Fast Production of Cellulose Nanocrystals by Hydrolytic-Oxidative Microwave-Assisted Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Experiment
2.3. Cellulose Nanocrystals Extraction Processes
2.3.1. Heating Programs
2.3.2. Purification Steps
2.4. CNCs Extraction Yield
2.5. Morphological Characterization of CNCs
2.6. Zeta Potential Measurement
2.7. Statistical Analysis
2.8. Fourier Transform Infrared Spectroscopy (FTIR)-Attenuated Total Reflectance (ATR) Analysis of Cellulose Nanocrystals
2.9. X-ray Diffraction (XRD) Characterization
2.10. Thermogravimetric Analysis (TGA)
3. Results
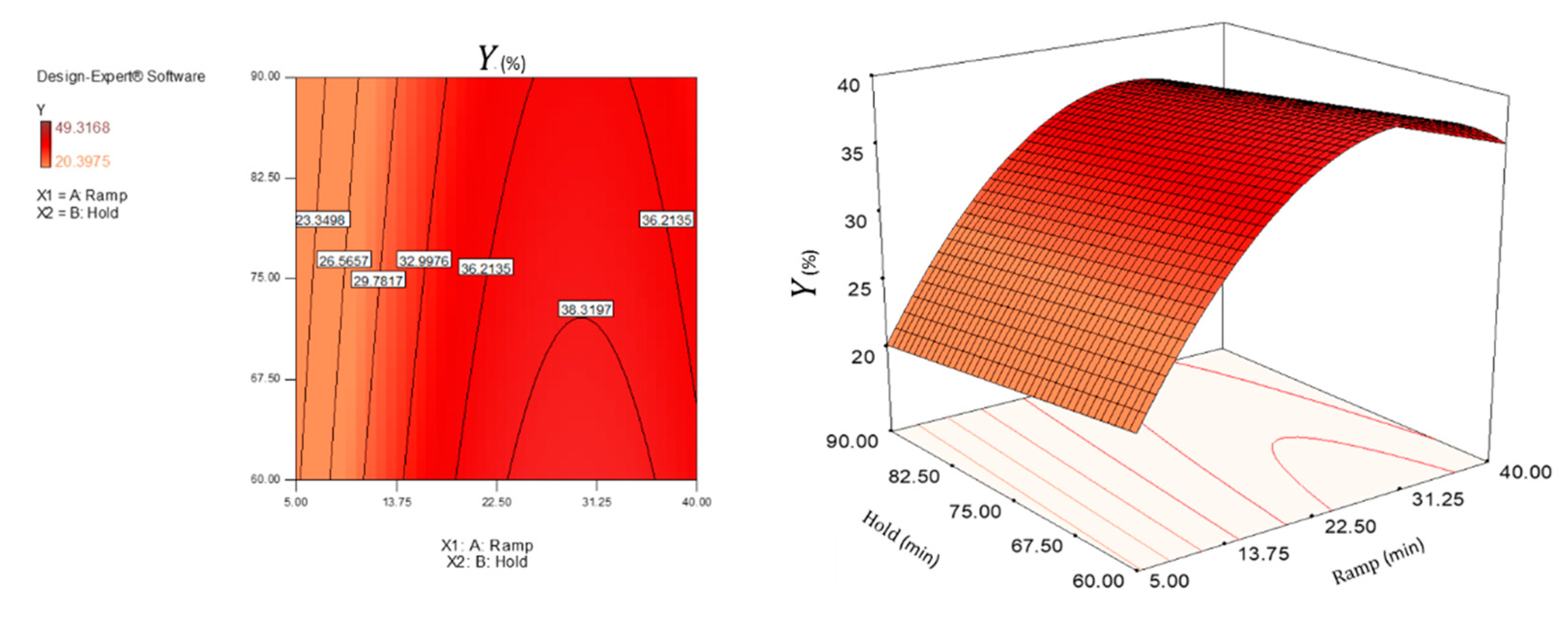
3.1. Evaluation of the Yield of Cellulose Nanocrystals
3.2. Morphology of Cellulose Nanocrystals
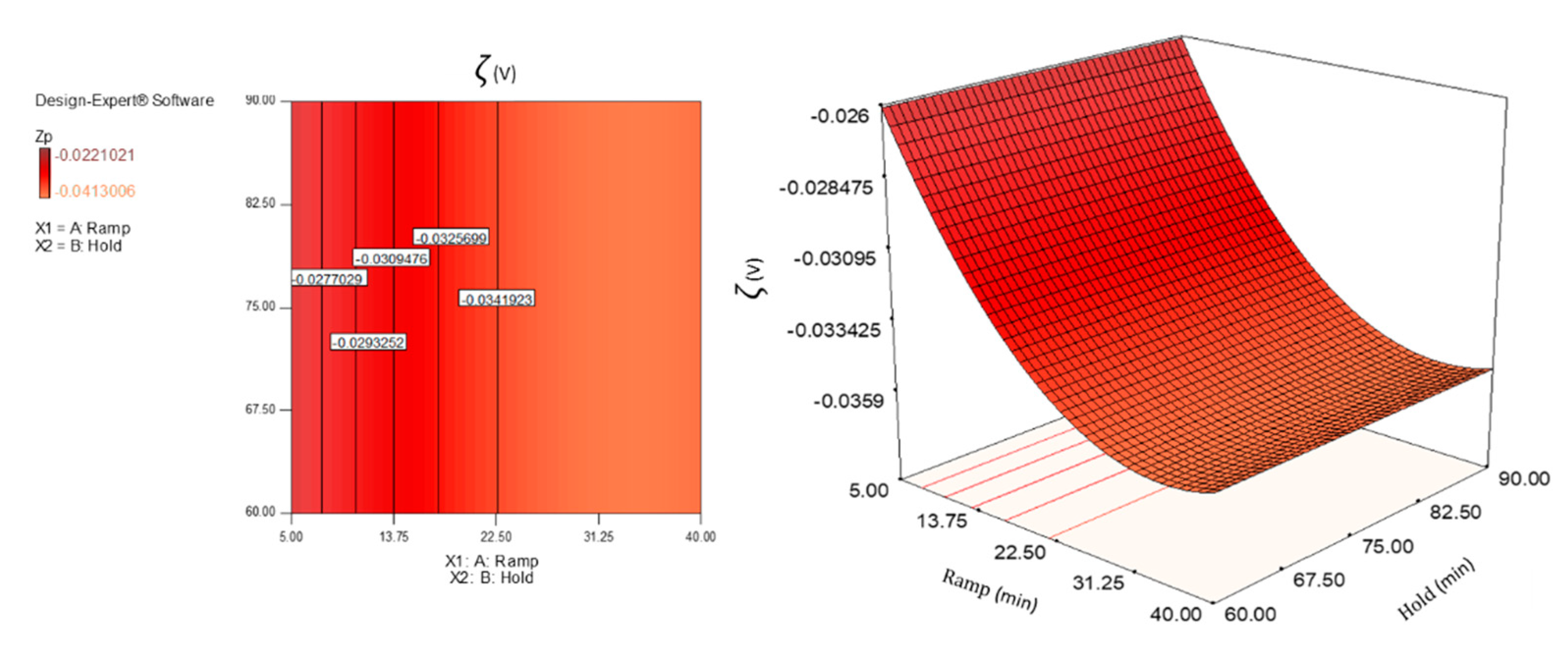
3.3. Zeta Potential Analysis
3.4. Statistical Evaluation of the Responses and Model Fitting
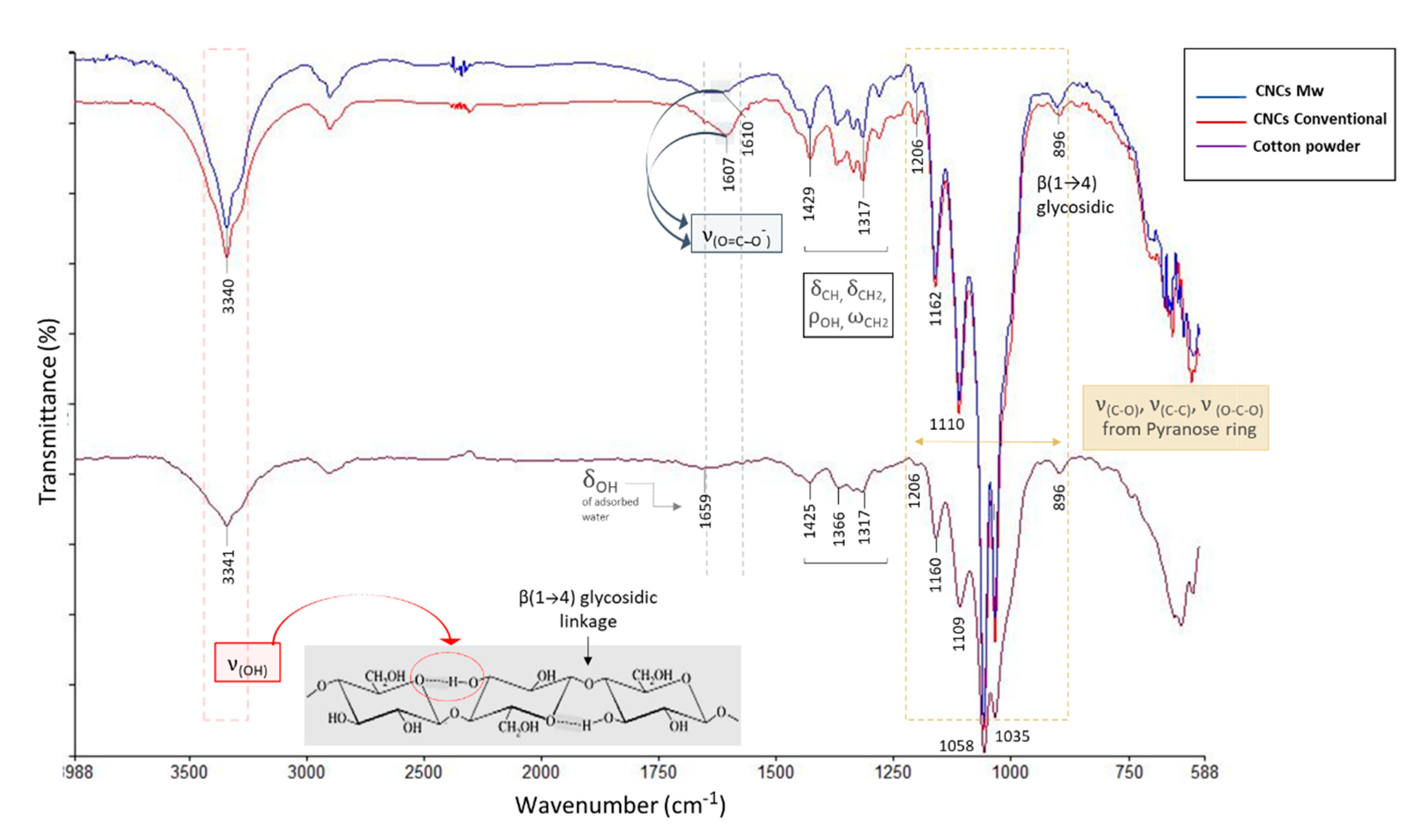
3.5. FTIR-ATR Analysis of Cellulose Nanocrystals
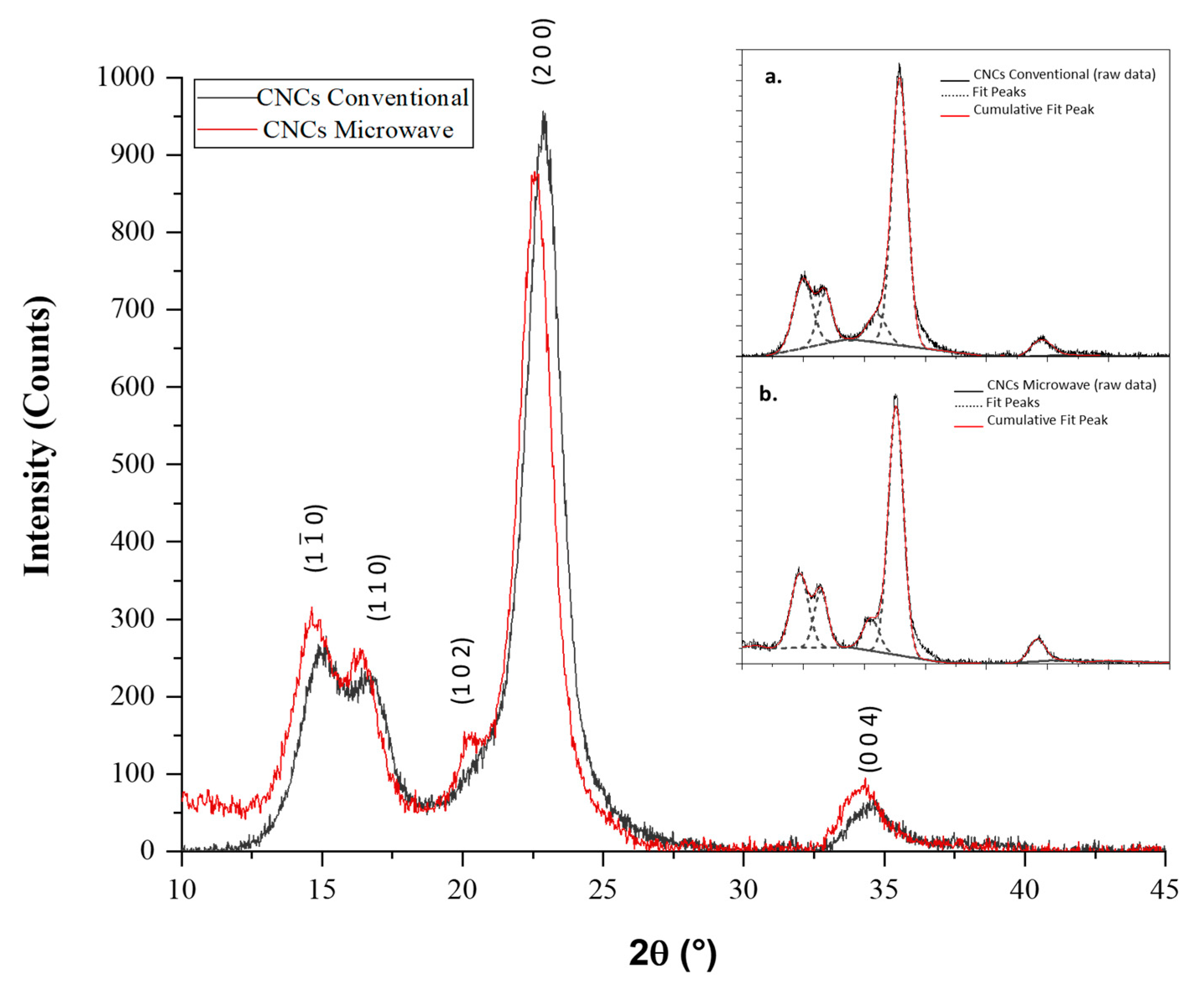
3.6. X-ray Powder Diffraction Analysis
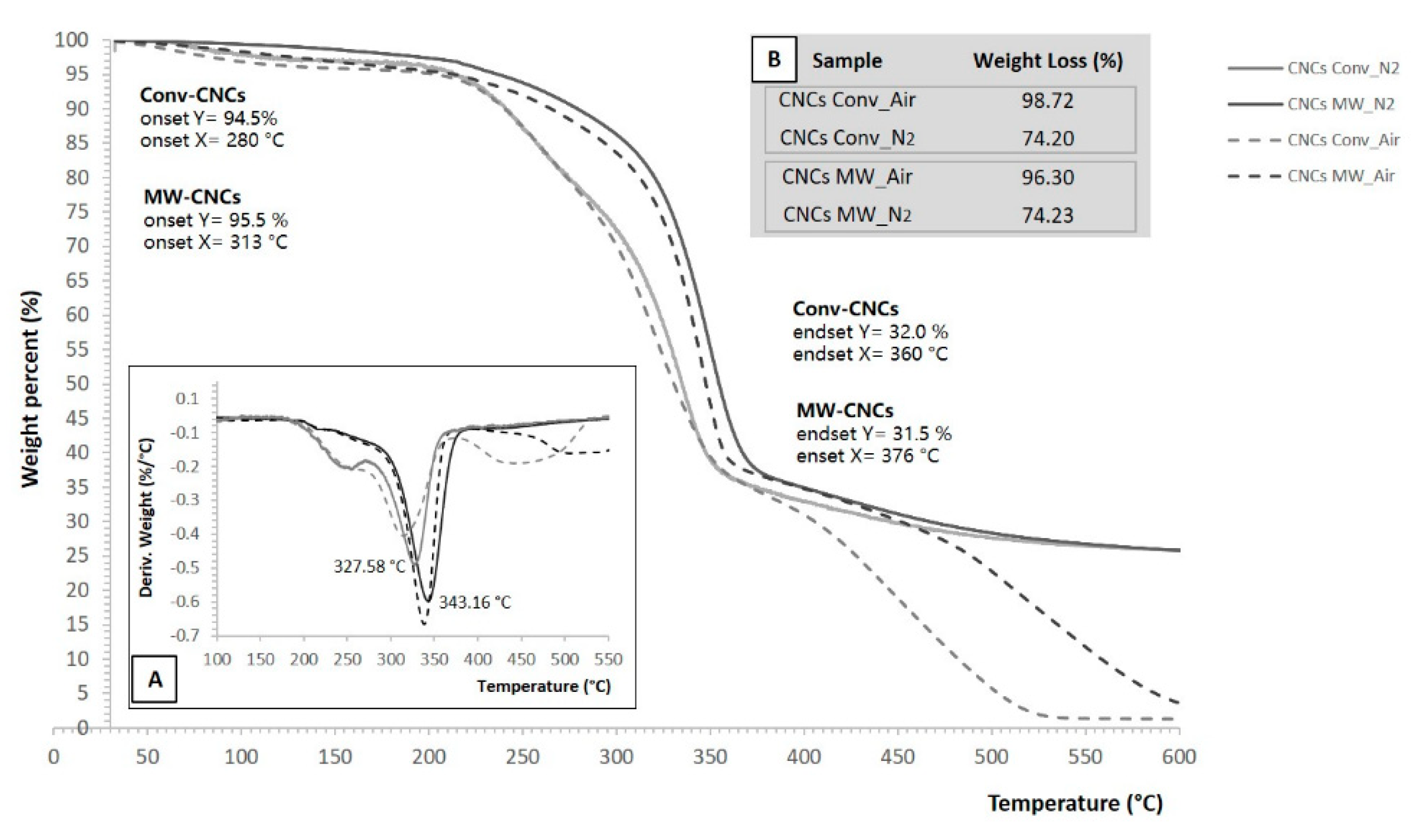
3.7. Thermal Properties of Cellulose Nanocrystals
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fotie, G.; Rampazzo, R.; Ortenzi, M.A.; Checchia, S.; Fessas, D.; Piergiovanni, L. The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials. Polymers 2017, 9, 415. [Google Scholar] [CrossRef]
- Rampazzo, R.; Alkan, D.; Gazzotti, S.; Ortenzi, M.A.; Piva, G.; Piergiovanni, L. Cellulose Nanocrystals from Lignocellulosic Raw Materials, for Oxygen Barrier Coatings on Food Packaging Films. Packag. Technol. Sci. 2017, 30, 645–661. [Google Scholar] [CrossRef] [Green Version]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Biagioni, P.; Bollani, M.; Maccagnan, A.; Piergiovanni, L. Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 2013, 20, 2491–2504. [Google Scholar] [CrossRef] [Green Version]
- Gómez, H.C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An insight into nanocellulose as soft condensed matter: Challenge and future prospective toward environmental sustainability. Sci. Total Environ. 2019, 650, 1309–1326. [Google Scholar] [CrossRef]
- MarketsandMarkets. Nanocellulose Market by Type (Microfibrillated Cellulose, Cellulose Nanocrystals), Application (Pulp& Paper, Composites & Packaging, Biomedical & Pharmaceuticals, Electronics & Sensors), and Region (Europe, North America, APAC)-Global Forecast to 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/nano-cellulose-market-56392090.html (accessed on 22 October 2019).
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Jongaroontaprangsee, S.; Chiewchan, N.; Devahastin, S. Production of nanocellulose from lime residues using chemical-free technology. Mater. Today Proc. 2018, 5, 11095–11100. [Google Scholar] [CrossRef]
- De Melo, E.M.; Clark, J.H.; Matharu, A.S. The Hy-MASS concept: Hydrothermal microwave assisted selective scissoring of cellulose for in situ production of (meso)porous nanocellulose fibrils and crystals. Green Chem. 2017, 19, 3408–3417. [Google Scholar] [CrossRef] [Green Version]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer Nanocomposites Reinforced by Cellulose Whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E.; Schwegler-Berry, D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009, 11, 1808–1814. [Google Scholar] [CrossRef]
- Kos, T.; Anžlovar, A.; Kunaver, M.; Huskić, M.; Žagar, E. Fast preparation of nanocrystalline cellulose by microwave-assisted hydrolysis. Cellulose 2014, 21, 2579–2585. [Google Scholar] [CrossRef]
- Leung, A.; Hrapovic, S.; Lam, E.; Liu, Y.L.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, C.W.; Luong, J.H.T.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.; Rho, D. Cellulose Nanocrystals from Renewable Biomass. U.S. Patent 8900706 B2, 2 December 2014. [Google Scholar]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef]
- Caddick, S.; Fitzmaurice, R. Microwave enhanced synthesis. Tetrahedron 2009, 65, 3325–3355. [Google Scholar] [CrossRef]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis—A critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Venkatesh, M.S.; Raghavan, G.S.V. An Overview of Microwave Processing and Dielectric Properties of Agri-food Materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-Assisted Green Synthesis of Silver Nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z. Recent advances in microwave initiated synthesis of nanocarbon materials. Nanoscale 2012, 4, 707–714. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef] [PubMed]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Pillai, S.K.; Ray, S.S.; Jalama, K.; Krause, R.W.M. Recent trends in the microwave-assisted synthesis of metal oxide nanoparticles supported on carbon nanotubes and their applications. J. Nanomater. 2012, 2012, 51. [Google Scholar] [CrossRef] [Green Version]
- Dariusz, B.; Aleksander, P.; Slawomir, M. Synthesis of Polymer Nanocomposites Under Microwave Irradiation. Curr. Org. Chem. 2011, 15, 178–188. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.-P.; Peng, F.; Bian, J.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Microwave-assisted solvent-free acetylation of cellulose with acetic anhydride in the presence of iodine as a catalyst. Molecules 2009, 14, 3551–3566. [Google Scholar] [CrossRef]
- Biswas, A.; Kim, S.; Selling, G.W.; Cheng, H.N. Conversion of agricultural residues to carboxymethylcellulose and carboxymethylcellulose acetate. Ind. Crop. Prod. 2014, 60, 259–265. [Google Scholar] [CrossRef]
- De la Hoz, A. Microwave Heating as a Tool for Sustainable Chemistry, 1st ed.; Leadbeater, N.E., Ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Hult, E.-L.; Iversen, T.; Sugiyama, J. Characterization of the supermolecular structure of cellulose in wood pulp fibres. Cellulose 2003, 10, 103–110. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3. [Google Scholar] [CrossRef] [Green Version]
- Ioelovich, M.Y. Models of supramolecular structure and properties of cellulose. Polym. Sci. Ser. A 2016, 58, 925–943. [Google Scholar] [CrossRef]
- Duchemin, B. Size, shape, orientation and crystallinity of cellulose Iβ by X-ray powder diffraction using a free spreadsheet program. Cellulose 2017, 24. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherrer, P. Bestimmung dergrosse und der inneren struktur yon kolloiteilchen mittels. Gott. Nachr Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Aguayo, M.G.; Pérez, A.; Reyes, G.; Oviedo, C.; Gacitua, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef] [Green Version]
- Oun, A.A.; Rhim, J.-W. Characterization of carboxymethyl cellulose-based nanocomposite films reinforced with oxidized nanocellulose isolated using ammonium persulfate method. Carbohydr. Polym. 2017, 174, 484–492. [Google Scholar] [CrossRef]
- Castro-Guerrero, C.F.; Gray, D.G. Chiral nematic phase formation by aqueous suspensions of cellulose nanocrystals prepared by oxidation with ammonium persulfate. Cellulose 2014, 21, 2567–2577. [Google Scholar] [CrossRef]
- Ye, S.; Yu, H.-Y.; Wang, D.; Zhu, J.; Gu, J. Green acid-free one-step hydrothermal ammonium persulfate oxidation of viscose fiber wastes to obtain carboxylated spherical cellulose nanocrystals for oil/water Pickering emulsion. Cellulose 2018, 25, 5139–5155. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Baalousha, M.; Lead, J. Rationalizing nanomaterial sizes measured by atomic force microscopy, flow field-flow fractionation, and dynamic light scattering: Sample preparation, polydispersity, and particle structure. Environ. Sci. Technol. 2012, 46, 6134–6142. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Fu, S.; Peng, X.; Zhan, H.; Sun, R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohydr. Polym. 2012, 90, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Shamskar, K.R.; Heidari, H.; Rashidi, A. Preparation and evaluation of nanocrystalline cellulose aerogels from raw cotton and cotton stalk. Ind. Crop. Prod. 2016, 93, 203–211. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibres. In Fourier Transform-Materials Analysis; Salih, S.M., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Xiong, R.; Zhang, X.; Tian, D.; Zhou, Z.; Lu, C. Comparing microcrystalline with spherical nanocrystalline cellulose from waste cotton fabrics. Cellulose 2012, 19. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Saha, N.R.; Sarkar, G.; Rana, D.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. A facile comparative approach towards utilization of waste cotton lint for the synthesis of nano-crystalline cellulose crystals along with acid recovery. Int. J. Biol. Macromol. 2018, 109, 1246–1252. [Google Scholar] [CrossRef]
- Zianor Azrina, Z.A.; Beg, M.D.H.; Rosli, M.Y.; Ramli, R.; Junadi, N.; Alam, A.K.M.M. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr. Polym. 2017, 162, 115–120. [Google Scholar] [CrossRef]
- Pirani, S.; Hashaikeh, R. Nanocrystalline cellulose extraction process and utilization of the byproduct for biofuels production. Carbohydr. Polym. 2013, 93, 357–363. [Google Scholar] [CrossRef]
- Potthast, A.; Kostic, M.; Schiehser, S.; Kosma, P.; Rosenau, T. Studies on oxidative modifications of cellulose in the periodate system: Molecular weight distribution and carbonyl group profiles. Holzforschung 2007, 61, 662–667. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Nassar, M.A. Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohydr. Polym. 2010, 79, 694–699. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Ioelovich, M.; Leykin, A.; Figovsky, O. Study of cellulose paracrystallinity. Bioresources 2010, 5, 1393–1407. [Google Scholar] [CrossRef]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the Interpretation of X-ray Diffraction Powder Patterns in Terms of the Nanostructure of Cellulose I Fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanomaterials: Size and surface influence on the thermal and rheological behavior. Polímeros 2018, 28, 93–102. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Cui, S.; Wang, S.-Y. Preparation and crystalline analysis of high-grade bamboo dissolving pulp for cellulose acetate. J. Appl. Polym. Sci. 2008, 107, 1029–1038. [Google Scholar] [CrossRef]
- Mokhena, T.; Sefadi, J.; Sadiku, E.; John, M.; Mochane, M.; Mtibe, A. Thermoplastic processing of PLA/cellulose nanomaterials composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Hsieh, Y.L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Morán, J.; Alvarez, V.; Cyras, V.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Ioelovich, M. Influence of hydrolysis conditions on production of cellulose nanocrystalline particles. Proc. SITA 2018, 20, 13–20. [Google Scholar]
- Li, F.; Mascheroni, E.; Piergiovanni, L. The Potential of NanoCellulose in the Packaging Field: A Review. Packag. Technol. Sci. 2015, 28, 475–508. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Thermal stability of cellulose and their nanoparticles: Effect of incremental increases in carboxyl and aldehyde groups. Carbohydr. Polym. 2014, 114, 339–343. [Google Scholar] [CrossRef] [PubMed]
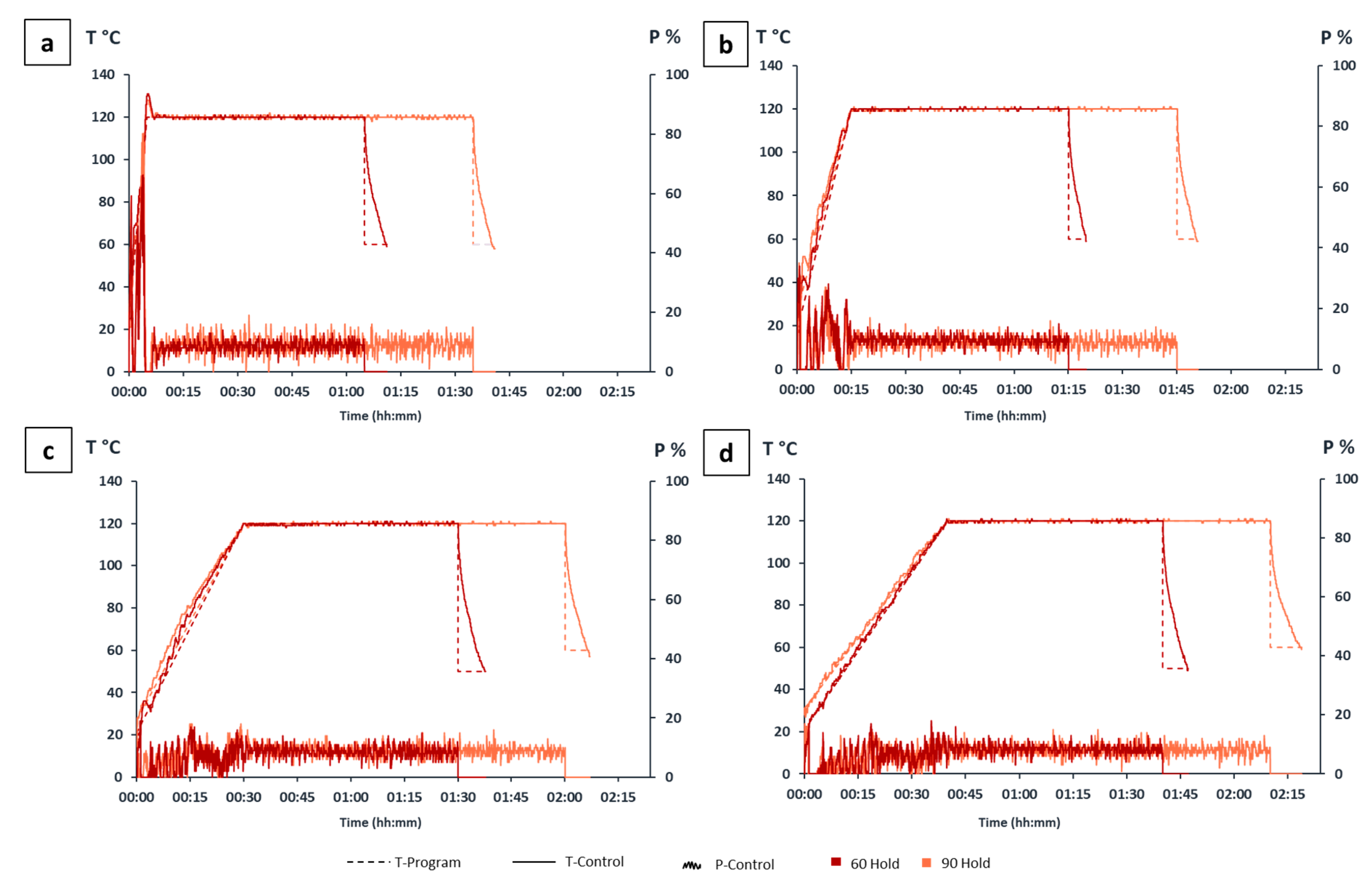



| Microwave Heating Method | Heating Program | Total Heating Time (hh:mm) | Cooling(°C) | |||
| Ramp (mm:ss) | ΔT/t (°C min−1) | Hold (mm:ss) | Temperature (°C) | |||
| M1 | 05:00 | 20 | 60:00 | 120 | 01:05 | 40 |
| M2 | 15:00 | 7 | 60:00 | 120 | 01:15 | 40 |
| M3 | 30:00 | 3 | 60:00 | 120 | 01:30 | 40 |
| M4 | 40:00 | 2.5 | 60:00 | 120 | 01:40 | 40 |
| M5 | 05:00 | 20 | 90:00 | 120 | 01:35 | 40 |
| M6 | 15:00 | 7 | 90:00 | 120 | 01:45 | 40 |
| M7 | 30:00 | 3 | 90:00 | 120 | 02:00 | 40 |
| M8 | 40:00 | 2.5 | 90:00 | 120 | 02:10 | 40 |
| Conventional Heating Method | Temperature (°C) | Total Heating Time (hh:mm) | Cooling(°C) | |||
| C1 | 75 | 16:00 | 40 | |||
| Heating Method | Yield Y (%) | Particle Size DHy (μm) | Polidispersity Index (PDI) (%) | Zeta Potential ζ (mV) | |
|---|---|---|---|---|---|
| Microwave | M1 | 22.093 ± 1.634 | 0.212 ± 0.014 | 23.491 ± 0.926 | −27.388 ± 3.280 |
| M2 | 32.314 ± 1.474 | 0.174 ± 0.008 a | 23.550 ± 1.637 | −30.520 ± 1.638 | |
| M3 | 45.814 ± 3.795 a | 0.153 ± 0.005 | 21.901 ± 1.172 | −40.575 ± 2.534 a | |
| M4 | 36.894 ± 3.236 | 0.165 ± 0.006 | 22.996 ± 2.649 | −35.735 ± 2.077 | |
| M5 | 23.370 ± 4.124 | 0.244 ± 0.026 | 22.760 ± 2.215 | −26.559 ± 1.171 | |
| M6 | 32.422 ± 2.985 | 0.178 ± 0.005 a | 21.842 ± 2.475 | −32.095 ±2.466 | |
| M7 | 34.411 ± 2.011 | 0.174 ± 0.009 a | 24.418 ± 2.999 | −33.229 ± 2.844 | |
| M8 | 35.031 ± 1.935 | 0.159 ± 0.008 | 22.714 ± 1.783 | −35.874 ± 2.127 | |
| Conventional | C1 | 48.850 ± 11.991 a | 0.176 ± 0.002 a | 25.067 ± 0.231 | −39.52 ± 1.141 a |
| Model | *** | *** | NS | *** | |
| Lack of Fit | NS | NS | NS | NS | |
| Main effect | |||||
| Ramp time (X1) | *** | *** | NS | *** | |
| Hold time (X2) | * | NS | NS | NS | |
| Ramp time2 (X12) | *** | *** | NS | ** | |
| Interaction | |||||
| Ramp × Hold (X1X2) | NS | NS | NS | NS | |
| Ramp2 × Hold (X12X2) | NS | NS | NS | NS | |
| Performance index of fitted model | |||||
| R2Adj | 0.8 | 0.8 | - | 0.7 |
| Crystalline Plane (Miller Index of Iβ) | FWHM (°) | Peak Position 2θ (°) | d-Spacing (nm) | Crystallite Size τ (nm) | |
|---|---|---|---|---|---|
| CNCs Conventional | 1.7 | 15.0 | 0.59 | 4.8 | |
| 1 1 0 | 1.4 | 16.7 | 0.52 | 6.0 | |
| 1 0 2 | 21.0 | ||||
| 2 0 0 | 1.5 | 22.9 | 0.39 | 5.6 | |
| 0 0 4 | 34.5 | ||||
| CNCs Microwave | 1.7 | 14.7 | 0.60 | 5.0 | |
| 1 1 0 | 1.4 | 16.4 | 0.53 | 6.1 | |
| 1 0 2 | 20.6 | ||||
| 2 0 0 | 1.5 | 22.6 | 0.39 | 5.7 | |
| 0 0 4 | 34.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoroso, L.; Muratore, G.; Ortenzi, M.A.; Gazzotti, S.; Limbo, S.; Piergiovanni, L. Fast Production of Cellulose Nanocrystals by Hydrolytic-Oxidative Microwave-Assisted Treatment. Polymers 2020, 12, 68. https://doi.org/10.3390/polym12010068
Amoroso L, Muratore G, Ortenzi MA, Gazzotti S, Limbo S, Piergiovanni L. Fast Production of Cellulose Nanocrystals by Hydrolytic-Oxidative Microwave-Assisted Treatment. Polymers. 2020; 12(1):68. https://doi.org/10.3390/polym12010068
Chicago/Turabian StyleAmoroso, Luana, Giuseppe Muratore, Marco Aldo Ortenzi, Stefano Gazzotti, Sara Limbo, and Luciano Piergiovanni. 2020. "Fast Production of Cellulose Nanocrystals by Hydrolytic-Oxidative Microwave-Assisted Treatment" Polymers 12, no. 1: 68. https://doi.org/10.3390/polym12010068






