamadaka, U.; Tappa, K.; Weisman, J. Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly (methyl methacrylate) beads. Bioact. Mater. 2018, 3, 157–166. [Google Scholar] [CrossRef]
- Bloch, O.; McDermott, M.W. In situ cranioplasty for hyperostosing meningiomas of the cranial vault. Can. J. Neurol. Sci. 2011, 38, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.A.; Fong, A.J.; Buchanan, E.P.; Monson, L.; Khechoyan, D.; Lam, S. History of synthetic materials in alloplastic cranioplasty. Neurosurg. Focus 2014, 36, E20. [Google Scholar] [CrossRef]
- De Santis, R.; Mollica, F.; Ambrosio, L.; Nicolais, L.; Ronca, D. Dynamic mechanical behavior of PMMA based bone cements in wet environment. J. Mater. Sci. Mater. Med. 2003, 14, 583–594. [Google Scholar] [CrossRef]
- Worm, P.V.; do Nascimento, T.L.; do Couto Nicola, F.; Sanches, E.F.; dos Santos Moreira, C.F.; Rogério, L.P.; dos Reis, M.M.; Finger, G.; Collares, M.V. Polymethylmethacrylate imbedded with antibiotics cranioplasty: An infection solution for moderate and large defects reconstruction? Surg. Neurol. Int. 2016, 7, S746. [Google Scholar] [CrossRef] [Green Version]
- De Santis, R.; Ambrogi, V.; Carfagna, C.; Ambrosio, L.; Nicolais, L. Effect of microencapsulated phase change materials on the thermo-mechanical properties of poly (methyl-methacrylate) based biomaterials. J. Mater. Sci. Mater. Med. 2006, 17, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Ronca, D.; Gloria, A.; De Santis, R.; Russo, T.; D’Amora, U.; Chierchia, M.; Nicolais, L.; Ambrosio, L. Critical analysis on dynamic-mechanical performance of spongy bone: The effect of an acrylic cement. Hard Tissue 2014, 3, 9–16. [Google Scholar]
- Cheng, C.H.; Chuang, H.Y.; Lin, H.L.; Liu, C.L.; Yao, C.H. Surgical results of cranioplasty using three-dimensional printing technology. Clin. Neurol. Neurosurg. 2018, 168, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Maricevich, J.P.; Cezar-Junior, A.B.; de Oliveira-Junior, E.X.; Silva, J.A.; da Silva, J.V.; Nunes, A.A.; Almeida, N.S.; Azevedo-Filho, H.R. Functional and aesthetic evaluation after cranial reconstruction with polymethyl methacrylate prostheses using low-cost 3D printing templates in patients with cranial defects secondary to decompressive craniectomies: A prospective study. Surg. Neurol. Int. 2019, 10, 1. [Google Scholar] [CrossRef]
- van Putten, M.C., Jr.; Yamada, S. Alloplastic cranial implants made from computed tomographic scan-generated casts. J. Prosthet. Dent. 1992, 68, 103–108. [Google Scholar] [CrossRef]
- Minelli, E.B.; Della Bora, T.; Benini, A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe 2011, 17, 380–383. [Google Scholar] [CrossRef]
- Fleaca, R.; Mitariu, S.I.; Oleksik, V.; Oleksik, M.; Roman, M. Mechanical Behaviour of Orthopaedic Cement Loaded with Antibiotics in the Operation Room. Mater. Plast. 2017, 54, 402. [Google Scholar]
- Pelletier, M.H.; Lau, A.C.; Smitham, P.J.; Nielsen, G.; Walsh, W.R. Pore distribution and material properties of bone cement cured at different temperatures. Acta Biomater. 2010, 6, 886–891. [Google Scholar] [CrossRef]
- Oei, J.D.; Zhao, W.W.; Chu, L.; DeSilva, M.N.; Ghimire, A.; Rawls, H.R.; Whang, K. Antimicrobial acrylic materials with in situ generated silver nanoparticles. J. Biomed. Mater. Res. B 2012, 100, 409–415. [Google Scholar] [CrossRef]
- Rau, J.; Fosca, M.; Graziani, V.; Egorov, A.; Zobkov, Y.; Fedotov, A.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.; Komlev, V. Silver-doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, T.; Gloria, A.; De Santis, R.; D’Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ledda, M.; Fosca, M.; De Bonis, A.; Curcio, M.; Teghil, R.; Lolli, M.G.; De Stefanis, A.; Marchese, R.; Rau, J.V.; Lisi, A. Placenta derived mesenchymal stem cells hosted on RKKP glass-ceramic: A tissue engineering strategy for bone regenerative medicine applications. BioMed Res. Int. 2016, 11, 2016. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Cochis, A.; Kumar, A.; Arciola, C.; Rimondini, L.; Verné, E. Copper-doped bioactive glass as filler for PMMA-based bone cements: Morphological, mechanical, reactivity, and preliminary antibacterial characterization. Materials 2018, 11, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itokawa, H.; Hiraide, T.; Moriya, M.; Fujimoto, M.; Nagashima, G.; Suzuki, R.; Fujimoto, T. A 12 month in vivo study on the response of bone to a hydroxyapatite–polymethylmethacrylate cranioplasty composite. Biomaterials 2007, 28, 4922–4927. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Wu, V.M.; Graziani, V.; Fadeeva, I.V.; Fomin, A.S.; Fosca, M.; Uskoković, V. The bone building blues: Self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. 2017, 79, 270–279. [Google Scholar] [CrossRef]
- Spierings, P.T. Testing and performance of bone cements. In The Well-Cemented Total Hip Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2005; pp. 67–78. [Google Scholar]
- Cheng, Y.K.; Weng, H.H.; Yang, J.T.; Lee, M.H.; Wang, T.C.; Chang, C.N. Factors affecting graft infection after cranioplasty. J. Clin. Neurosci. 2008, 15, 1115–1119. [Google Scholar] [CrossRef]
- Rensing, C.; Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Espírito, S.C.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, T.; De Santis, R.; Gloria, A.; Barbaro, K.; Altigeri, A.; Fadeeva, I.V.; Rau, J.V. Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles. Polymers 2020, 12, 37. https://doi.org/10.3390/polym12010037
Russo T, De Santis R, Gloria A, Barbaro K, Altigeri A, Fadeeva IV, Rau JV. Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles. Polymers. 2020; 12(1):37. https://doi.org/10.3390/polym12010037
Chicago/Turabian StyleRusso, Teresa, Roberto De Santis, Antonio Gloria, Katia Barbaro, Annalisa Altigeri, Inna V. Fadeeva, and Julietta V. Rau. 2020. "Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles" Polymers 12, no. 1: 37. https://doi.org/10.3390/polym12010037





