Melt Rheology and Mechanical Characteristics of Poly(Lactic Acid)/Alkylated Graphene Oxide Nanocomposites
Abstract
1. Introduction
2. Experimental
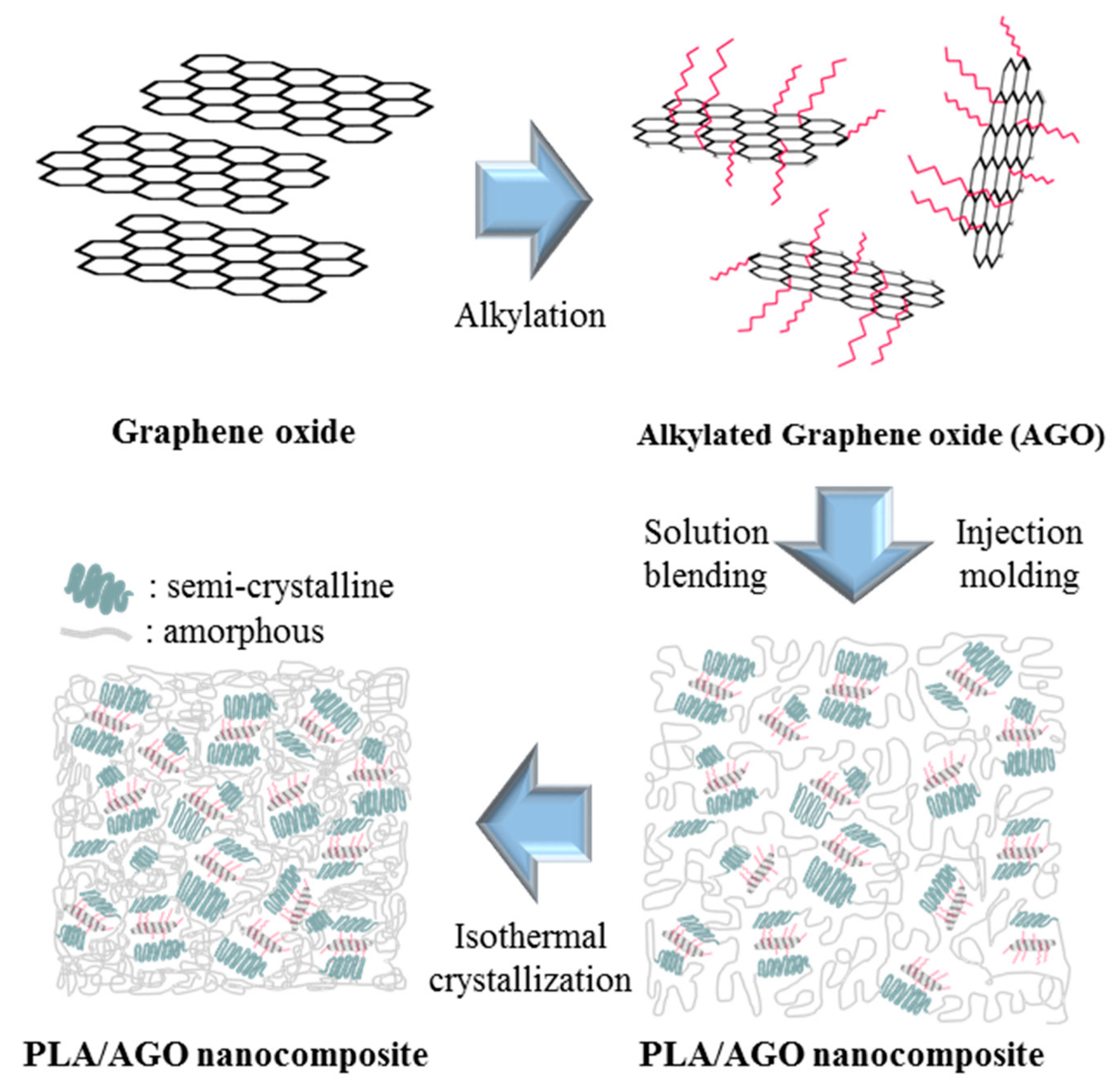
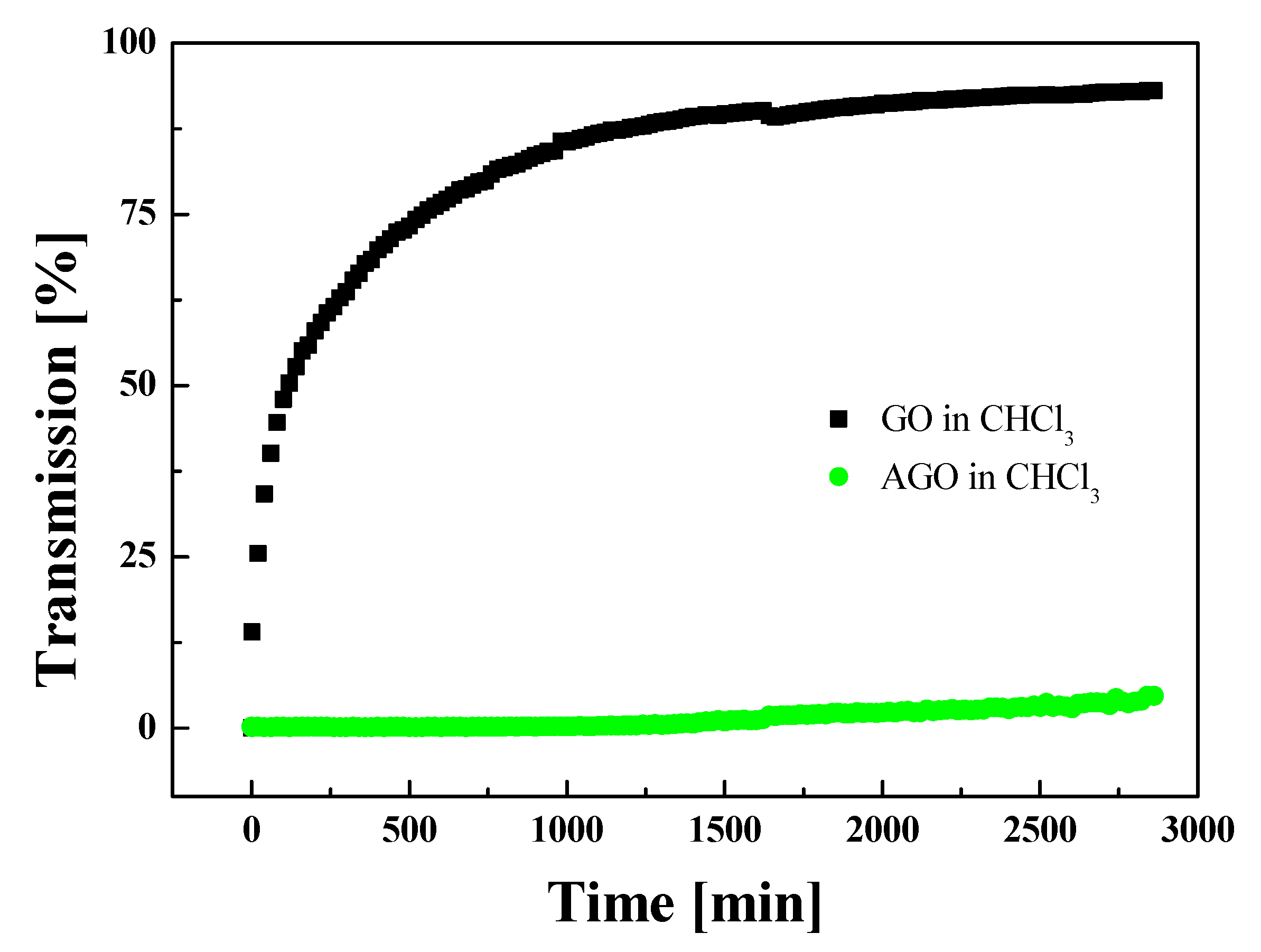
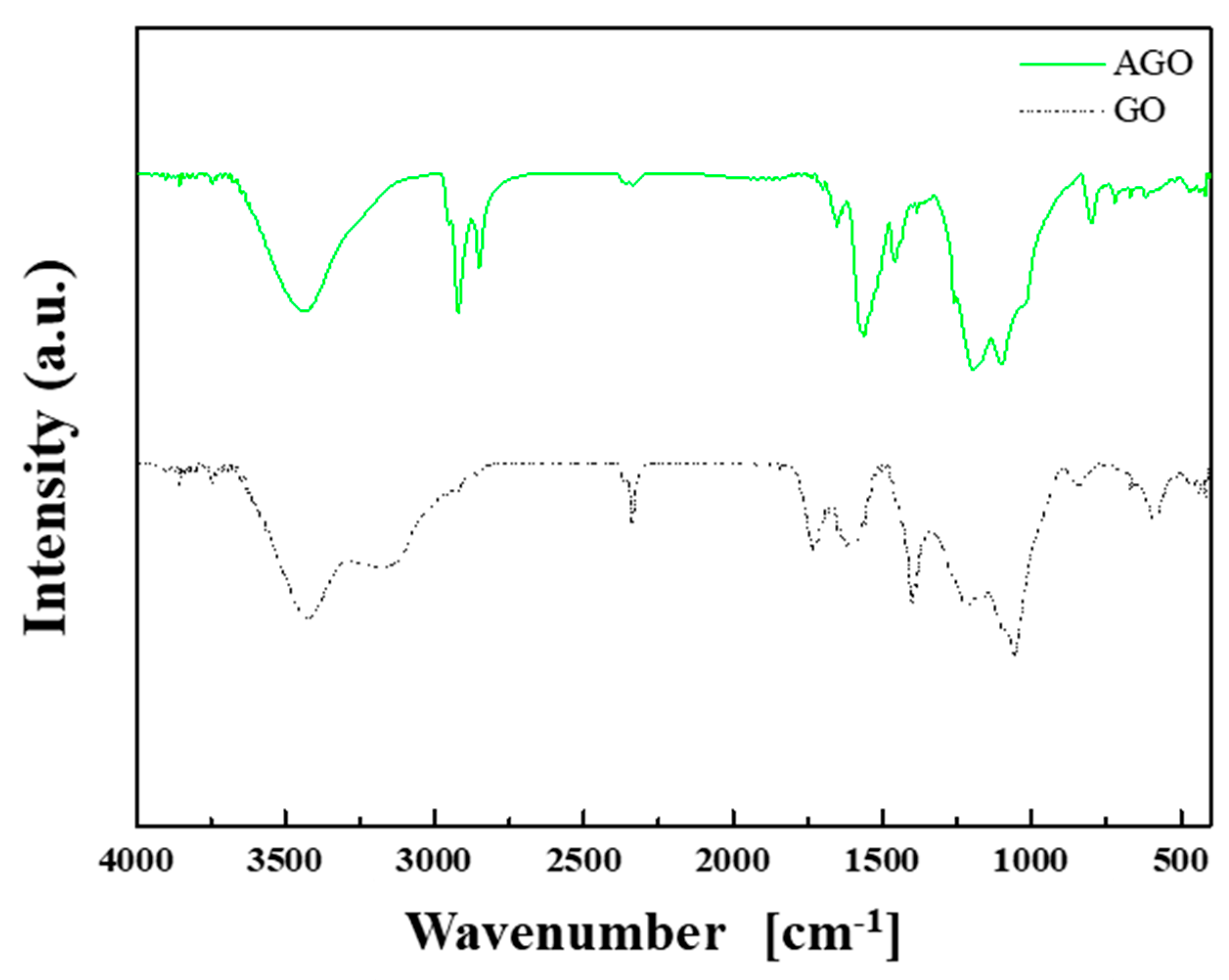
2.1. Materials and Synthesis
2.2. Characterization
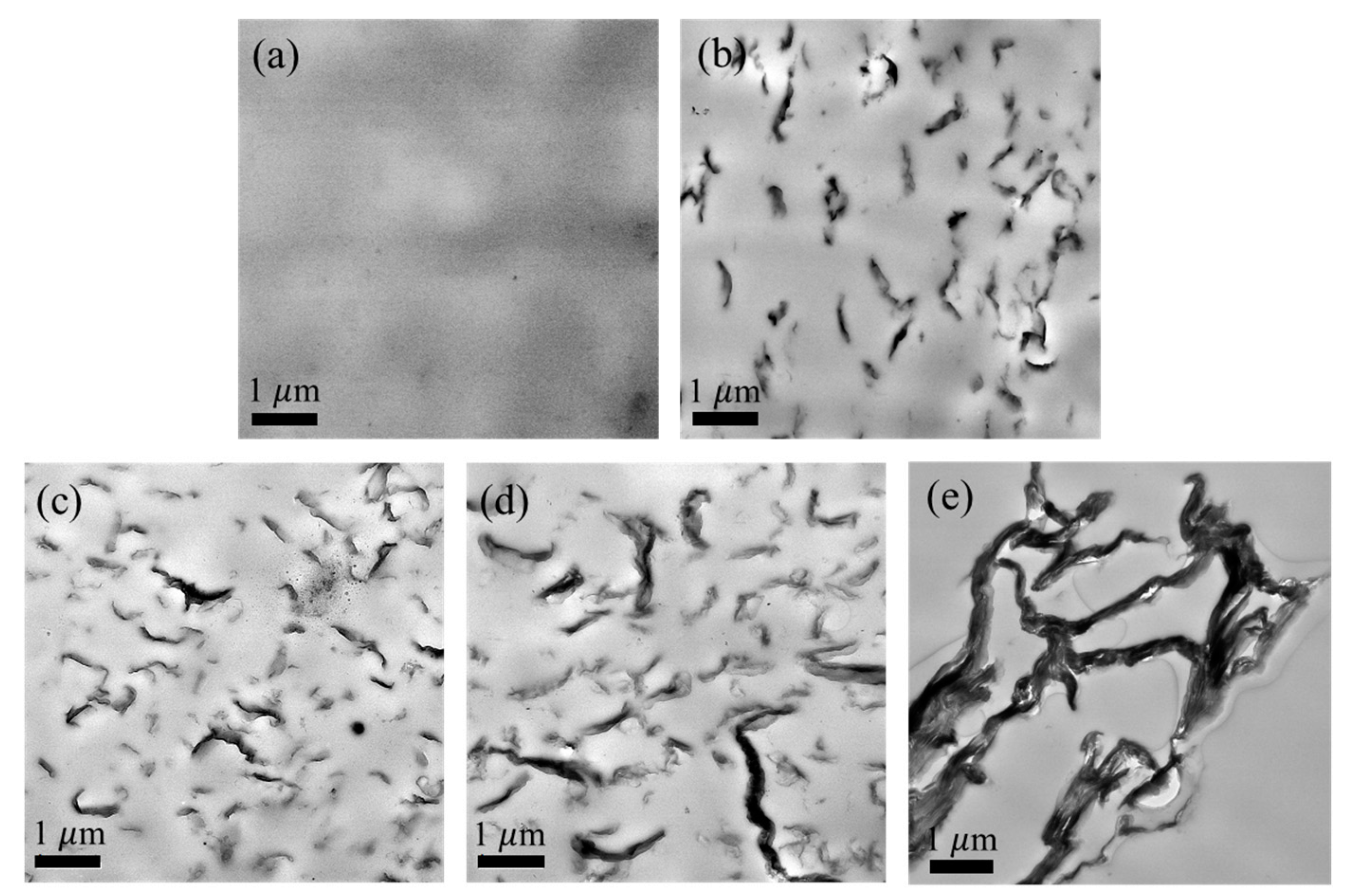
3. Results and Discussions
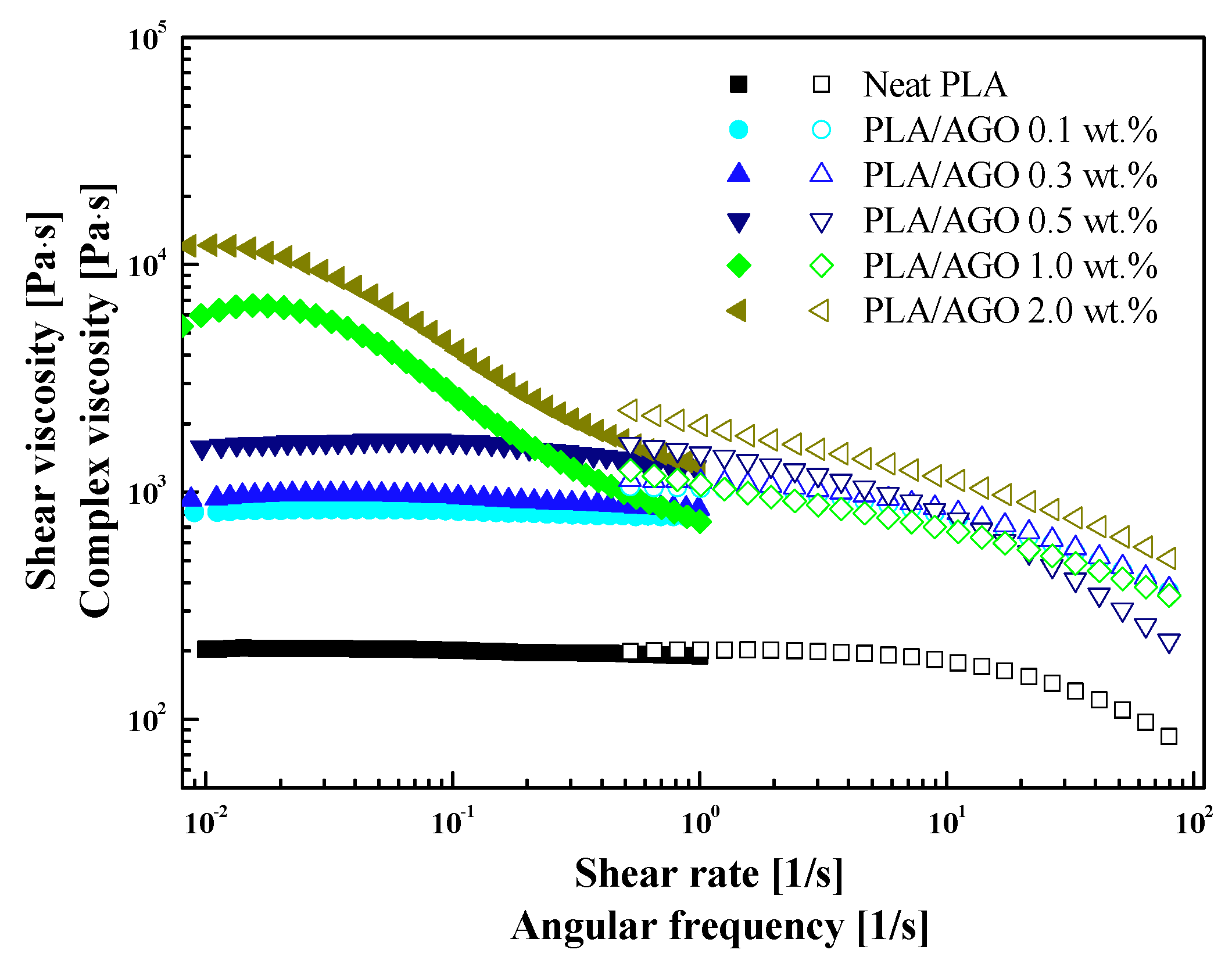
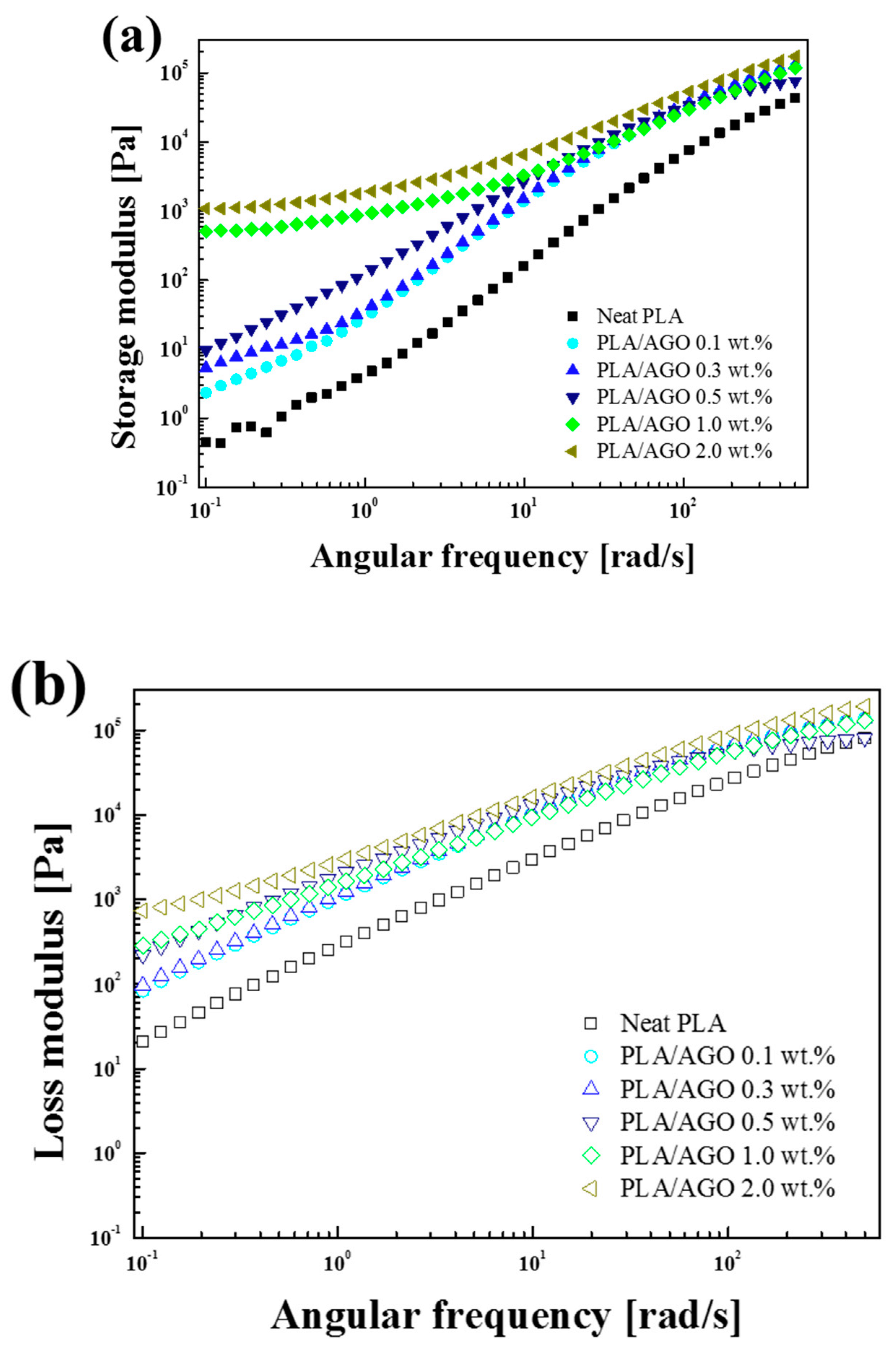
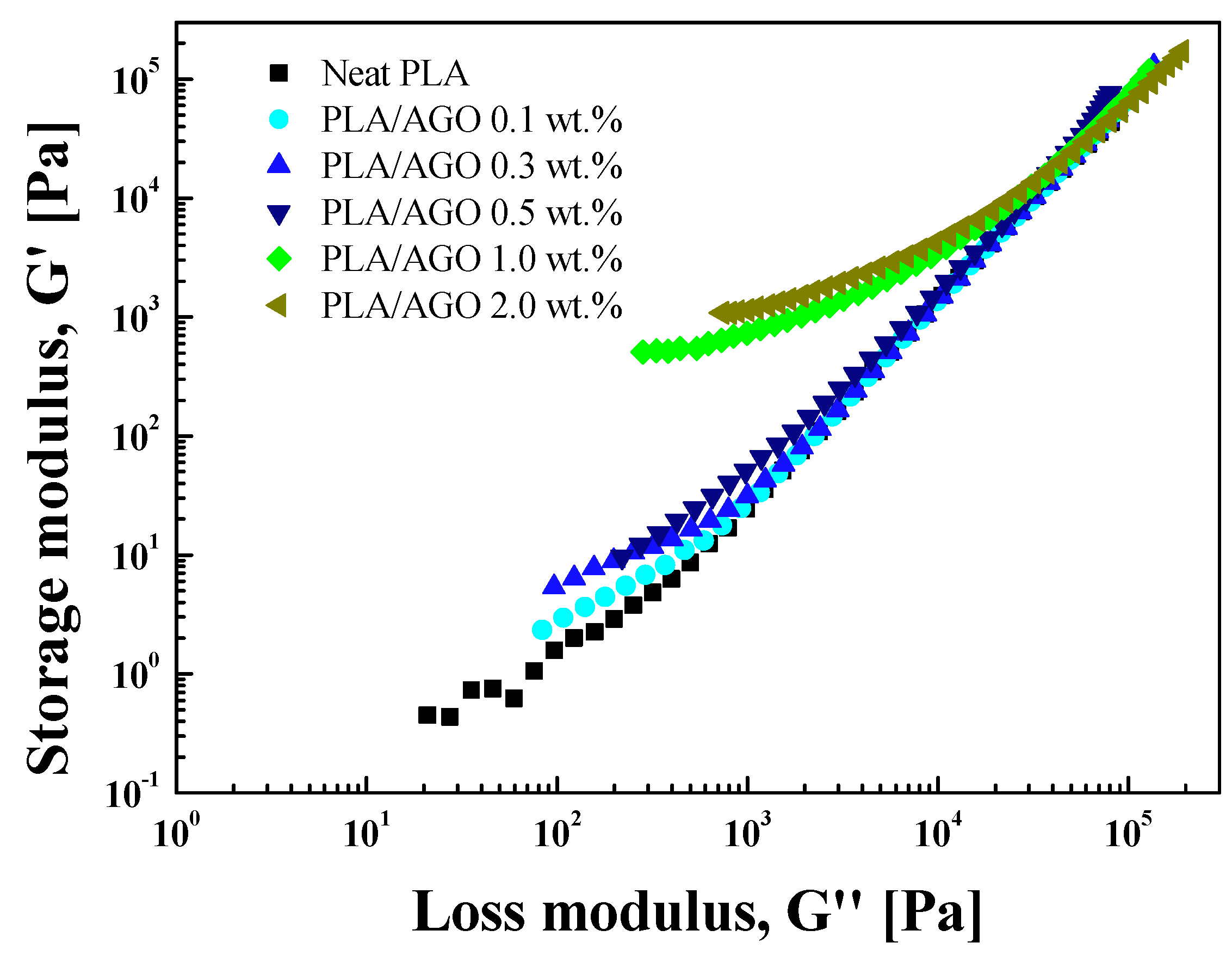
Material Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Ruiz-Mercado, G.J.; Smith, R.L.; Gonzalez, M.A. Sustainability indicators for chemical processes: II Data needs. Ind. Eng. Chem. Res. 2012, 51, 2329–2353. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G. Flame retardancy of polylactide: An overview. Polym. Chem. 2010, 1, 1413–1422. [Google Scholar] [CrossRef]
- Narimissa, E.; Gupta, R.K.; Choi, H.J.; Kao, N.; Jollands, M. Morphological, Mechanical, and Thermal Characterization of Biopolymer Composites Based on Polylactide and Nanographite Platelets. Polym. Compos. 2012, 33, 1505–1515. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Durability of polylactide-based polymer blends for injection-molded applications. J. Appl. Polym. Sci. 2013, 128, 2136–2144. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(lactic acid)-based biomaterials: Synthesis, modification and applications. Biomed. Sci. Eng. Technol. 2012, 11, 247–282. [Google Scholar]
- Li, Z.; Tan, B.H.; Lin, T.; He, C. Recent advances in stereocomplexation of enantiomeric PLA-based copolymers and applications. Prog. Polym. Sci. 2016, 62, 22–72. [Google Scholar] [CrossRef]
- Mustapa, I.R.; Shanks, R.A.; Daud, N. Morphological structure and thermomechanical properties of hemp fibre reinforced poly(lactic acid) Nanocomposites plasticized with tributyl citrate. Mater. Today Proc. 2018, 5, 3211–3218. [Google Scholar] [CrossRef]
- Mosanenzadeh, S.G.; Khalid, S.; Cui, Y.; Naguib, H.E. High thermally conductive PLA based composites with tailored hybrid network of hexagonal boron nitride and graphene nanoplatelets. Polym. Compos. 2016, 37, 2196–2205. [Google Scholar] [CrossRef]
- Piekarska, K.; Piorkowska, E.; Bojda, J. The influence of matrix crystallinity, filler grain size and modification on properties of PLA/calcium carbonate composites. Polym. Test. 2017, 62, 203–209. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Pizzi, E.; Puopolo, M.; Vesco, S. Wear resistance of injection moulded PLA-talc engineered bio-composites: Effect of material design, thermal history and shear stresses during melt processing. Wear 2017, 390, 184–197. [Google Scholar] [CrossRef]
- Keshtkar, M.; Nofar, M.; Park, C.B.; Carreau, P.J. Extruded PLA/clay nanocomposite foams blown with supercritical CO2. Polymer 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Kuan, C.F.; Kuan, H.C.; Ma, C.C.M.; Chen, C.H. Mechanical and electrical properties of multi-wall carbon nanotube/poly(lactic acid) composites. J. Phys. Chem. Solids 2008, 69, 1395–1398. [Google Scholar] [CrossRef]
- Dhar, P.; Bhasney, S.M.; Kumar, A.; Katiyar, V. Acid functionalized cellulose nanocrystals and its effect on mechanical, thermal, crystallization and surfaces properties of poly(lactic acid) bionanocomposites films: A comprehensive study. Polymer 2016, 101, 75–92. [Google Scholar] [CrossRef]
- Sabzi, M.; Jiang, L.; Liu, F.; Ghasemi, I.; Atai, M. Graphene nanoplatelets as poly(lactic acid) modifier: Linear rheological behavior and electrical conductivity. J. Mater. Chem. A 2013, 1, 8253–8261. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Zhang, W.L.; Park, B.J.; Choi, H.J. Colloidal graphene oxide/polyaniline nanocomposite and its electrorheology. Chem. Commun. 2010, 46, 5596–5598. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Graphene oxide based smart fluids. Soft Matter 2014, 10, 6601–6608. [Google Scholar] [CrossRef]
- Xu, H.; Wu, D.; Yang, X.; Xie, L.; Hakkarainen, M. Thermostable and impermeable “nano-barrier walls” constructed by poly(lactic acid) stereocomplex crystal decorated graphene oxide nanosheets. Macromolecules 2015, 48, 2127–2137. [Google Scholar] [CrossRef]
- Xu, H.; Feng, Z.X.; Xie, L.; Hakkarainen, M. Graphene oxide-driven design of strong and flexible biopolymer barrier films: From smart crystallization control to affordable engineering. Sustain. Chem. Eng. 2016, 4, 334–349. [Google Scholar] [CrossRef]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Wei, T.; Meng, Z.; Jia, X.; Xi, K. Greatly enhanced mechanical properties and heat distortion resistance of poly(L-lactic acid) upon compositing with functionalized reduced graphene oxide. J. Mater. Chem. A 2013, 1, 9028–9032. [Google Scholar] [CrossRef]
- Yang, L.; Zhen, W. Poly(lactic acid)/p-phenylenediamine functionalized graphene oxidized nanocomposites: Preparation, rheological behavior and biodegradability. Eur. Polym. J. 2019, 121, 109341. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, C.; Shi, X.; Zhang, G.; Chen, P.; Wang, J. Crystallization, rheology behavior, and antibacterial application of graphene oxide-graft-poly(L-lactide)/poly(L-lactide) nanocomposites. Appl. Surf. Sci. 2018, 451, 315–324. [Google Scholar] [CrossRef]
- Xu, Z.; Niu, Y.; Yang, L.; Xie, W.; Li, H.; Gan, Z.; Wang, Z. Morphology, rheology and crystallization behavior of polylactide composites prepared through addition of five-armed star polylactide grafted multiwalled carbon nanotubes. Polymer 2010, 51, 730–737. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. An investigation of melt rheology and thermal stability of poly(lactic acid)/poly(butylene succinate) nanocomposites. J. Appl. Polym. Sci. 2009, 114, 2837–2847. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically conductive “alkylated” graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef]
- Kim, H.; Kobayashi, S.; AbdurRahim, M.A.; Zhang, M.J.; Khusainova, A.; Hillmyer, M.A.; Abdala, A.A.; Macosko, C.W. Graphene/polyethylene nanocomposites: Effect of polyethylene functionalization and blending methods. Polymer 2011, 52, 1837–1846. [Google Scholar] [CrossRef]
- Gong, L.; Yin, B.; Li, L.P.; Yang, M.B. Nylon-6/Graphene composites modified through polymeric modification of graphene. Compos. B Eng. 2015, 73, 49–56. [Google Scholar] [CrossRef]
- The Graphene Catalog: Your Source for Graphene Materials. Available online: https://catalog.graphene-info.com/view/30/ABX-ADCP-GO (accessed on 12 October 2020).
- Zhang, H.; Hines, D.; Akins, D.L. Synthesis of a nanocomposite composed of reduced graphene oxide and gold nanoparticles. Dalton Trans. 2014, 43, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Gwon, J.G.; Cho, H.J.; Chun, S.J.; Lee, S.; Wu, Q.; Li, M.C.; Lee, S.Y. Mechanical and thermal properties of toluene diisocyanate-modified cellulose nanocrystal nanocomposites using semi-crystalline poly(lactic acid) as a base matrix. RSC Adv. 2016, 6, 73879–73886. [Google Scholar] [CrossRef]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, H.; Qiao, Y.; Chen, J.; Cao, S. Strong and ductile poly(lactic acid) nanocomposite films reinforced with alkylated graphene nanosheets. Chem. Eng. J. 2015, 264, 538–546. [Google Scholar] [CrossRef]
- Oh, H.; Green, P.F. Polymer chain dynamics and glass transition in athermal polymer/nanoparticle mixtures. Nat. Mater. 2009, 8, 139–143. [Google Scholar] [CrossRef]
- Choe, J.H.; Jeon, J.; Lee, M.E.; Wie, J.J.; Jin, H.J.; Yun, Y.S. Nanoconfinement effects of chemically reduced graphene oxide nanoribbons on poly(vinyl chloride). Nanoscale 2018, 10, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ma, P.; Xu, P.; Wang, R.; Dong, W.; Chen, M.; Joziasse, C. The crystallization behavior of poly (lactic acid) with different types of nucleating agents. Int. J. Biol. Macromol. 2018, 106, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, J.B.; Wang, Y.; Xu, J.Z.; Hsiao, B.S.; Zhong, G.J.; Li, Z.M. Shear flow and carbon nanotubes synergistically induced nonisothermal crystallization of poly(lactic acid) and its application in injection molding. Biomacromolecules 2012, 13, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Zhang, Q.; Fu, Q. Enhancing mechanical performance of polylactide by tailoring crystal morphology and lamellae orientation with the aid of nucleating agent. Polymer 2014, 55, 6924–6934. [Google Scholar] [CrossRef]
- Sun, Y.; He, C. Synthesis and stereocomplex crystallization of poly(lactide)–graphene oxide nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Investigation of phase transitional behavior of poly (L-lactide)/poly (D-lactide) blend used to prepare the highly-oriented stereocomplex. Macromolecules 2007, 40, 1049–1054. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhang, Z.J.; Xu, H.; Chen, J.B.; Ran, R.; Li, Z.M. Highly enhanced crystallization kinetics of poly(l-lactic acid) by poly(ethylene glycol) grafted graphene oxide simultaneously as heterogeneous nucleation agent and chain mobility promoter. Macromolecules 2015, 48, 4891–4900. [Google Scholar] [CrossRef]
- Chartarrayawadee, W.; Molloy, R.; Ratchawet, A.; Janmee, N.; Butsamran, M.; Panpai, K. Fabrication of poly(lactic acid)/graphene oxide/stearic acid composites with improved tensile strength. Polym. Compos. 2017, 38, 2272–2282. [Google Scholar] [CrossRef]
- Qian, S.; Sheng, K. PLA toughened by bamboo cellulose nanowhiskers: Role of silane compatibilization on the PLA bionanocomposite properties. Compos. Sci. Technol. 2017, 148, 59–69. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Z.; Ma, C. Bioinspired approaches for optimizing the strength and toughness of graphene-based polymer nanocomposites. J. Mater. Chem. C 2012, 22, 16182–16190. [Google Scholar] [CrossRef]
- Narimissa, E.; Gupta, R.K.; Kao, N.; Choi, H.J.; Jollands, M.; Bhattacharya, S.N. Melt rheological investigation of polylactide-nanographite platelets biopolymer composites. Polym. Eng. Sci. 2014, 54, 175–188. [Google Scholar] [CrossRef]
- Das, D.; Sethy, S.; Satapathy, B.K. Matrix tacticity controlled tuning of microstructure, constitutive behavior and rheological percolation effect of melt-mixed amino-functionalized MWCNT/PP nanocomposites. Polym. Eng. Sci. 2018, 58, 1115–1126. [Google Scholar] [CrossRef]
- Hyun, Y.H.; Lim, S.T.; Choi, H.J.; Jhon, M.S. Rheology of poly(ethylene oxide)/organoclay nanocomposites. Macromolecules 2001, 34, 8084–8093. [Google Scholar] [CrossRef]
- Cox, W.P.; Merz, E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Rostami, A.; Nazockdast, H.; Karimi, M. Graphene induced microstructural changes of PLA/MWCNT biodegradable nanocomposites: Rheological, morphological, thermal and electrical properties. RSC Adv. 2016, 6, 49747–49759. [Google Scholar] [CrossRef]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Hylton, D.; Mintz, E.A. Rheological and thermo-mechanical properties of poly(lactic acid)/lignin-coated cellulose nanocrystal composites. ACS Sustain. Chem. Eng. 2017, 5, 1711–1720. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwon, S.H.; Choi, H.J.; Choi, K.; Kao, N.; Bhattacharya, S.N.; Gupta, R.K. Thermal, mechanical, and rheological characterization of polylactic acid/halloysite nanotube nanocomposites. J. Macromol. Sci. B 2016, 55, 680–692. [Google Scholar] [CrossRef]
- Harrell, E.R.; Nakajima, N. Modified Cole–Cole plot based on viscoelastic properties for characterizing molecular architecture of elastomers. J. Appl. Polym. Sci. 1984, 29, 995–1010. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Phase transition and anomalous rheological behaviour of polylactide/graphene nanocomposites. Compos. B Eng. 2018, 135, 25–34. [Google Scholar] [CrossRef]
- Nair, S.T.; Vijayan, P.P.; Xavier, P.; Bose, S.; George, S.C.; Thomas, S. Selective localisation of multi walled carbon nanotubes in polypropylene/natural rubber blends to reduce the percolation threshold. Compos. Sci. Technol. 2015, 116, 9–17. [Google Scholar] [CrossRef]
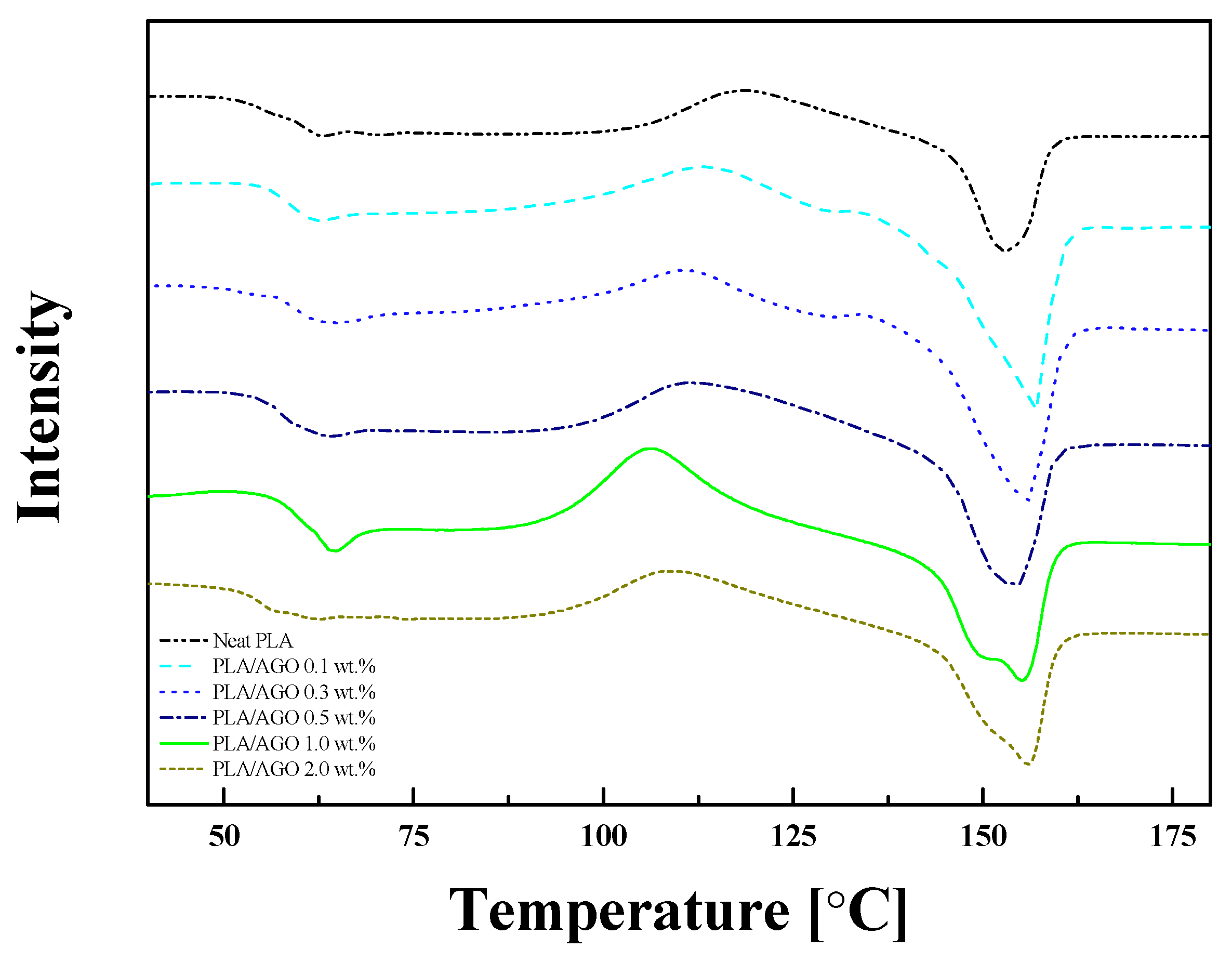
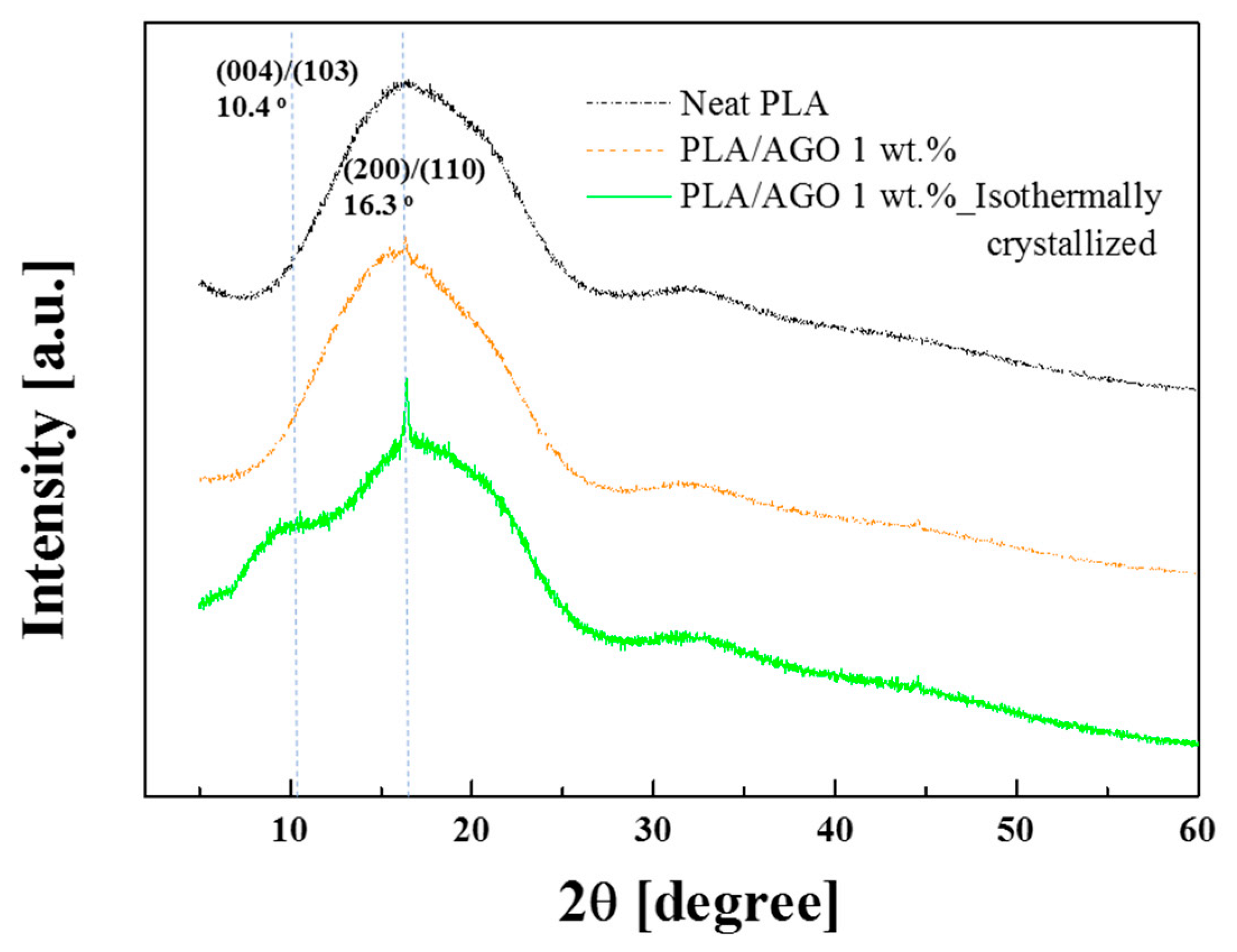
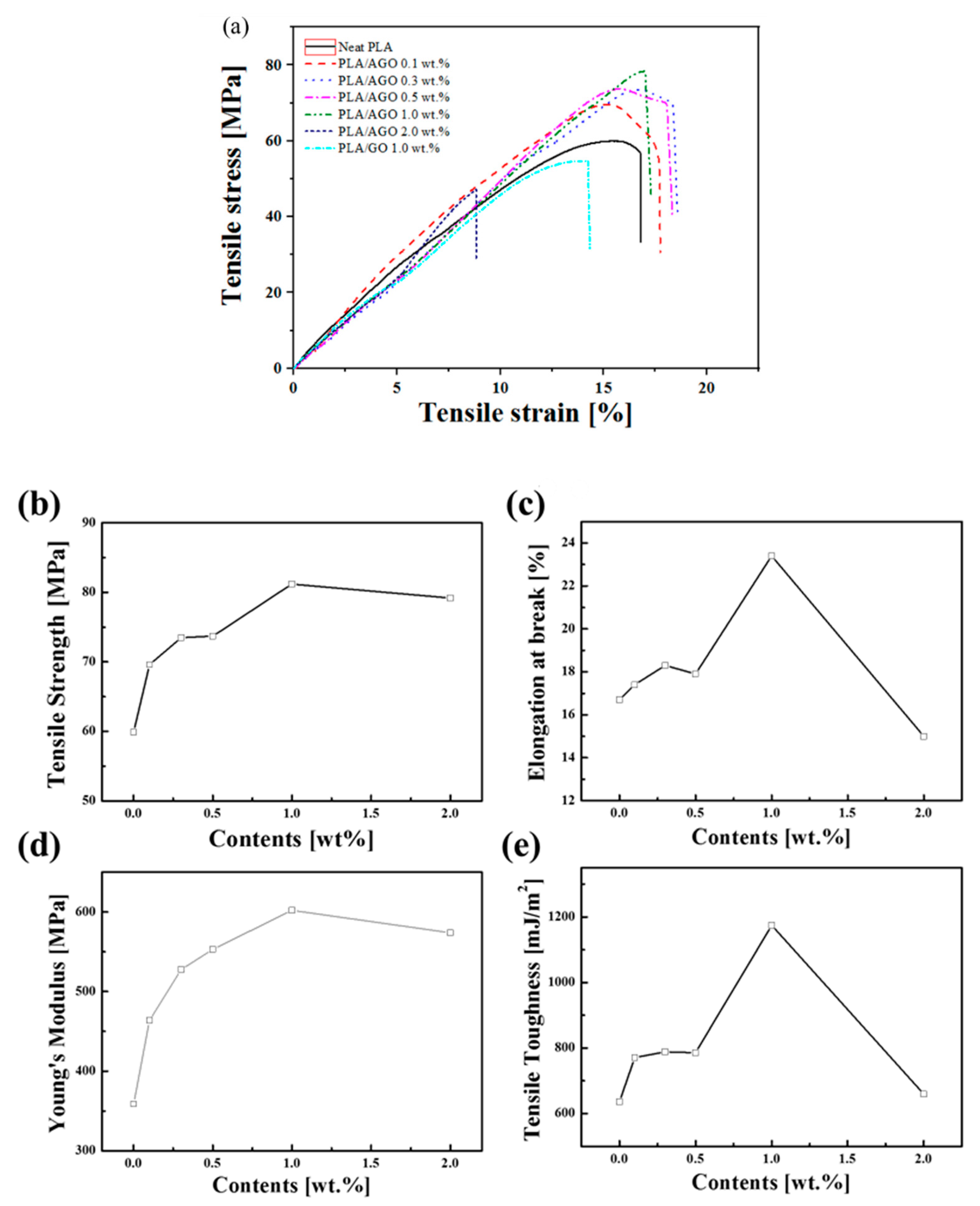

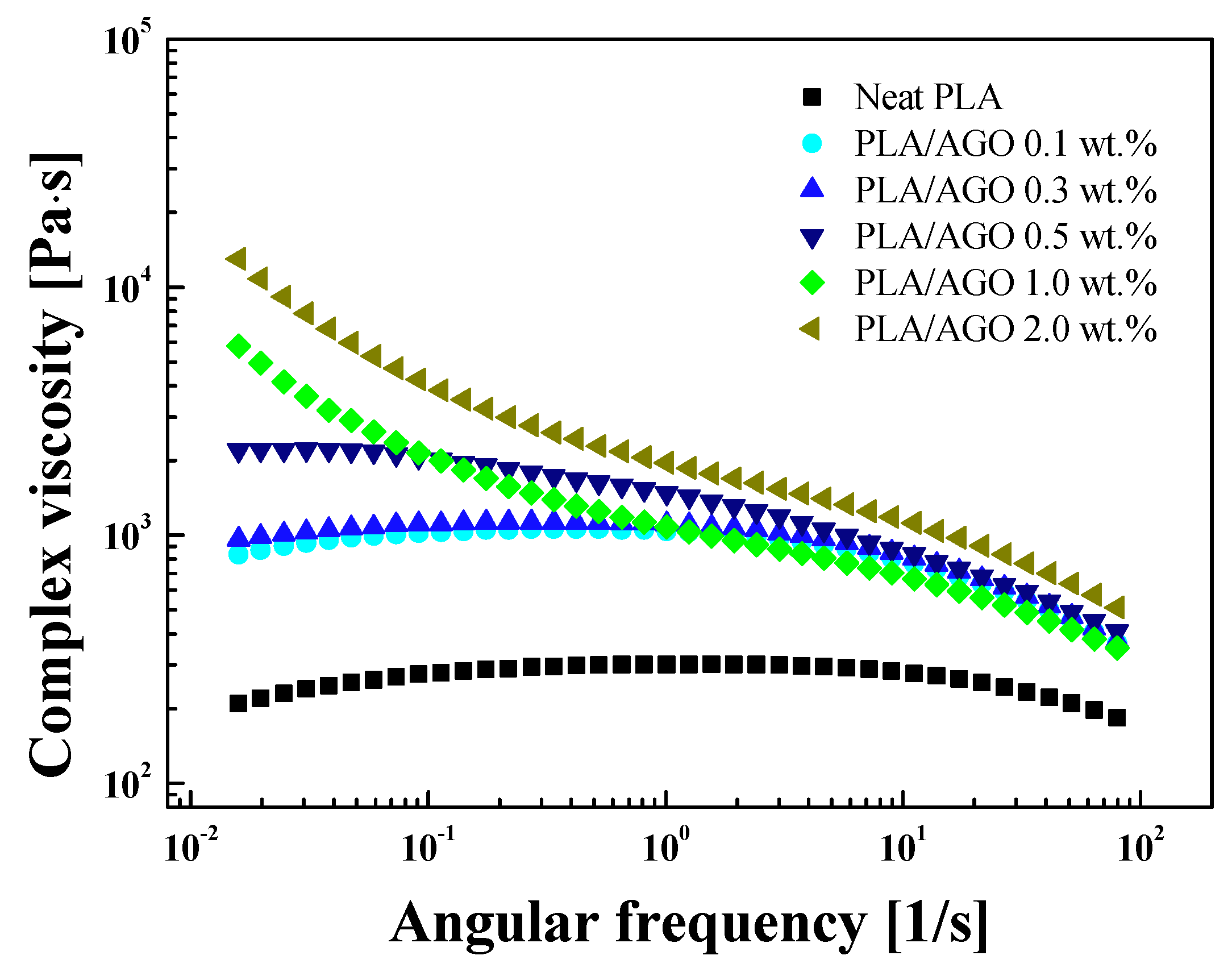

| Samples | Tg [°C] | Tcc [°C] | Tm [°C] | [J/g] | [%] | |
|---|---|---|---|---|---|---|
| Tm1 | Tm2 | |||||
| Neat PLA | 56.5 | 117.9 | - | 152.8 | 15.44 | 19.77 |
| PLA/AGO 0.1 | 58.6 | 113.2 | - | 156.4 | 29.1 | 30.34 |
| PLA/AGO 0.3 | 58.9 | 110.3 | - | 155.1 | 30.36 | 31.72 |
| PLA/AGO 0.5 | 57.2 | 111.2 | - | 154.2 | 24.13 | 25.39 |
| PLA/AGO 1.0 | 59.8 | 106.2 | 149.8 | 155 | 23.15 | 24.23 |
| PLA/AGO 2.0 | 55.1 | 108.9 | 150.8 | 156 | 20.93 | 22.25 |
| Sample | Λ | n | R2 | ||
|---|---|---|---|---|---|
| Neat | 203 | 6.8 | 0.3656 | 186 | 0.93 |
| PLA/AGO 0.1 | 833 | 5.627 | 0.1032 | 764 | 0.96 |
| PLA/AGO 0.3 | 976 | 6.186 | 0.4511 | 823 | 0.98 |
| PLA/AGO 0.5 | 2290 | 0.7193 | 0.1874 | 1120 | 0.99 |
| PLA/AGO 1.0 | 6610 | 22.86 | 0.2123 | 374 | 0.99 |
| PLA/AGO 2.0 | 12,200 | 29.89 | 0.1002 | 834 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.H.; Lee, J.Y.; Ahn, S.J.; Choi, H.J. Melt Rheology and Mechanical Characteristics of Poly(Lactic Acid)/Alkylated Graphene Oxide Nanocomposites. Polymers 2020, 12, 2402. https://doi.org/10.3390/polym12102402
Park IH, Lee JY, Ahn SJ, Choi HJ. Melt Rheology and Mechanical Characteristics of Poly(Lactic Acid)/Alkylated Graphene Oxide Nanocomposites. Polymers. 2020; 12(10):2402. https://doi.org/10.3390/polym12102402
Chicago/Turabian StylePark, In Hye, Jae Yoon Lee, Seung Jae Ahn, and Hyoung Jin Choi. 2020. "Melt Rheology and Mechanical Characteristics of Poly(Lactic Acid)/Alkylated Graphene Oxide Nanocomposites" Polymers 12, no. 10: 2402. https://doi.org/10.3390/polym12102402
APA StylePark, I. H., Lee, J. Y., Ahn, S. J., & Choi, H. J. (2020). Melt Rheology and Mechanical Characteristics of Poly(Lactic Acid)/Alkylated Graphene Oxide Nanocomposites. Polymers, 12(10), 2402. https://doi.org/10.3390/polym12102402





