Voltammetric pH Measurements Using Azure A-Containing Layer-by-Layer Film Immobilized Electrodes
Abstract
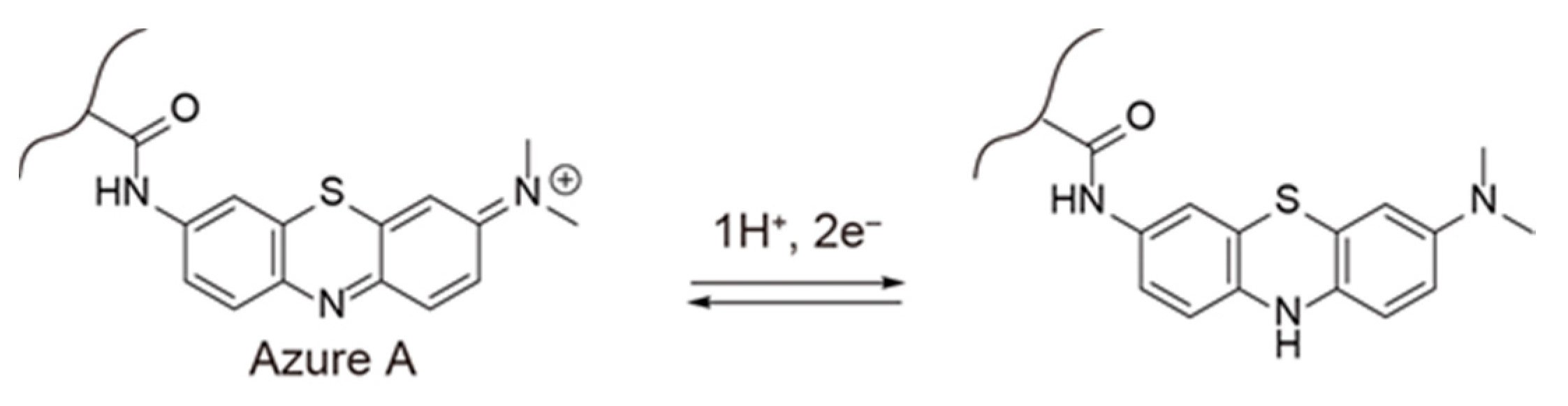
1. Introduction
2. Materials
3. Apparatus
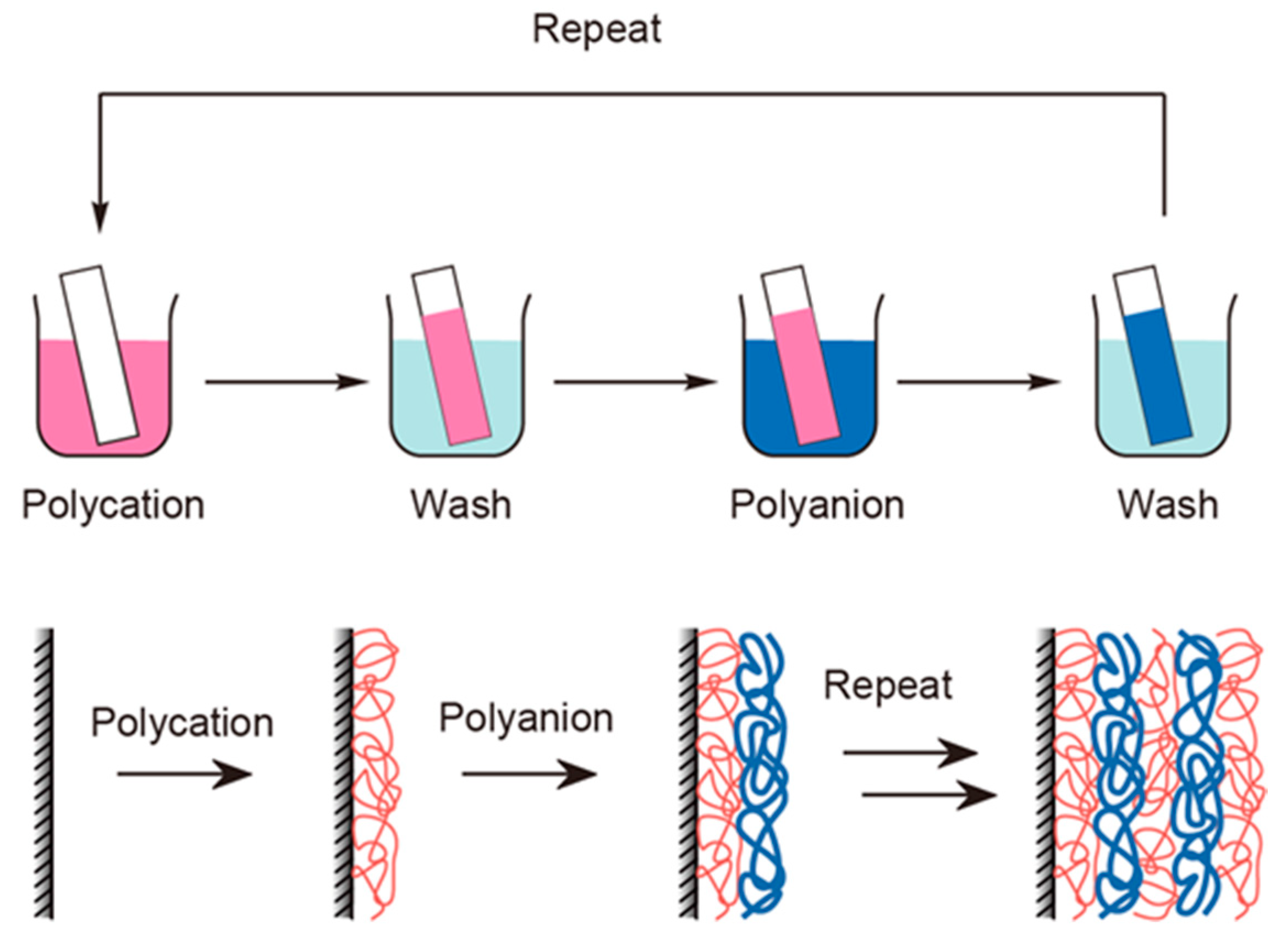
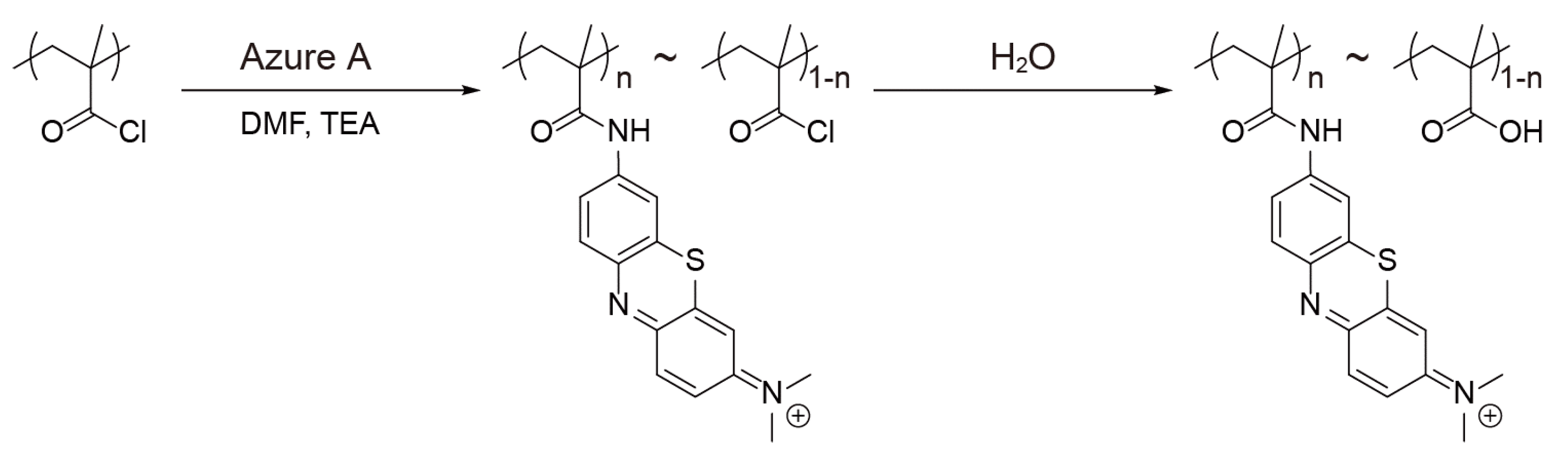
4. Preparation of (PAH/AA-PMA)5-Multilayer Films
5. Results and Discussion
5.1. Preparation of (PAH/AA-PMA)5-Multilayer Films
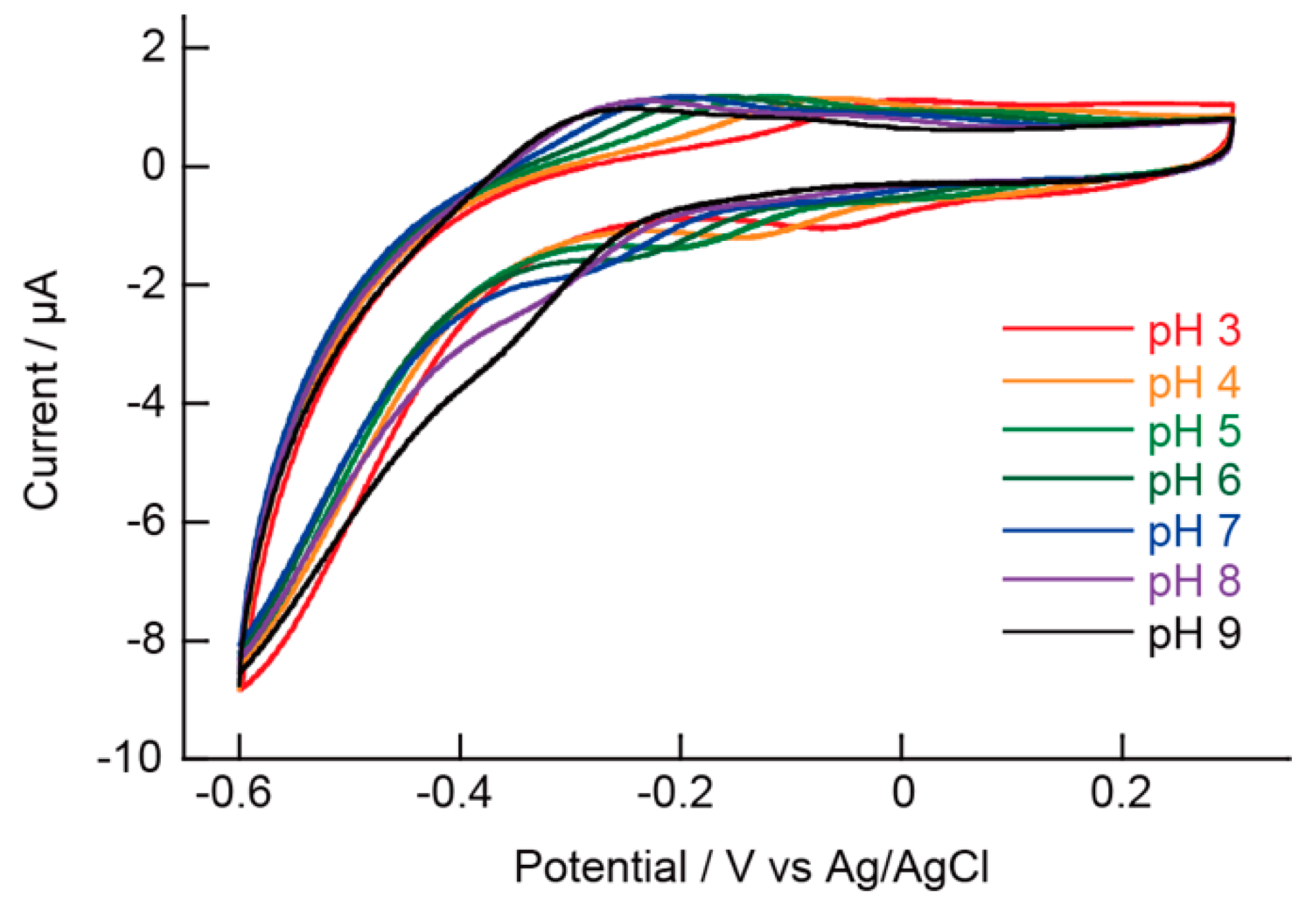
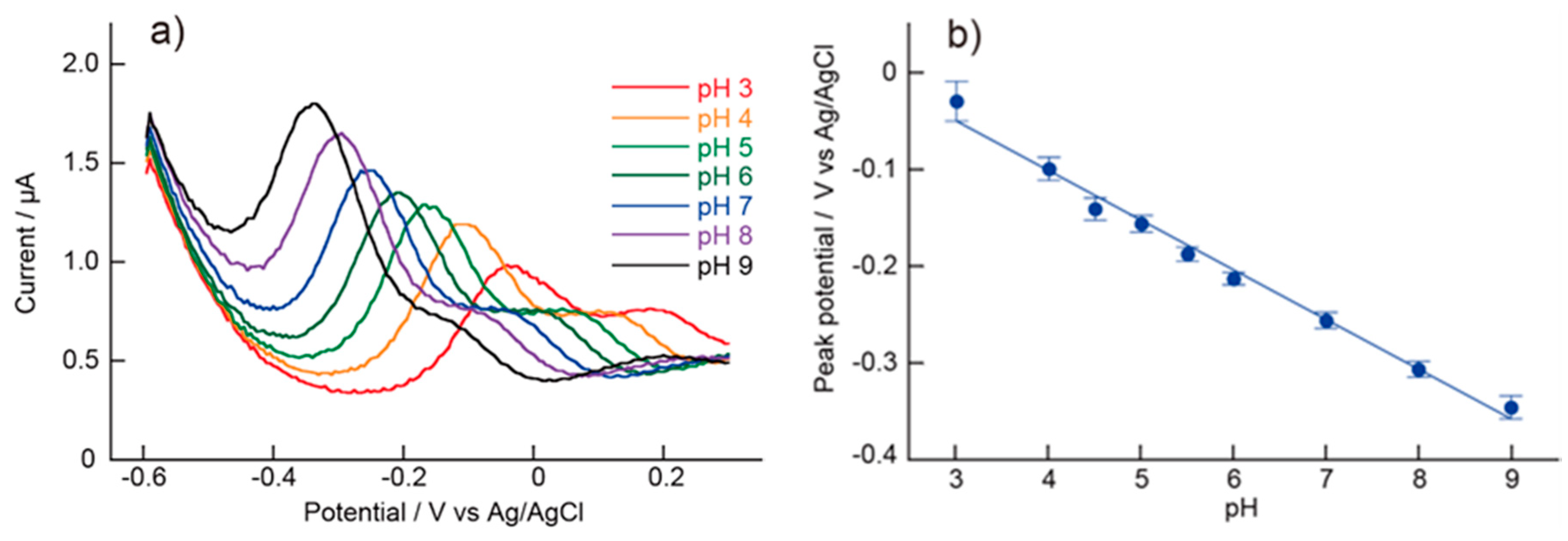
5.2. pH Measurements Using the (PAH/AA-PMA)5-Modified Electrode
5.3. pH Measurements Using the (PAH/AA-PMA)5-Modified Electrode
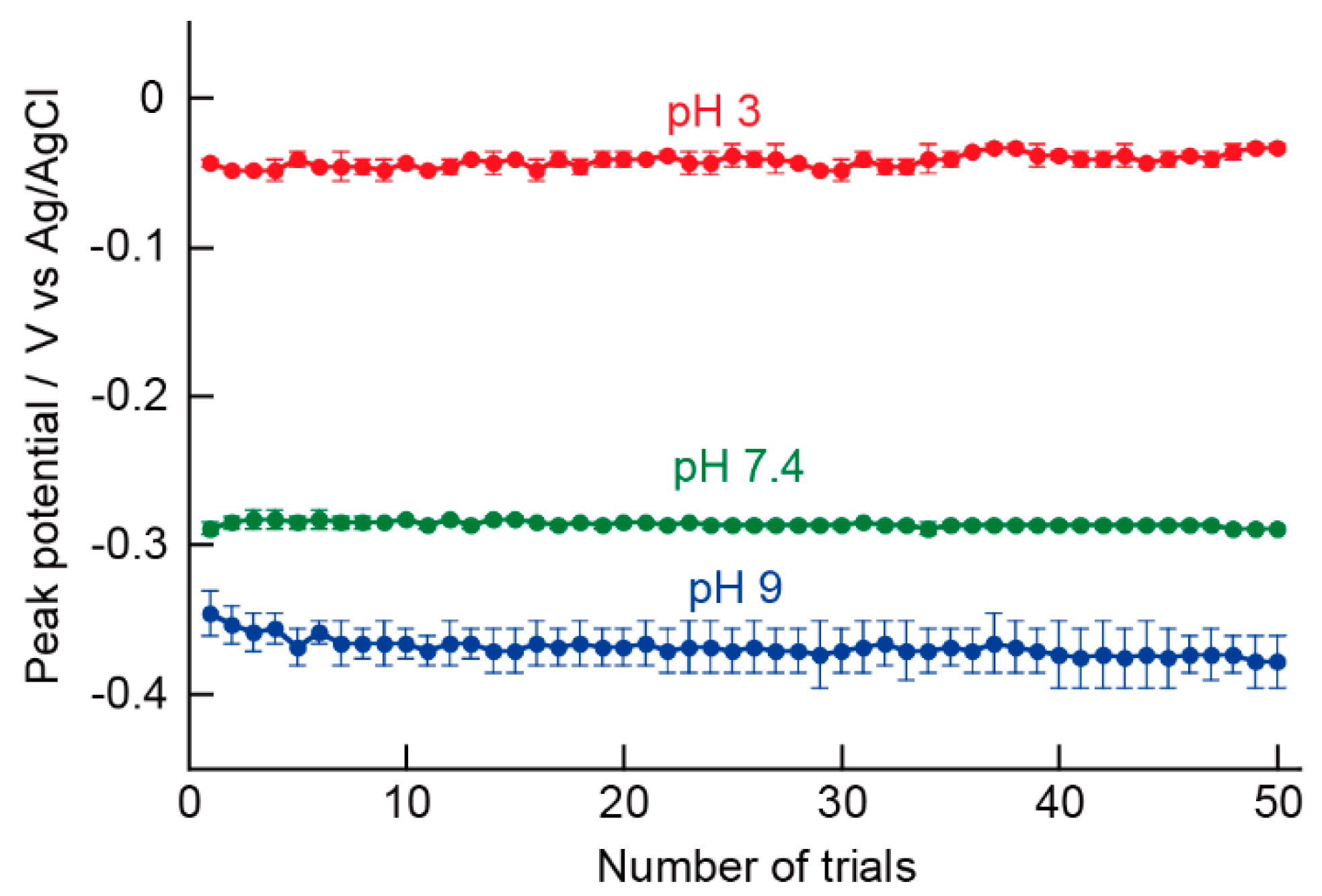
5.4. Repeated Measurements
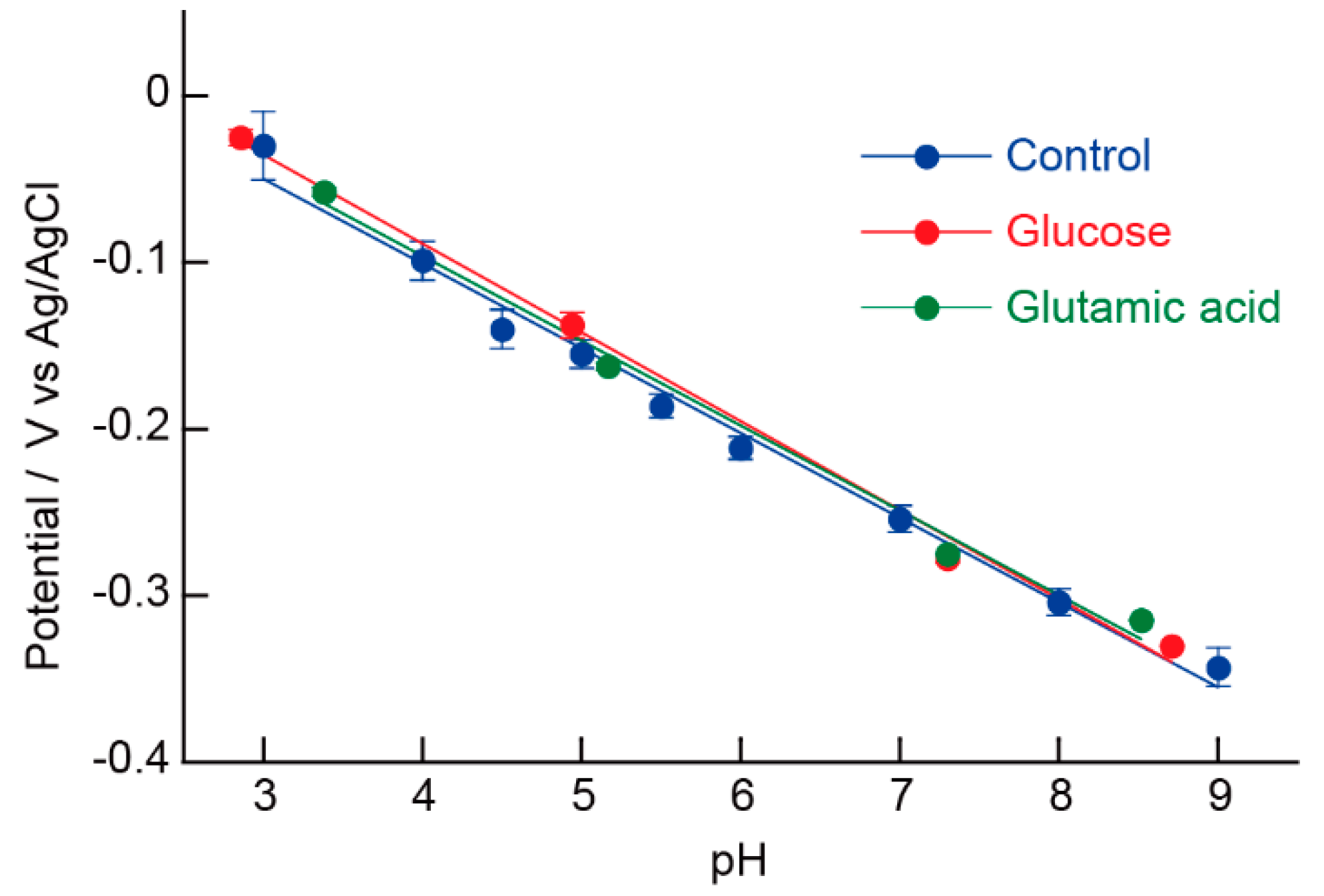
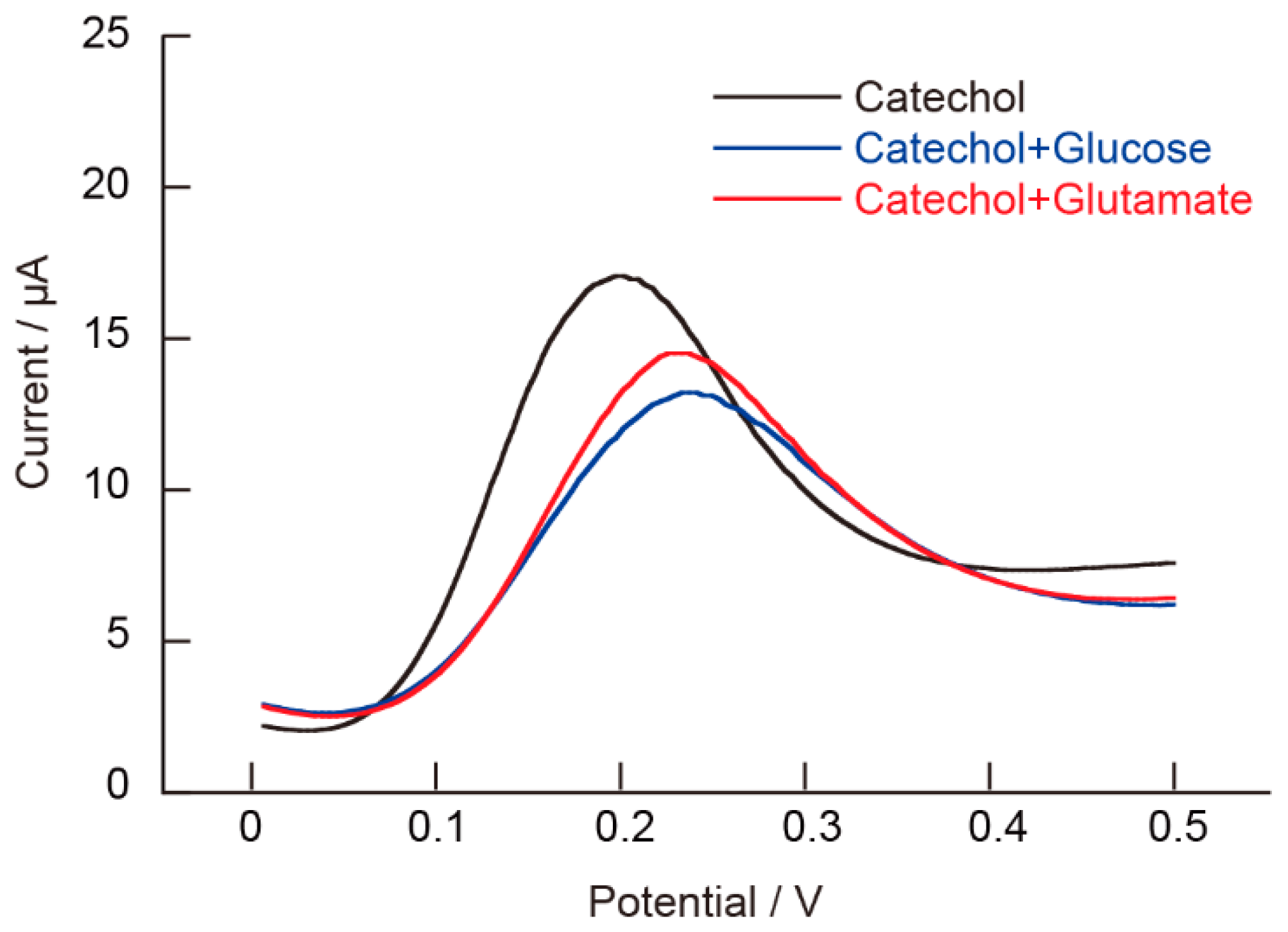
5.5. Influence of Additives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Das, B.P.; Tsianou, M. From polyelectrolyte complexes to polyelectrolyte multilayers: Electrostatic assembly, nanostructure, dynamics, and functional properties. Adv. Colloid. Interface. Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Matsusaki, M.; Akashi, M. Effectiveness of nanometer-sized extracellular matrix layer-by-layer assembled films for a cell membrane coating protecting cells from physical stress. Langmuir 2013, 29, 7362–7368. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ajiro, H.; Akashi, M. Hydrogen-bonded multilayer films based on poly(N-vinylamide) derivatives and tannic acid. Langmuir 2015, 31, 6863–6869. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Guan, Y.; Tan, S.; Xu, J.; Cheng, S.; Zhang, X. Water uptake behavior of hydrogen-bonded PVPON-PAA LbL film. Soft Matter 2006, 2, 699–704. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, A.; Midorikawa, H.; Kamijo, T.; Ono, T.; Dairaku, T.; Sato, T.; Fujimura, T.; Kashiwagi, Y.; Sato, K. Adsorption and release of rose bengal on layer-by-layer films of poly(vinyl alcohol) and poly(amidoamine) dendrimers bearing 4-carboxyphenylboronic acid. Polymers 2020, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
- Watahiki, R.; Sato, K.; Niina, S.; Suwa, K.; Egawa, Y.; Seki, T.; Anzai, J. Multilayer films composed of phenylboronic acid-modified dendrimers sensitive to glucose under physiological conditions. J. Mater. Chem. B. 2014, 2, 5809–5817. [Google Scholar] [CrossRef]
- Akiba, U.; Minaki, D.; Anzai, J. Host-guest chemistry in layer-by-layer assemblies containing calix[n]arenes and cucurbit[n]urils: A review. Polymers 2018, 10, 130. [Google Scholar] [CrossRef]
- Raposo, M.; Pontes, R.S.; Mattoso, L.H.C.; Oliveira, O.N., Jr. Kinetics of Adsorption of Poly(o-methoxyaniline) Self-Assemble Films. Macromolecules 1997, 30, 6095–6101. [Google Scholar] [CrossRef]
- Pontes, R.S.; Raposo, M.; Camilo, C.S.; Dhanabalan, A.; Ferreira, M.; Oliveira, O.N., Jr. Non-Equilibrium Adsorbed Polymer Layers via Hydrogen Bonding. Phys. Status. Solidi. A. 1999, 173, 41–50. [Google Scholar] [CrossRef]
- Raposo, M.; Oliveira, O.N., Jr. Energies of Adsorption of Poly(o-methoxyaniline) Layer-by-Layer Films. Langmuir 2000, 16, 2839–2844. [Google Scholar] [CrossRef]
- Straeten, A.V.; Bratek-Skicki, A.; Jonas, A.M.; Fustin, C.-A.; Dupont-Gillain, C. Integrating proteins in layer-by-layer assemblies independently of their electrical charge. ACS. Nano 2018, 12, 8372–8381. [Google Scholar] [CrossRef]
- Hashide, R.; Yoshida, K.; Hasebe, Y.; Seno, M.; Takahashi, S.; Sato, K.; Anzai, J. Poly(lactic acid) microparticles coated with insulin-containing layer-by-layer films and their pH-dependent insulin release. J. Nanosci. Nanotechnol. 2014, 14, 3100–3105. [Google Scholar] [CrossRef]
- Xia, L.; Long, Y.; Li, D.; Huang, L.; Wang, Y.; Dai, F.; Tao, F.; Cheng, Y.; Deng, H. LBL deposition of chitosan and silk fibroin on nanofibers for improving physical and biological performance of patches. Int. J. Biol. Macromol. 2019, 130, 348–356. [Google Scholar] [CrossRef]
- Bhalerao, U.M.; Valiveti, A.K.; Acharya, J.; Halve, A.K.; Kaushik, M.P. Controlled release studies of antimalarial 1, 3, 5-trisubstituted-2-pyrazolines from biocompatible chitosan-heparin Layer-by-Layer (LbL) self assembled thin films. Colloids Surf. B. 2015, 125, 151–159. [Google Scholar] [CrossRef]
- Fang, R.; Zhang, H.; Yang, L.; Wang, H.; Tian, Y.; Zhang, X.; Jiang, L. Supramolecular self-assembly induced adjustable multiple gating states of nanofluidic diodes. J. Am. Chem. Soc. 2016, 138, 16372–16379. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, L.; Shen, Q.; Nie, Z.; Guo, M.; Yao, S. Shouzhuo Layer-by-layer assembly of polyelectrolyte and nanoparticles, monitored by capillary electrophoresis. Chemistry 2009, 15, 12828–12836. [Google Scholar] [CrossRef]
- Wang, B.; Yoshida, K.; Sato, K.; Anzai, J.-I. Phenylboronic acid-functionalized layer-by-layer assemblies for biomedical applications. Polymers 2017, 9, 202. [Google Scholar] [CrossRef]
- Silva, J.M.; Caridade, S.G.; Costa, R.R.; Alves, N.M.; Groth, T.; Picart, C.; Reis, R.L.; Mano, J.F. pH responsiveness of multilayered films and membranes made of polysaccharides. Langmuir 2015, 31, 11318–11328. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sato, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y. Preparation of nafion/polycation layer-by-layer films for adsorption and release of insulin. Polymers 2018, 10, 812. [Google Scholar] [CrossRef]
- Schmidt, D.J.; Moskowitz, J.S.; Hammond, P.T. Electrically triggered release of a small molecule drug from a polyelectrolyte multilayer coating. Chem. Mater. 2010, 22, 6416–6425. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J. H2O2-induced decomposition of layer-by-layer films consisting of phenylboronic acid-bearing poly(allylamine) and Ppoly(vinyl alcohol). Langmuir 2014, 30, 9247–9250. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J. Glucose-induced decomposition of layer-by-layer films composed of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol) under physiological conditions. J. Mater. Chem. B. 2015, 3, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shimizu, S.; Awaji, K.; Hitomi, O.; Anzai, J. Lactate-induced decomposition of layer-by-layer films composed of phenylboronic acid-modified poly(allylamine) and poly(vinyl alcohol) under extracellular tumor conditions. J. Colloid Interface Sci. 2018, 510, 302–307. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, C.; Van der Bruggen, B. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, L.; Carroll, S.; Muniz, M.; Gibson, H.; Wei, W.Z.; Liu, H.; Mao, G. Layer-by-layer films with bioreducible and nonbioreducible polycations for sequential DNA release. Biomacromolecules 2014, 15, 3965–3975. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Layer-by-Layer Nano-assembly: A Powerful Tool for Optical Fiber Sensing Applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef]
- Dhanjai; Yu, N.; Mugo, S.M. A flexible-imprinted capacitive sensor for rapid detection of adrenaline. Talanta 2019, 204, 602–606. [Google Scholar]
- Sato, K.; Yoshida, K.; Takahashi, S.; Anzai, J.-I. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Kastania, G.; Volodkin, D. Encapsulation of low-molecular-weight drugs into polymer multilayer capsules templated on vaterite CaCO3 Crystals. Micromachines 2020, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Guzmána, E.; Mateos-Marotoa, A.; Ruanob, M.; Ortegaa, F.; Rubioac, R.G. Layer-by-layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid. Interface. Sci. 2017, 249, 290–307. [Google Scholar]
- Jing, H.; Das, S. Electric double layer electrostatics of lipid-bilayer-encapsulated nanoparticles: Toward a better understanding of protocell electrostatics. Electrophoresis 2018, 39, 752–759. [Google Scholar] [CrossRef]
- Schiebe, M.; Jaeger, U. Sampling membrane potential, membrane resistance and electrode resistance with a glass electrode impaled into a single cell. J. Neurosci. Methods 1980, 2, 191–202. [Google Scholar] [CrossRef]
- Velmurugan, J.; Zhan, D.; Mirkin, M.V. Electrochemistry through glass. Nat. Chem. 2010, 2, 498–502. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.K.; Kim, M. Ion-sensitive field-effect transistor for biological sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef]
- Yang, H.; Sakata, T. Molecular-charge-contact-based ion-sensitive field-effect transistor sensor in microfluidic system for protein sensing. Sensors 2019, 19, 3393. [Google Scholar] [CrossRef]
- Eisner, D.A.; Kenning, N.A.; O Neill, S.C.; Pocock, G.; Richards, C.D.; Valdeolmillos, M. A novel method for absolute calibration of intracellular pH indicators. Pflugers. Arch. 1989, 413, 553–558. [Google Scholar] [CrossRef]
- Ogoh, K.; Kinebuchi, T.; Murai, M.; Takahashi, T.; Ohmiya, Y.; Suzuki, H. Dual-color-emitting green fluorescent protein from the sea cactus Cavernularia obesa and its use as a pH indicator for fluorescence microscopy. Luminescence 2013, 28, 582–591. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Zhu, A.; Shi, G.; Tian, Y. In vivo monitoring of local pH values in a live rat brain based on the design of a specific electroactive molecule for H+. Chem. Commun. 2016, 52, 3717–3720. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Tian, Y. Micro Electrochemical pH Sensor Applicable for Real-Time Ratiometric Monitoring of pH Values in Rat Brains. Anal. Chem. 2016, 88, 2113–2118. [Google Scholar] [CrossRef]
- Amiri, M.; Amali, E.; Nematollahzadeh, A.; Salehniya, H. Poly-dopamine films: Voltammetric sensor for pH monitoring. Sens. Actuators B Chem. 2016, 228, 53–58. [Google Scholar] [CrossRef]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Sagara, T.; Iizuka, J.; Niki, K. Electroreflectance study of the redox reaction of thylene blue adsorbed on a pyrolytic graphite electrode. Langmuir 1992, 8, 1018–1025. [Google Scholar] [CrossRef]
- Leventis, N.; Chen, M. Electrochemically assisted sol-gel process for the synthesis of polysiloxane films incorporating phenothiazine dyes analogous to methylene blue. Structure and ion-transport properties of the films via spectroscopic and electrochemical characterization. Chem. Mater. 1997, 9, 2621–2631. [Google Scholar] [CrossRef]
- Ning, L.; Li, X.; Yang, D.; Miao, P.; Ye, Z.; Li, G. Measurement of intracellular pH changes based on DNA-templated capsid protein nanotubes. Anal. Chem. 2014, 86, 8042–8047. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Oguchi, T.; Watanabe, K.; Abe, H.; Kanno, S.; Ishikawa, M.; Katoh, T. Highly efficient total synthesis of the marine natural products (+)-avarone, (+)-avarol, (-)-Neoavarone, (-)-neoavarol, and (+)-aureol. Chem. Eur. J. 2008, 14, 829–837. [Google Scholar] [CrossRef]
- Oguchi, T.; Watanabe, K.; Ohkubo, K.; Abe, H.; Katoh, T. Enantioselective total synthesis of (-)-candelalides A, B and C: Potential Kv1.3 blocking immunosuppressive agents. Chem. Eur. J. 2009, 15, 2826–2845. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sugizaki, T.; Tozawa, Y.; Katoh, T. A new entry to the synthesis of primin via a B-alkyl Suzuki-Miyaura cross-coupling reaction. Heterocycles 2012, 86, 985–989. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Sugiyama, K.; Komatsu, S.; Yoshida, K.; Ono, T.; Fujimura, T.; Kashiwagi, Y.; Sato, K. Voltammetric pH Measurements Using Azure A-Containing Layer-by-Layer Film Immobilized Electrodes. Polymers 2020, 12, 2328. https://doi.org/10.3390/polym12102328
Watanabe K, Sugiyama K, Komatsu S, Yoshida K, Ono T, Fujimura T, Kashiwagi Y, Sato K. Voltammetric pH Measurements Using Azure A-Containing Layer-by-Layer Film Immobilized Electrodes. Polymers. 2020; 12(10):2328. https://doi.org/10.3390/polym12102328
Chicago/Turabian StyleWatanabe, Kazuhiro, Kyoko Sugiyama, Sachiko Komatsu, Kentaro Yoshida, Tetsuya Ono, Tsutomu Fujimura, Yoshitomo Kashiwagi, and Katsuhiko Sato. 2020. "Voltammetric pH Measurements Using Azure A-Containing Layer-by-Layer Film Immobilized Electrodes" Polymers 12, no. 10: 2328. https://doi.org/10.3390/polym12102328
APA StyleWatanabe, K., Sugiyama, K., Komatsu, S., Yoshida, K., Ono, T., Fujimura, T., Kashiwagi, Y., & Sato, K. (2020). Voltammetric pH Measurements Using Azure A-Containing Layer-by-Layer Film Immobilized Electrodes. Polymers, 12(10), 2328. https://doi.org/10.3390/polym12102328






