Vitrimer-Like Shape Memory Polymers: Characterization and Applications in Reshaping and Manufacturing
Abstract
:1. Introduction
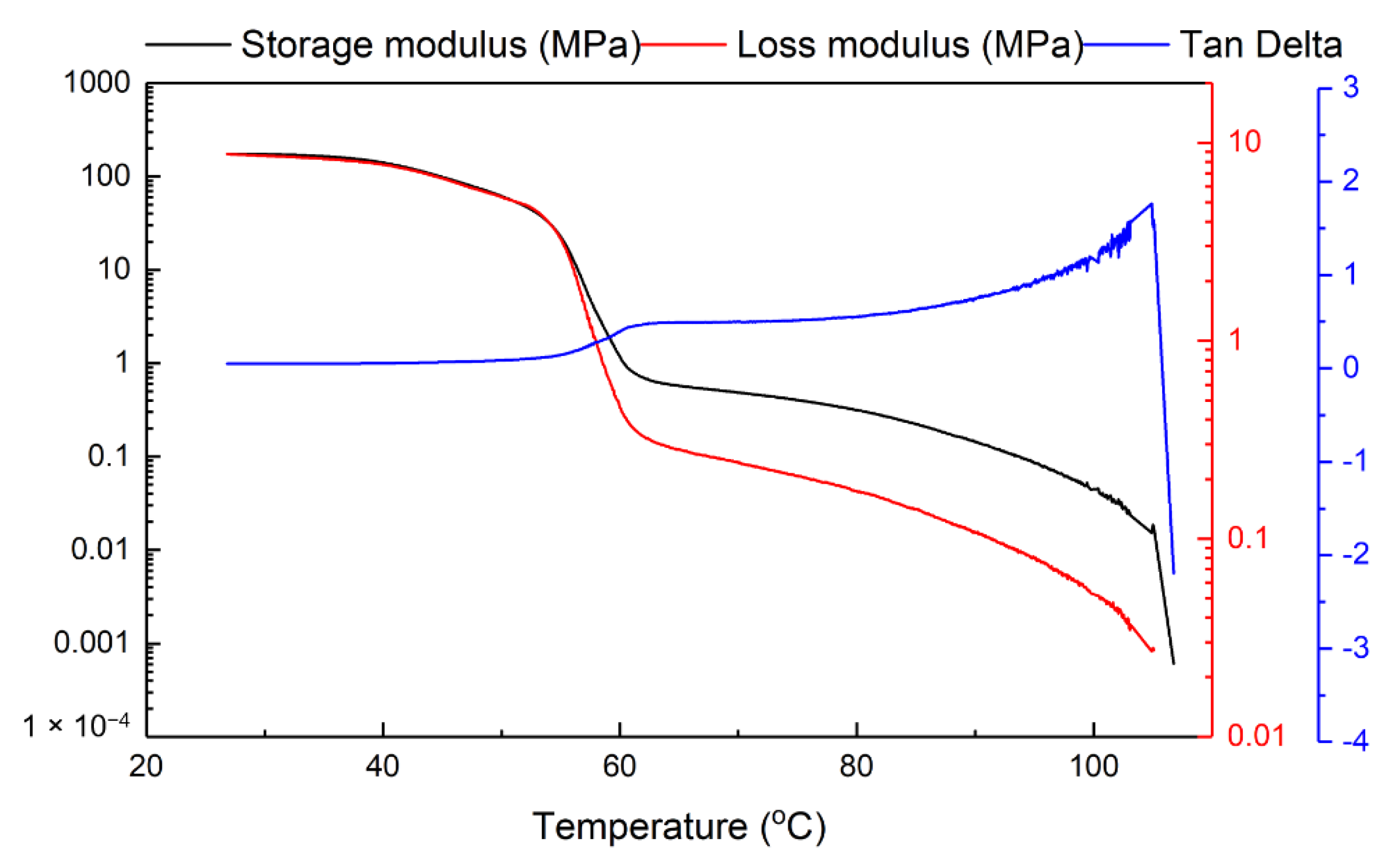
2. Characterization of Vitrimer-Like Behavior via SME
2.1. Material and Sample Preparation
2.2. Characterization
- (1)
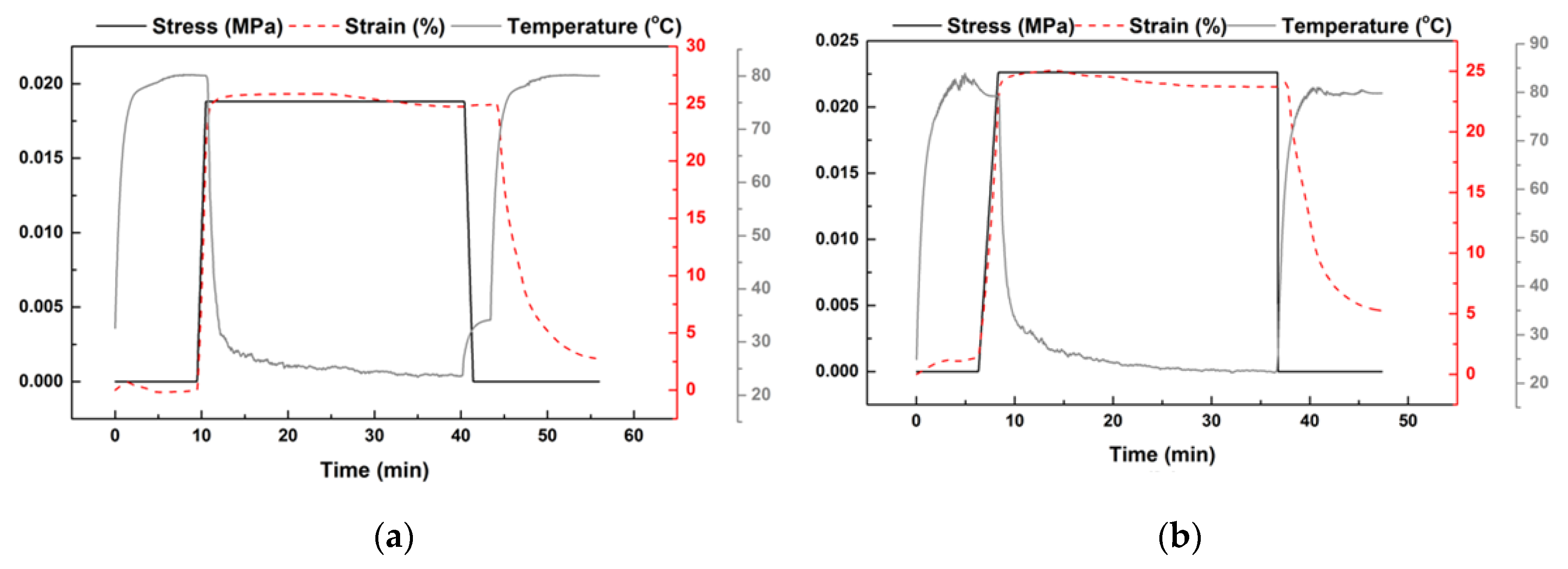
- Heat the sample to a prescribed pre-heating temperature (Tp). Three pre-heating temperatures (namely, 75 °C, 90 °C, and 120 °C) were applied in the course of this study.
- (2)
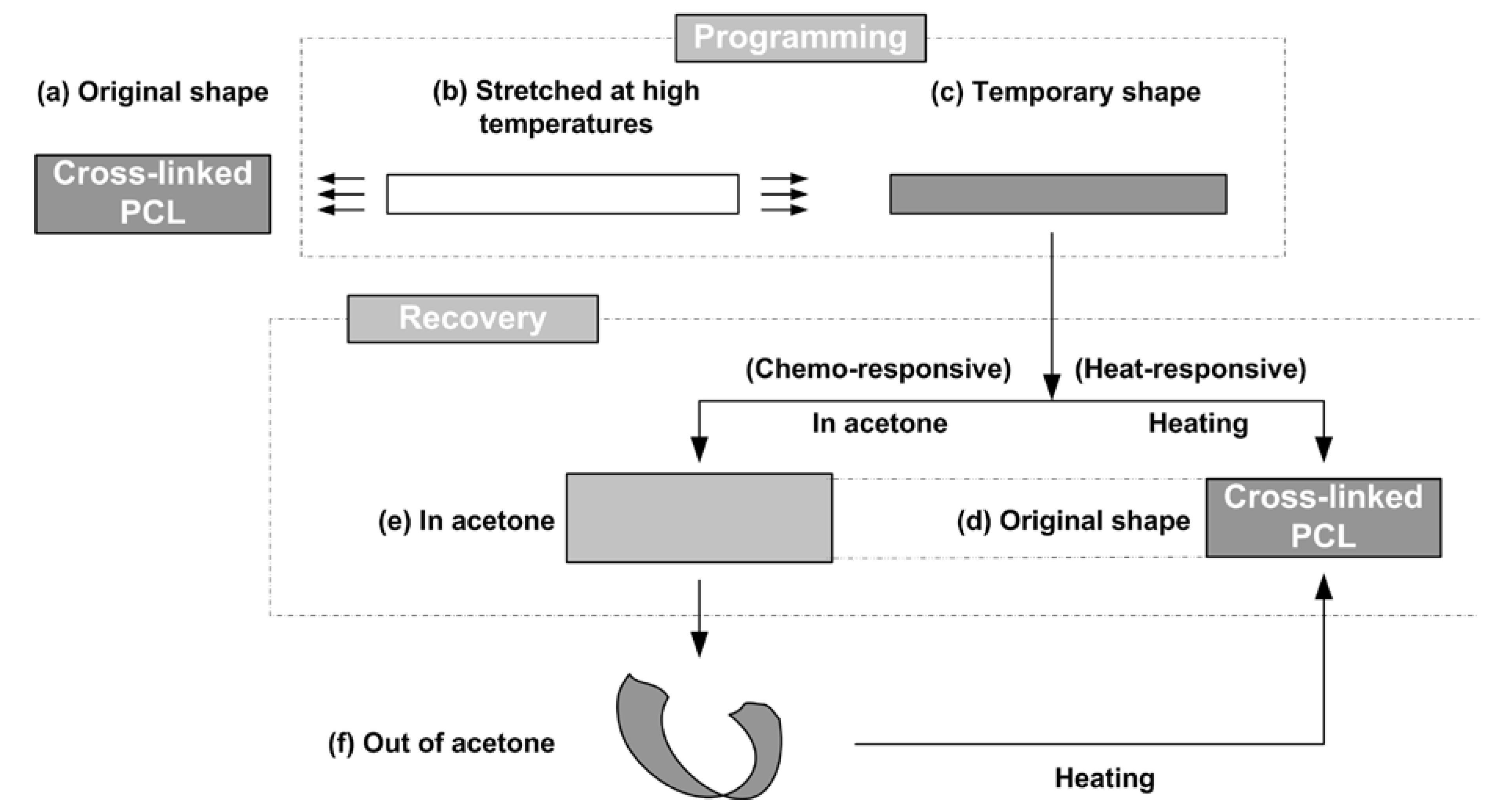
- Cool the sample to a predetermined programming temperature, Td (≤Tp), and then gently and slowly stretch it by hands so that the strain between the two marked dots reaches around 100%. Three programming temperatures (namely, 50 °C, 75 °C, and 90 °C) were applied. According to Figure 2, these three temperatures are all well above the crystallization temperature of this material.
- (3)
- Further cool the sample back to room temperature for full crystallization.
- (4)
- Heat the sample in a step-by-step manner to five reheating temperatures (Tr) from 80 °C, 90 °C, 100 °C, 110 °C, to finally 120 °C. The corresponding shape recovery ratios after each heating are plotted in Figure 8. Legend in Figure 8 is in (Tp:Td) format to indicate the actual thermal history before final step-by-step heating of a sample.
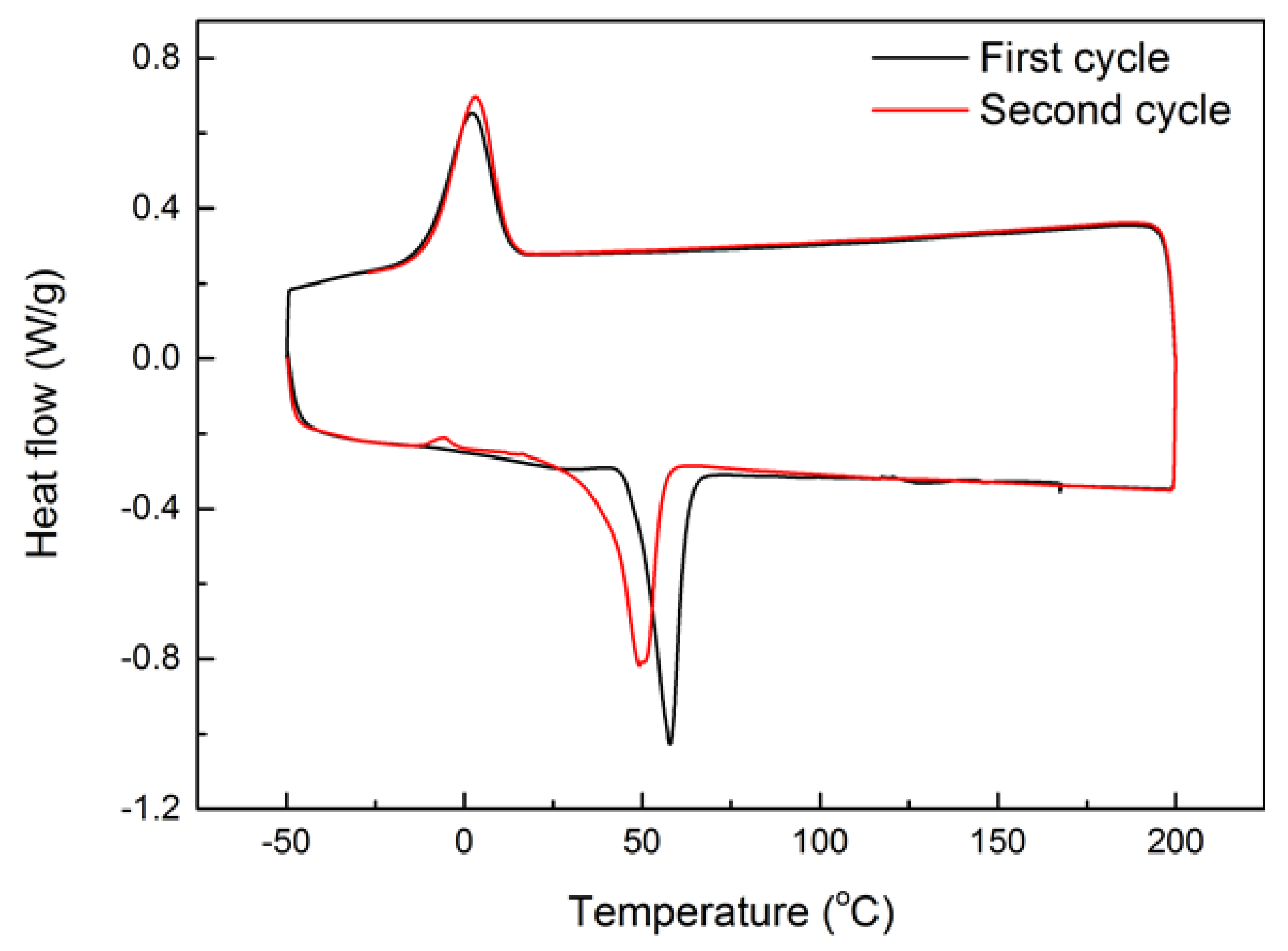
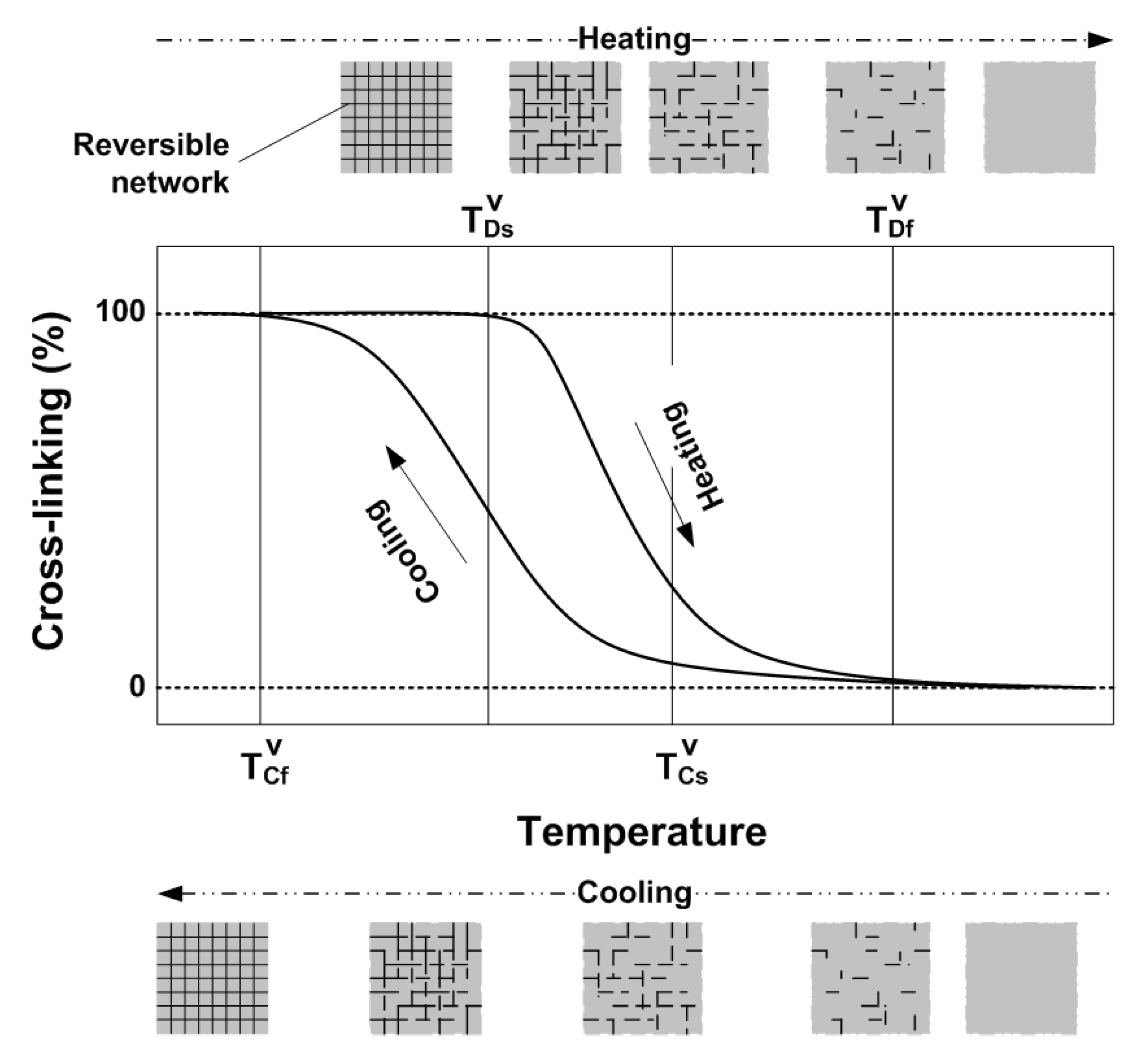
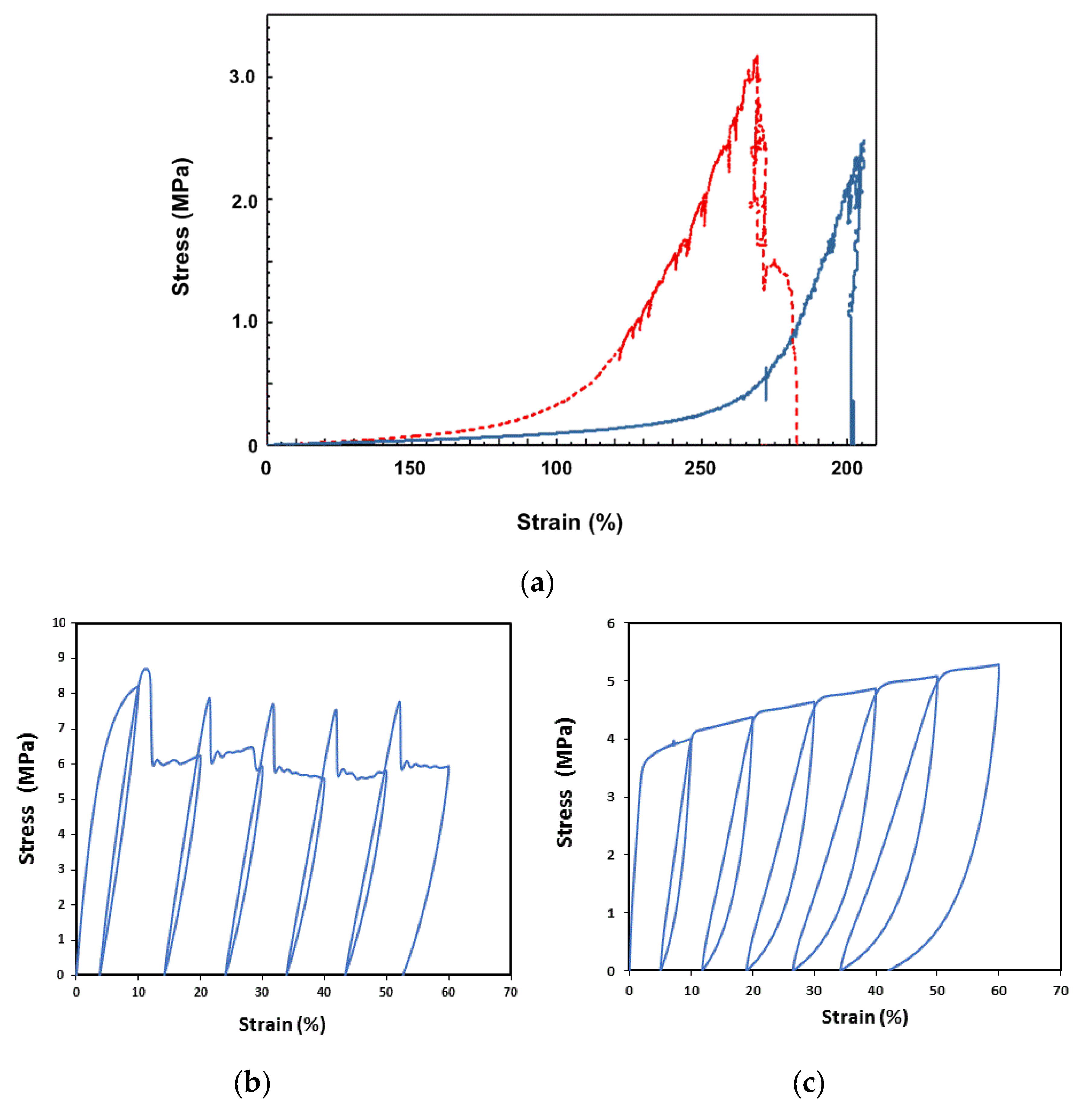
2.3. Vitrimer-Like Behavior
3. Applications of Vitrimer-Like SMPs and Their Composites
3.1. Superimposing of Permanent and Temporary Shapes
3.2. Heat-Assisted Healing without Altering Surface Feature
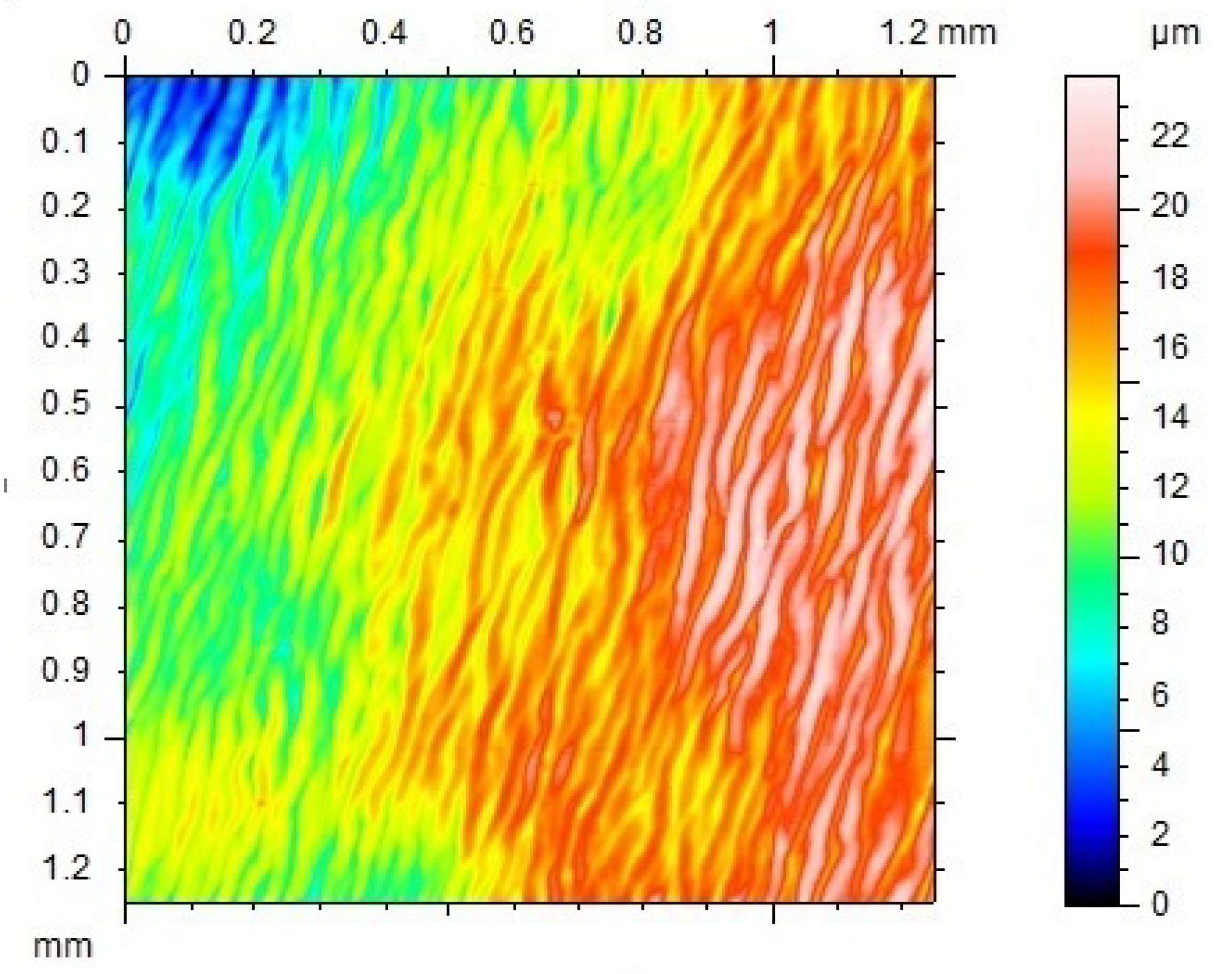
3.3. From 2D-Shape to Surface Wrinkling/3D-Shape
3.4. Vitrimer Composites
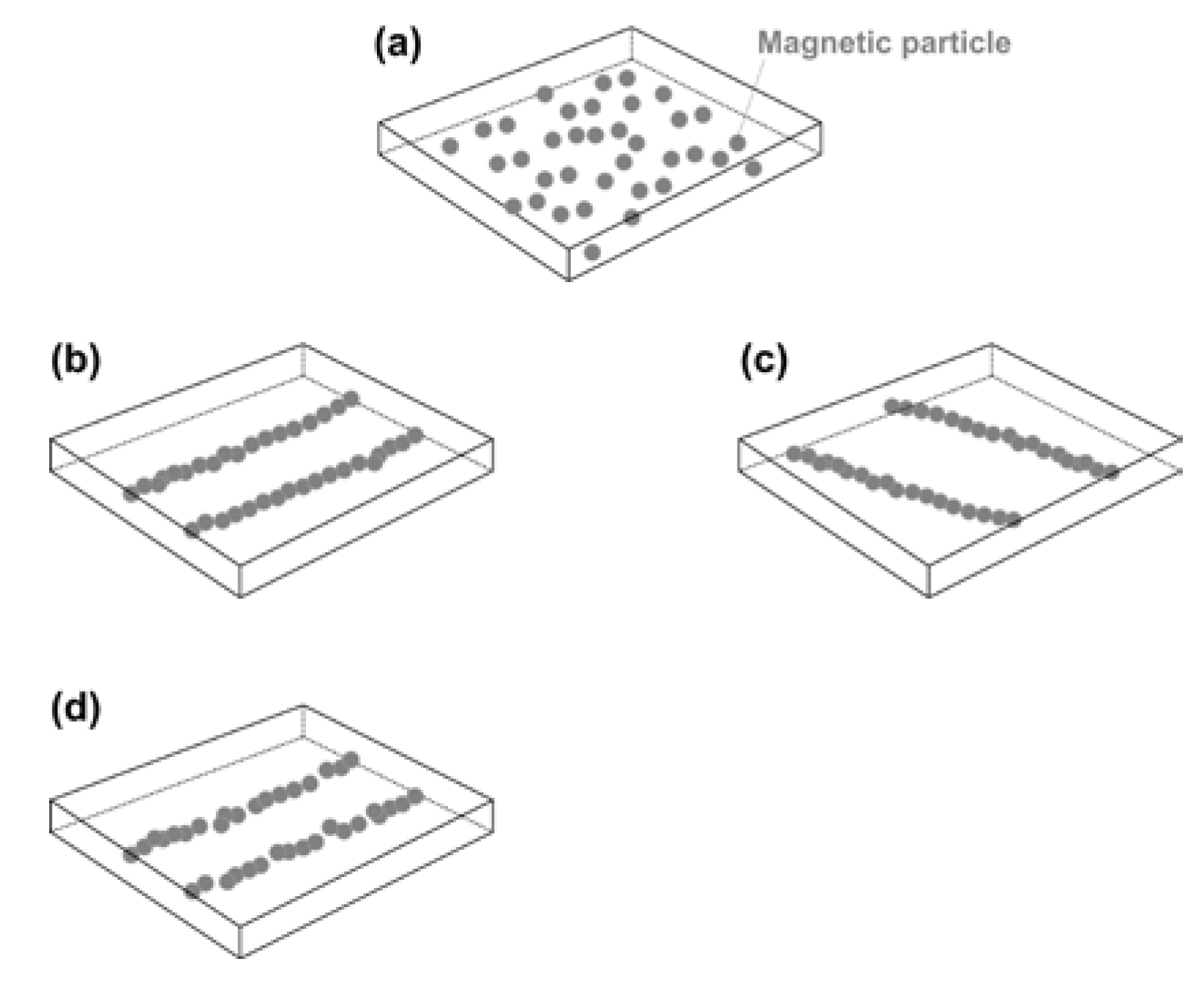
3.4.1. Formation and Realignment/Healing of Embedded Magnetic Particle Chains
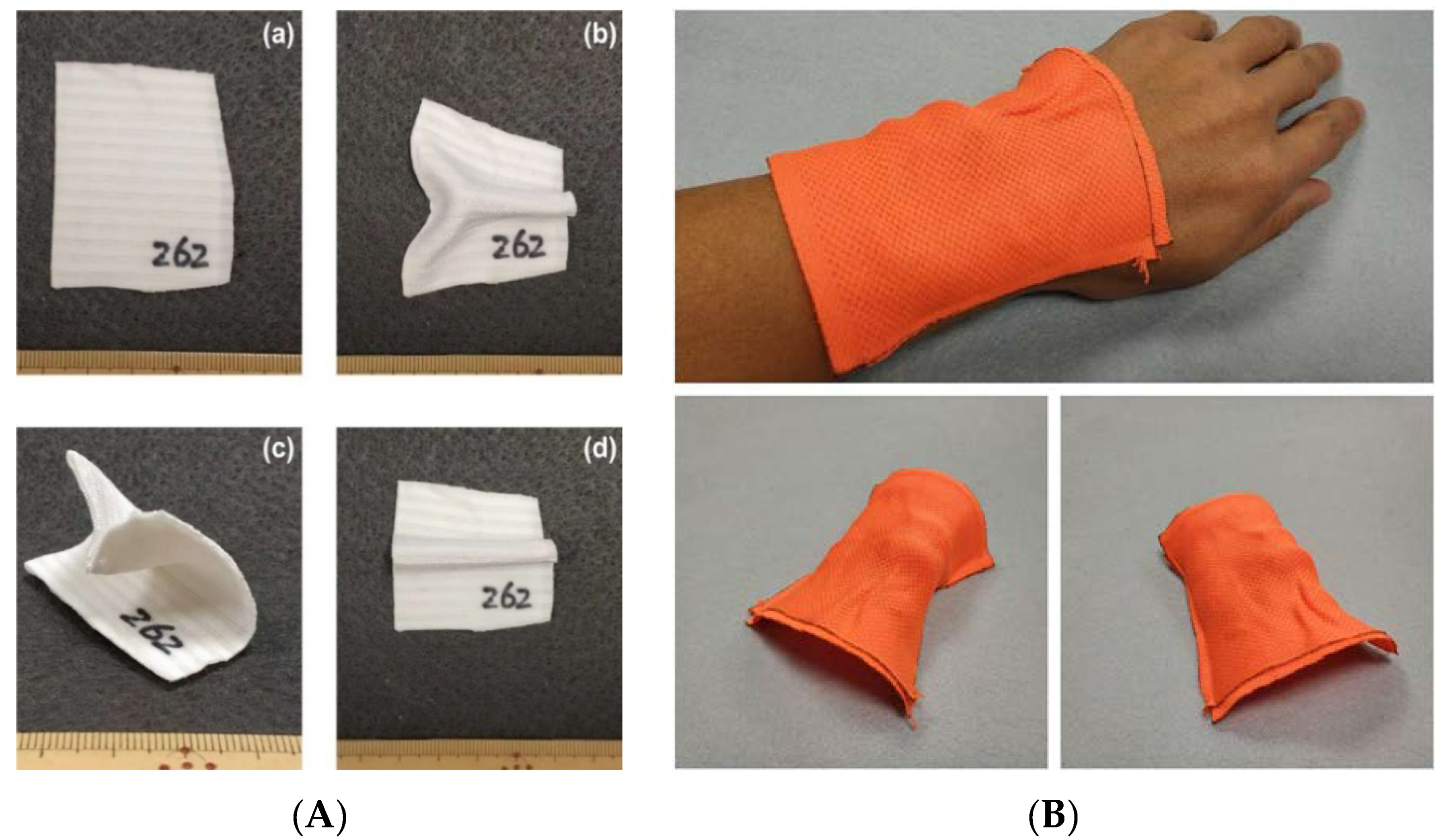
3.4.2. Rapid Permanent/Temporary Reshaping with Reinforcement Layer in the Middle
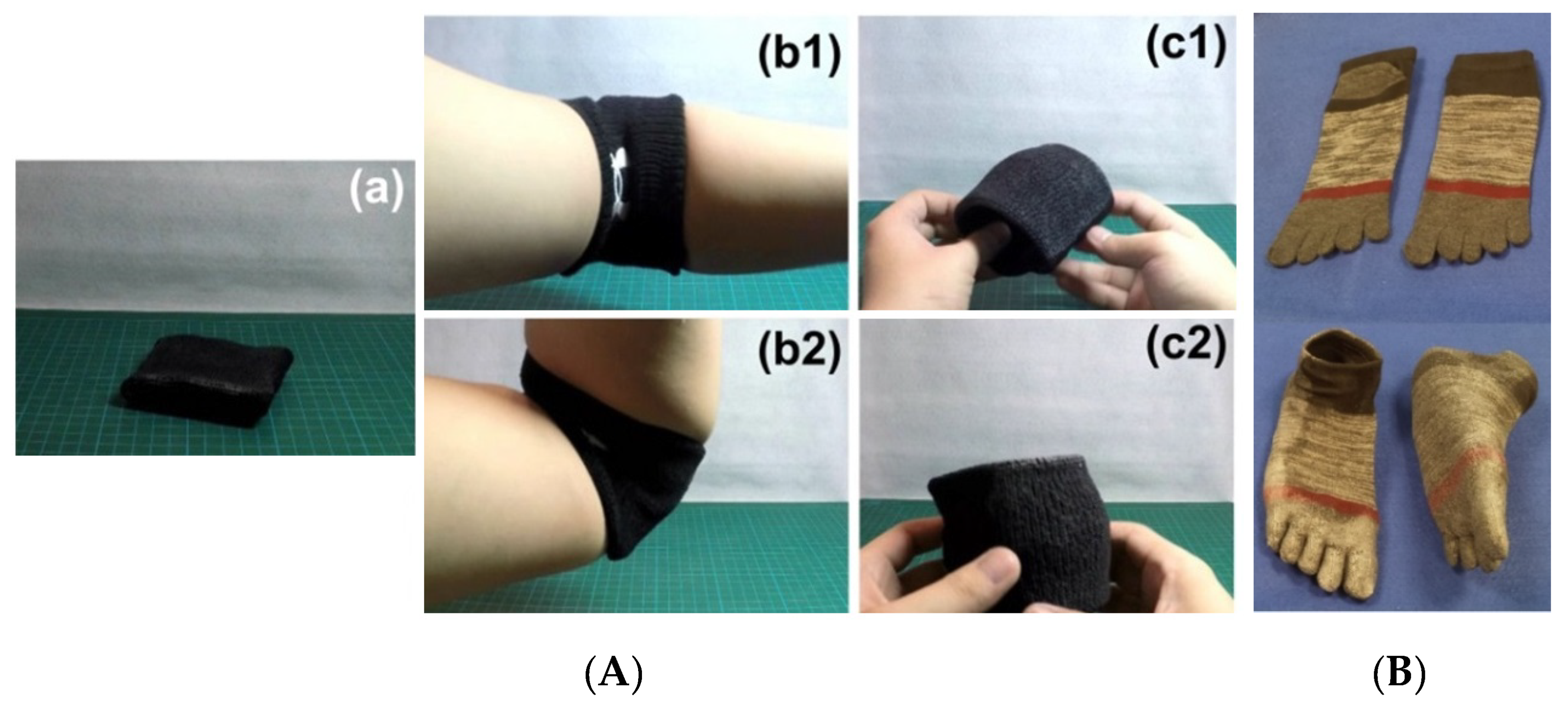
3.4.3. Comfort Fitting of Wearable Items around Body-Temperature
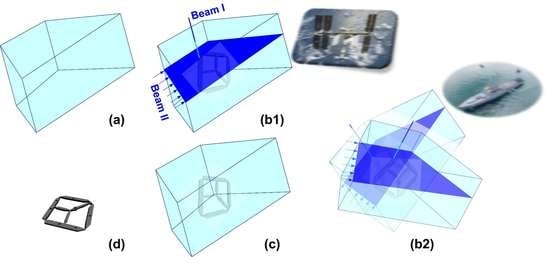
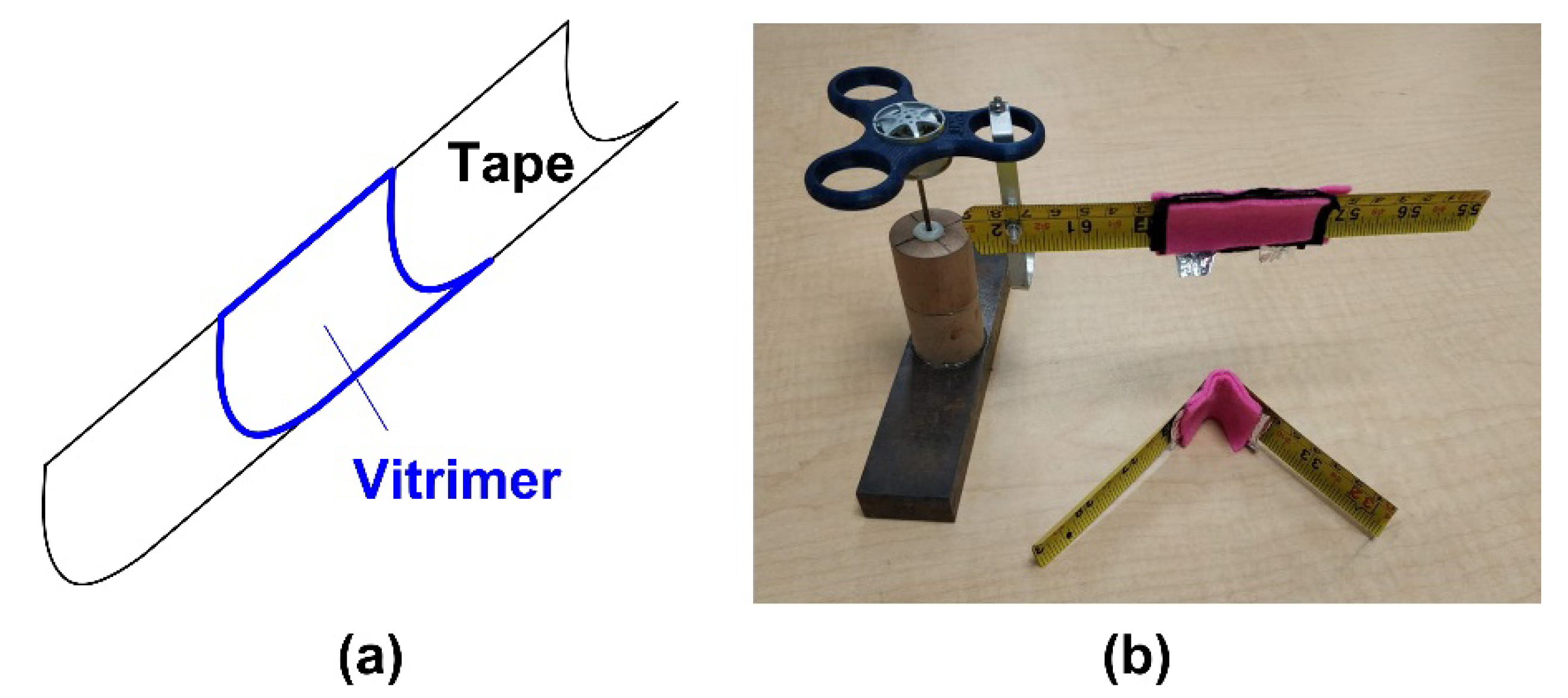
3.4.4. Controlled Unfolding to Minimize Impact
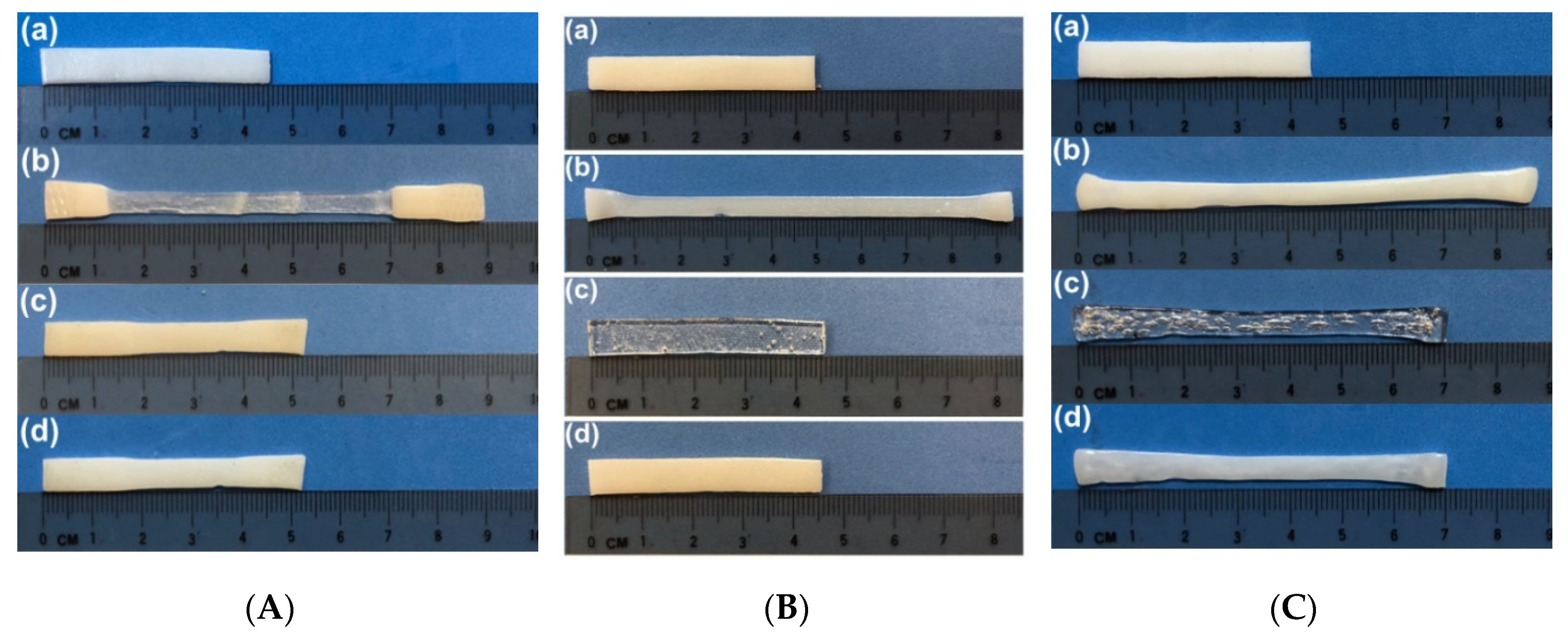
3.5. Additional Permanent Cross-Linking
3.5.1. Reconfigurable Two-Way Actuation
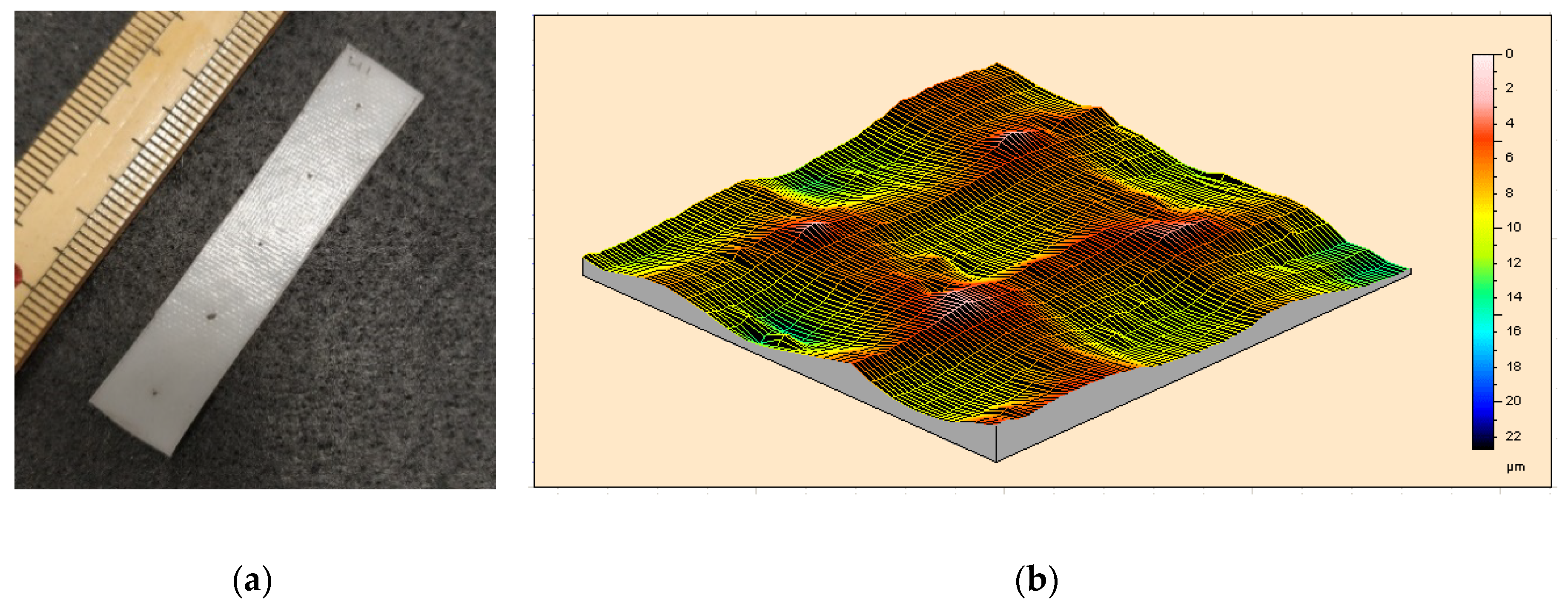
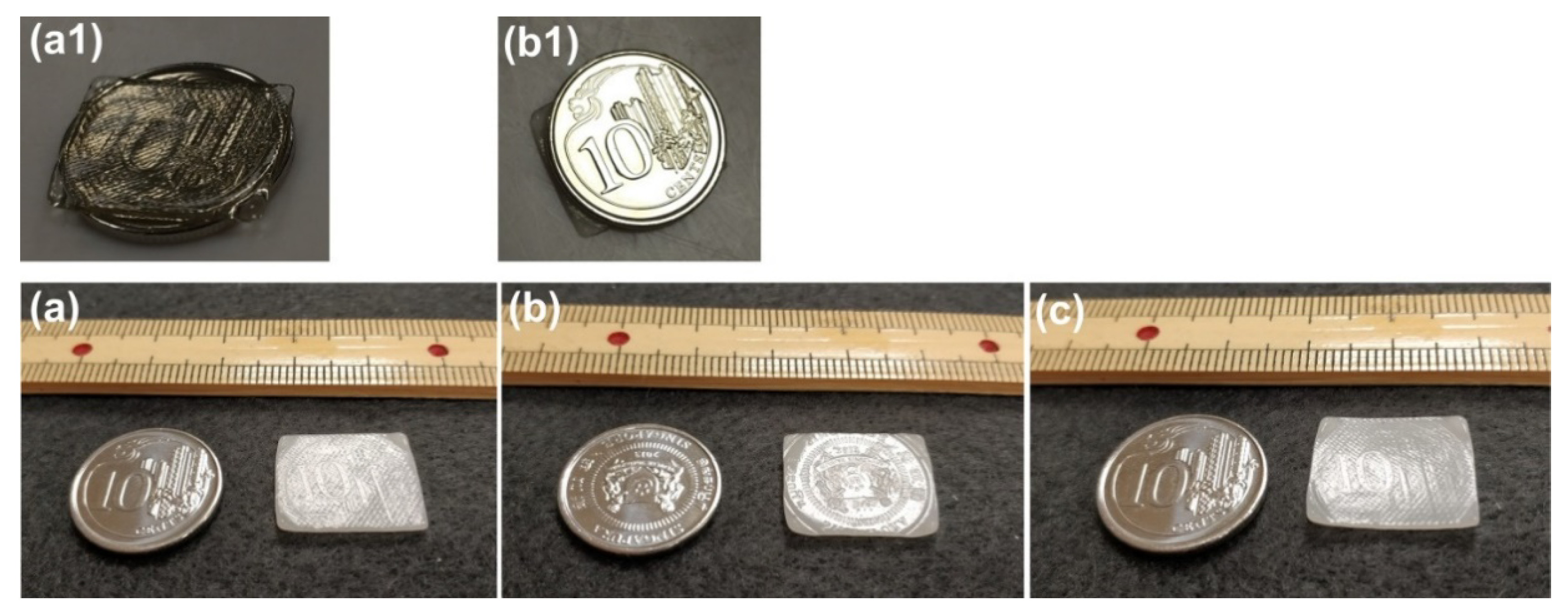
3.5.2. Rapid Additive Manufacturing in Solid State
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salvekar, A.V.; Zhou, Y.; Huang, W.M.; Wong, Y.S.; Venkatraman, S.S.; Shen, Z.; Zhu, G.; Cui, H.P. Shape/temperature memory phenomena in un-crosslinked poly-ɛ-caprolactone (PCL). Eur. Polym. J. 2015, 72, 282–295. [Google Scholar] [CrossRef]
- Otsuka, K.; Wayman, C.M. Shape memory materials; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Huang, W.M.; Ding, Z.; Wang, C.C.; Wei, J.; Zhao, Y.; Purnawali, H. Shape memory materials. Mater. Today 2010, 13, 54–61. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandström, R.; Miyazaki, S. Shape memory materials and hybrid composites for smart systems—Part II Shape-memory hybrid composites. J. Mater. Sci. 1998, 33, 3763–3783. [Google Scholar] [CrossRef]
- Lu, H.; Du, S. A phenomenological thermodynamic model for the chemo-responsive shape memory effect in polymers based on Flory–Huggins solution theory. Polym. Chem. 2014, 5, 1155–1162. [Google Scholar] [CrossRef]
- Huang, W.M.; Lu, H.B.; Zhao, Y.; Ding, Z.; Wang, C.C.; Zhang, J.L.; Sun, L.; Fu, J.; Gao, X.Y. Instability/collapse of polymeric materials and their structures in stimulus-induced shape/surface morphology switching. Mater. Des. 2014, 59, 176–192. [Google Scholar] [CrossRef]
- Zhu, G.M.; Xu, S.G.; Wang, J.H.; Zhang, L.B. Shape memory behaviour of radiation-crosslinked PCL/PMVS blends. Radiat. Phys. Chem. 2006, 75, 443–448. [Google Scholar] [CrossRef]
- Wang, L.; Di, S.; Wang, W.; Chen, H.-M.; Yang, X.; Gong, T.; Zhou, S. Tunable temperature memory effect of photo-cross-linked star PCL–PEG networks. Macromolecules 2014, 47, 1828–1836. [Google Scholar] [CrossRef]
- Chern, M.-J.; Yang, L.-Y.; Shen, Y.-K.; Hung, J.-H. 3D scaffold with PCL combined biomedical ceramic materials for bone tissue regeneration. Int. J. Precis. Eng. Manuf. 2013, 14, 2201–2207. [Google Scholar] [CrossRef]
- Inoue, K.; Yamashiro, M.; Iji, M. Recyclable shape-memory polymer: Poly (lactic acid) crosslinked by a thermoreversible Diels–Alder reaction. J. Appl. Polym. Sci. 2009, 112, 876–885. [Google Scholar] [CrossRef]
- Ahmad, M.; Luo, J.; Purnawali, H.; Huang, W.M.; King, P.J.; Chalker, P.; Mireftab, M.; Geng, J. Making shape memory polymers reprocessable and reusable by a simple chemical method. J. Mater. Chem. 2012, 22, 8192–8194. [Google Scholar] [CrossRef]
- Huang, W.M.; Zhao, Y.; Wang, C.C.; Ding, Z.; Purnawali, H.; Tang, C.; Zhang, J.L. Thermo/chemo-responsive shape memory effect in polymers: A sketch of working mechanisms, fundamentals and optimization. J. Polym. Res. 2012, 19, 9952. [Google Scholar] [CrossRef]
- Lendlein, A. Shape-Memory Polymers; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Sun, L.; Huang, W.M.; Wang, C.C.; Zhao, Y.; Ding, Z.; Purnawali, H. Optimization of the shape memory effect in shape memory polymers. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3574–3581. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M. Mechanisms of the multi-shape memory effect and temperature memory effect in shape memory polymers. Soft Matter 2010, 6, 4403–4406. [Google Scholar] [CrossRef]
- Dai, L.; Tian, C.; Xiao, R. Modeling the thermo-mechanical behavior and constrained recovery performance of cold-programmed amorphous shape-memory polymers. Int. J. Plast. 2020, 127, 102654. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Kaiser, S.; Wurzer, S.; Pilz, G.; Kern, W.; Schlögl, S. Stress relaxation and thermally adaptable properties in vitrimer-like elastomers from HXNBR rubber with covalent bonds. Soft Matter 2019, 15, 6062–6072. [Google Scholar] [CrossRef]
- Jurowska, A.; Jurowski, K. Vitrimers-the miracle polymer materials combining the properties of glass and plastic? Chemik 2015, 69, 392–394. [Google Scholar]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, G.; Zheng, N.; Zhao, Q.; Xie, T. A metallosupramolecular shape-memory polymer with gradient thermal plasticity. Angew. Chem. Int. Ed. 2017, 56, 12599–12602. [Google Scholar] [CrossRef]
- Zhao, Q.; Zou, W.; Luo, Y.; Xie, T. Shape memory polymer network with thermally distinct elasticity and plasticity. Sci. Adv. 2016, 2, e1501297. [Google Scholar] [CrossRef] [Green Version]
- Kasemsiri, P.; Lorwanishpaisarn, N.; Pongsa, U.; Ando, S. Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes. Polymers 2018, 10, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Z.; Yang, Y.; Chen, Q.; Wei, Y.; Ji, Y. Regional shape control of strategically assembled multishape memory vitrimers. Adv. Mater. 2015, 28, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, J.; Wen, D.; Yuan, B.; Gong, P.; Liu, J.; Chen, X. Fabrication of remote controllable devices with multistage responsiveness based on NIR light-induced shape memory ionomer containing various bridge ions. J. Mater. Chem. A 2019, 7, 20723–20732. [Google Scholar] [CrossRef]
- Lei, H.; Wang, S.; Liaw, D.-J.; Cheng, Y.; Yang, X.; Tan, J.; Chen, X.; Gu, J.; Zhang, Y. Tunable and processable shape-memory materials based on solvent-free, catalyst-free polycondensation between formaldehyde and diamine at room temperature. ACS Macro Lett. 2019, 2019, 582–587. [Google Scholar] [CrossRef]
- Yang, Y.; Terentjev, E.M.; Wei, Y.; Ji, Y. Solvent-assisted programming of flat polymer sheets into reconfigurable and self-healing 3D structures. Nat. Commun. 2018, 9, 1906. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yu, X.; Pei, Z.; Yang, Y.; Wei, Y.; Ji, Y. Multi-stimuli responsive and multi-functional oligoaniline-modified vitrimers. Chem. Sci. 2017, 8, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Zhao, W.; Fu, X.; Liu, Z.; Kong, W.; Zhou, C.; Lei, J. Multifunctional polyurethane-vitrimers completely based on transcarbamoylation of carbamates: Thermally-induced dual-shape memory effect and self-welding. RSC Adv. 2017, 7, 26858–26866. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Zhao, W.; Jiang, L.; Wu, B.; Hu, K.; Yuan, Y.; Lei, J. Reconfiguration and shape memory triggered by heat and light of carbon nanotube–polyurethane vitrimer composites. J. Appl. Polym. Sci. 2017, 135, 45784. [Google Scholar] [CrossRef]
- Zhang, B.; Kowsari, K.; Serjouei, A.; Dunn, M.L.; Ge, Q. Reprocessable thermosets for sustainable three-dimensional printing. Nat. Commun. 2018, 9, 1831. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Jones, G.; Virwani, K.; McCloskey, B.; Boday, D.; ter Huurne, G.M.; Horn, H.W.; Coady, D.J.; Bintaleb, A.M.; Alabdulrahman, A.M.S.; et al. Recyclable, strong thermodest and organogels via paraformaldehyde condensation with diamines. Science 2014, 344, 732–735. [Google Scholar] [CrossRef]
- Röttger, M.; Domenech, T.; van der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically activated, catalyst-free polyhydroxyurethane vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef] [PubMed]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.L.; Huang, W.M.; Lu, H.B.; Wang, C.C.; Cui, H.P. Characterization of polymeric shape memory materials. J. Polym. Eng. 2017, 37, 1–20. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Lu, H.; Lim, K.J.; Zhou, Y.; Wang, T.X.; Gao, X.Y. Heating-responsive shape-memory effect in thermoplastic polyurethanes with low melt-flow index. Macromol. Chem. Phys. 2014, 215, 2430–2436. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Wang, T.; Chen, H.; Renata, C.; He, L.; Lv, P.; Wang, C. An overview of elastic polymeric shape memory materials for comfort fitting. Mater. Des. 2017, 136, 238–248. [Google Scholar] [CrossRef]
- Wang, T.X.; Renata, C.; Chen, H.M.; Huang, W.M. Elastic shape memory hybrids programmable at around body-temperature for comfort fitting. Polymers 2017, 9, 674. [Google Scholar] [CrossRef]
- Pritchard, R. The transparency of crystalline polymers. Polym. Eng. Sci. 1964, 4, 66–71. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Wang, T. Dual-triggered and thermally reconfigurable shape memory graphene-vitrimer composites. ACS Appl. Mater. Interfaces 2016, 8, 21691–21699. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Thermoset shape-memory polyurethane with intrinsic plasticity enabled by transcarbamoylation. Angew. Chem. Int. Ed. 2016, 55, 11421–11425. [Google Scholar] [CrossRef]
- Lu, H.; Huang, W.M.; Wu, X.L.; Ge, Y.C.; Zhang, F.; Zhao, Y.; Geng, J. Heating/ethanol-response of poly methyl methacrylate (PMMA) with gradient pre-deformation and potential temperature sensor and anti-counterfeit applications. Smart Mater. Struct. 2014, 23, 067002. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.X.; Chen, H.; Salvekar, A.V.; Naveen, B.S.; Xu, Q.; Li, M.; Guo, X.; Chen, Y.; Huang, W. A brief review of the shape memory phenomena in polymers and their typical sensor applications. Polymers 2019, 11, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.F.; Mather, P.T. Shape memory assisted self-healing coating. ACS Macro Lett. 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Wang, C.C.; Huang, W.M.; Ding, Z.; Zhao, Y.; Purnawali, H.; Zheng, L.; Fan, H.; He, C.-B. Rubber-like shape memory polymeric materials with repeatable thermal-assisted healing function. Smart Mater. Struct. 2012, 21, 115010. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Luo, X.F.; Mather, P.T. Linear/network poly(epsilon-caprolactone) blends exhibiting shape memory assisted self-healing (SMASH). ACS Appl. Mater. Interfaces 2011, 3, 152–161. [Google Scholar] [CrossRef]
- Zhang, P.F.; Li, G.Q. Structural relaxation behavior of strain hardened shape memory polymer fibers for self-healing applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 966–977. [Google Scholar] [CrossRef]
- Wu, X.L.; Huang, W.M.; Seow, Z.G.; Chin, W.S.; Yang, W.G.; Sun, K.Y. Two-step shape recovery in heating-responsive shape memory polytetrafluoroethylene and its thermally assisted self-healing. Smart Mater. Struct. 2013, 22, 125023. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; de Luzuriaga, A.R.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horizons 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.M.; Phee, S.J. A secret garden of micro butterflies: Phenomenon and mechanism. Surf. Rev. Lett. 2007, 14, 1187–1190. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.X.; Zakee, M.H.I.B.M.; Bin Rosli, M.S.; Lee, Y.X.; Huang, W.M. Self-surface wrinkling atop acrylonitrile butadiene styrene (ABS) via heating-responsive shape memory effect. Surf. Rev. Lett. 2019, 26, 1950044. [Google Scholar] [CrossRef]
- Zhang, G.; Peng, W.; Wu, J.; Zhao, Q.; Xie, T. Digital coding of mechanical stress in a dynamic covalent shape memory polymer network. Nat. Commun. 2018, 9, 4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horizons 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon fiber reinforced thermoset composite with near 100% recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of epoxy thermoset and composites via good solvent assisted and small molecules participated exchange reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]
- Wang, S.; Xing, X.; Zhang, X.; Wang, X.; Jing, X. Room-temperature fully recyclable carbon fibre reinforced phenolic composites through dynamic covalent boronic ester bonds. J. Mater. Chem. A 2018, 6, 10868–10878. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jia, L. Multiply fully recyclable carbon fibre reinforced heat-resistant covalent thermosetting advanced composites. Nat. Commun. 2017, 8, 14657. [Google Scholar] [CrossRef]
- Denissen, W.; De Baere, I.; Van Paepegem, W.; Leibler, L.; Winne, J.; Du Prez, F.E. Vinylogous urea vitrimers and their application in fiber reinforced composites. Macromolecules 2018, 51, 2054–2064. [Google Scholar] [CrossRef] [Green Version]
- Witten, T., Jr.; Cohen, M.H. Crosslinking in shear-thickening ionomers. Macromol. 1985, 18, 1915–1918. [Google Scholar] [CrossRef]
- Chen, H.M.; Huang, W.M. A Kind of Manufacturing Method for the Elasticity Hinge Brake that Elasticity Can Gradually Discharge. China Patent Application CN108644272A, 28 April 2018. [Google Scholar]
- Lendlein, A.; Gould, O.E. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Behl, M.; Kratz, K.; Zotzmann, J.; Nochel, U.; Lendlein, A. Reversible bidirectional shape-memory polymers. Adv. Mater. 2013, 25, 4466–4469. [Google Scholar] [CrossRef]
- Westbrook, K.K.; Mather, P.T.; Parakh, V.; Dunn, M.L.; Ge, Q.; Lee, B.M.; Qi, H.J. Two-way reversible shape memory effects in a free-standing polymer composite. Smart Mater. Struct. 2011, 20, 065010. [Google Scholar] [CrossRef]
- Bothe, M.; Pretsch, T. Two-way shape changes of a shape-memory poly(ester urethane). Macromol. Chem. Phys. 2012, 213, 2378–2385. [Google Scholar] [CrossRef]
- Huang, W. On the selection of shape memory alloys for actuators. Mater. Des. 2002, 23, 11–19. [Google Scholar] [CrossRef]
- Renata, C.; Huang, W.M.; Yang, J.J. Shape change/memory actuators based on shape memory materials. J. Mech. Sci. Technol. 2017, 31, 4863–4873. [Google Scholar] [CrossRef]
- Behl, M.; Kratz, K.; Noechel, U.; Sauter, T.; Lendlein, A. Temperature-memory polymer actuators. Proc. Natl. Acad. Sci. USA 2013, 110, 12555–12559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Turner, S.A.; Brosnan, S.M.; Li, Q.; Carrillo, J.-M.Y.; Nykypanchuk, D.; Gang, O.; Ashby, V.S.; Dobrynin, A.V.; Sheiko, S.S. Shapeshifting: Reversible shape memory in semicrystalline elastomers. Macromolecules 2014, 47, 1768–1776. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Li, J.; Yang, Z.; Wang, Q.; Wang, T.; Li, S.; Chen, S.; Zhang, X. Shape memory properties of interpenetrating polymer networks (IPNs) based on hyperbranched polyurethane (HBPU). Eur. Polym. J. 2020, 123, 109393. [Google Scholar] [CrossRef]
- Sacco, E.; Moon, S.K. Additive manufacturing for space: Status and promises. Int. J. Adv. Manuf. Technol. 2019, 105, 4123–4146. [Google Scholar] [CrossRef]
- Tian, X.; Li, D.; Lu, B. Status and propsect of 3D printing technology in space. Manned Spacefl. 2016, 22, 471–476. [Google Scholar]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef]
- Wang, H.; He, C.; Luo, H.; Huang, W.; Yang, Z.; Chen, X.; He, J.F. Method for 3D Printing of Polymer in Condensed State. China Patent Application CN111070673A, 24 December 2019. [Google Scholar]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.X.; Chen, H.M.; Salvekar, A.V.; Lim, J.; Chen, Y.; Xiao, R.; Huang, W.M. Vitrimer-Like Shape Memory Polymers: Characterization and Applications in Reshaping and Manufacturing. Polymers 2020, 12, 2330. https://doi.org/10.3390/polym12102330
Wang TX, Chen HM, Salvekar AV, Lim J, Chen Y, Xiao R, Huang WM. Vitrimer-Like Shape Memory Polymers: Characterization and Applications in Reshaping and Manufacturing. Polymers. 2020; 12(10):2330. https://doi.org/10.3390/polym12102330
Chicago/Turabian StyleWang, Tao Xi, Hong Mei Chen, Abhijit Vijay Salvekar, Junyi Lim, Yahui Chen, Rui Xiao, and Wei Min Huang. 2020. "Vitrimer-Like Shape Memory Polymers: Characterization and Applications in Reshaping and Manufacturing" Polymers 12, no. 10: 2330. https://doi.org/10.3390/polym12102330