Radiation Processing and Characterization of Some Ethylene-propylene-diene Terpolymer/Butyl (Halobutyl) Rubber/Nanosilica Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparationof EPDM Composites
2.3. Measurements
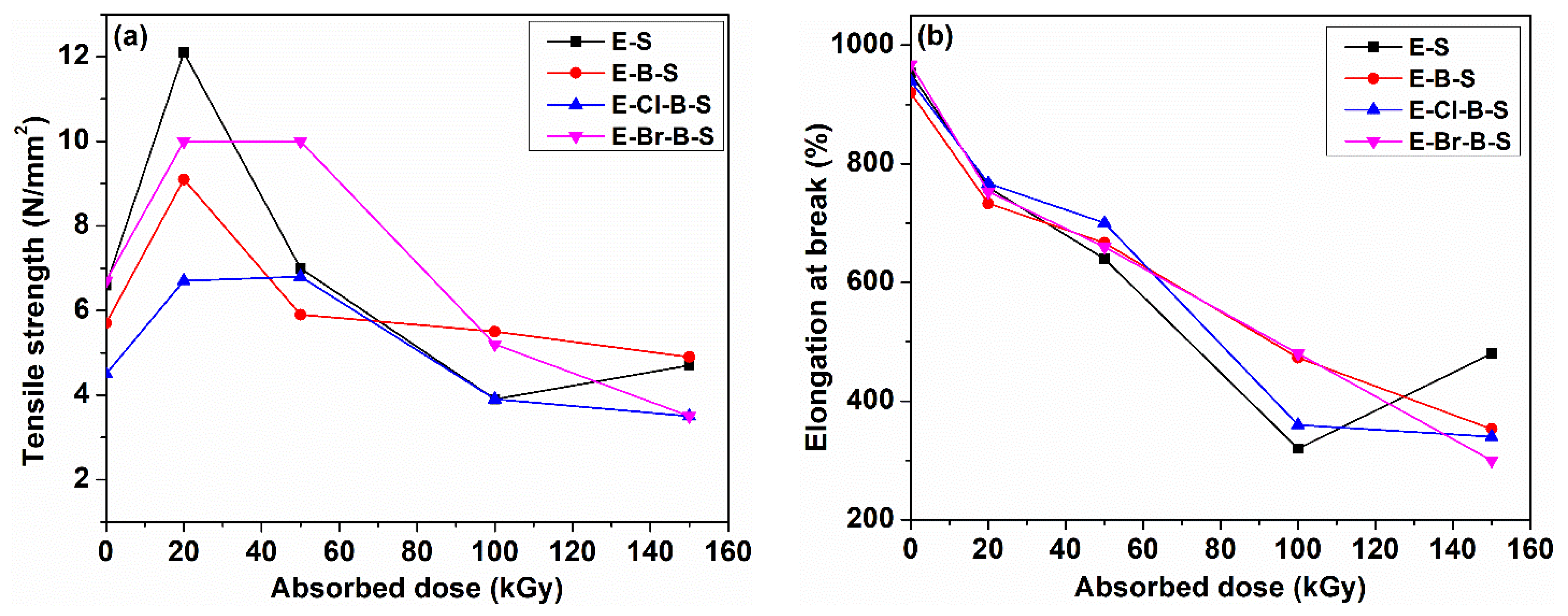
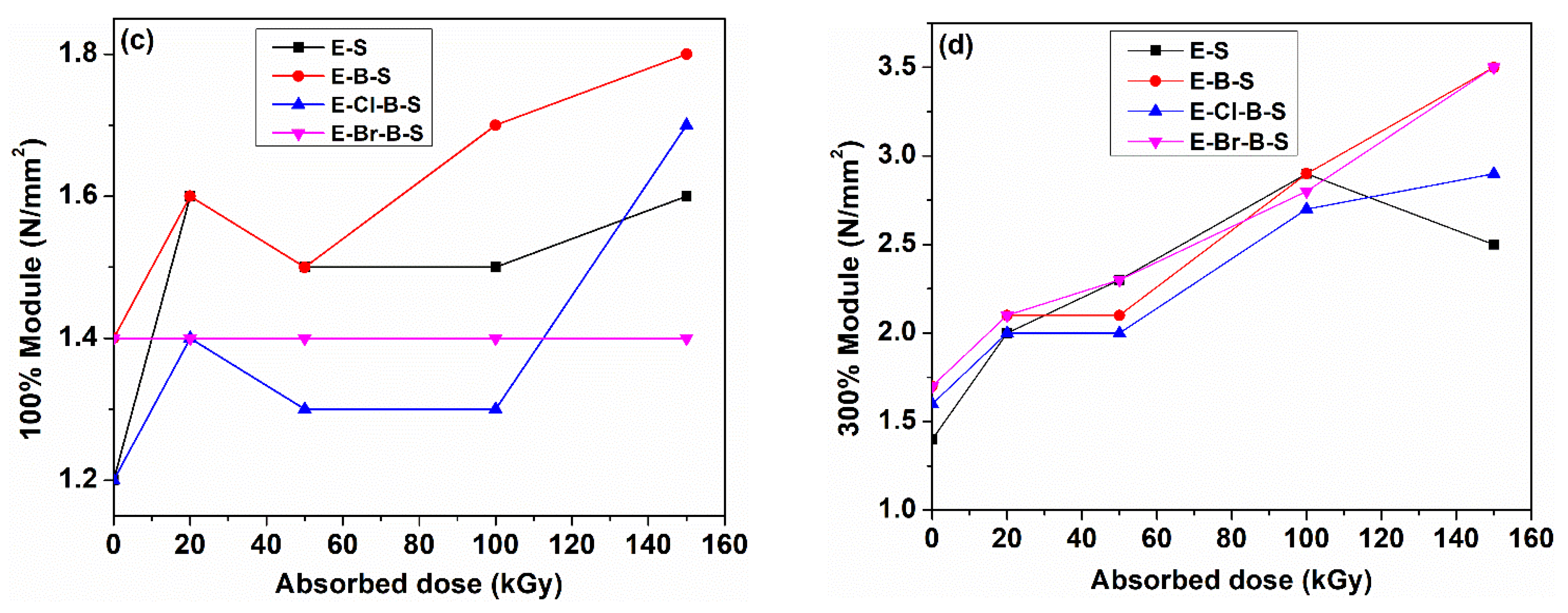
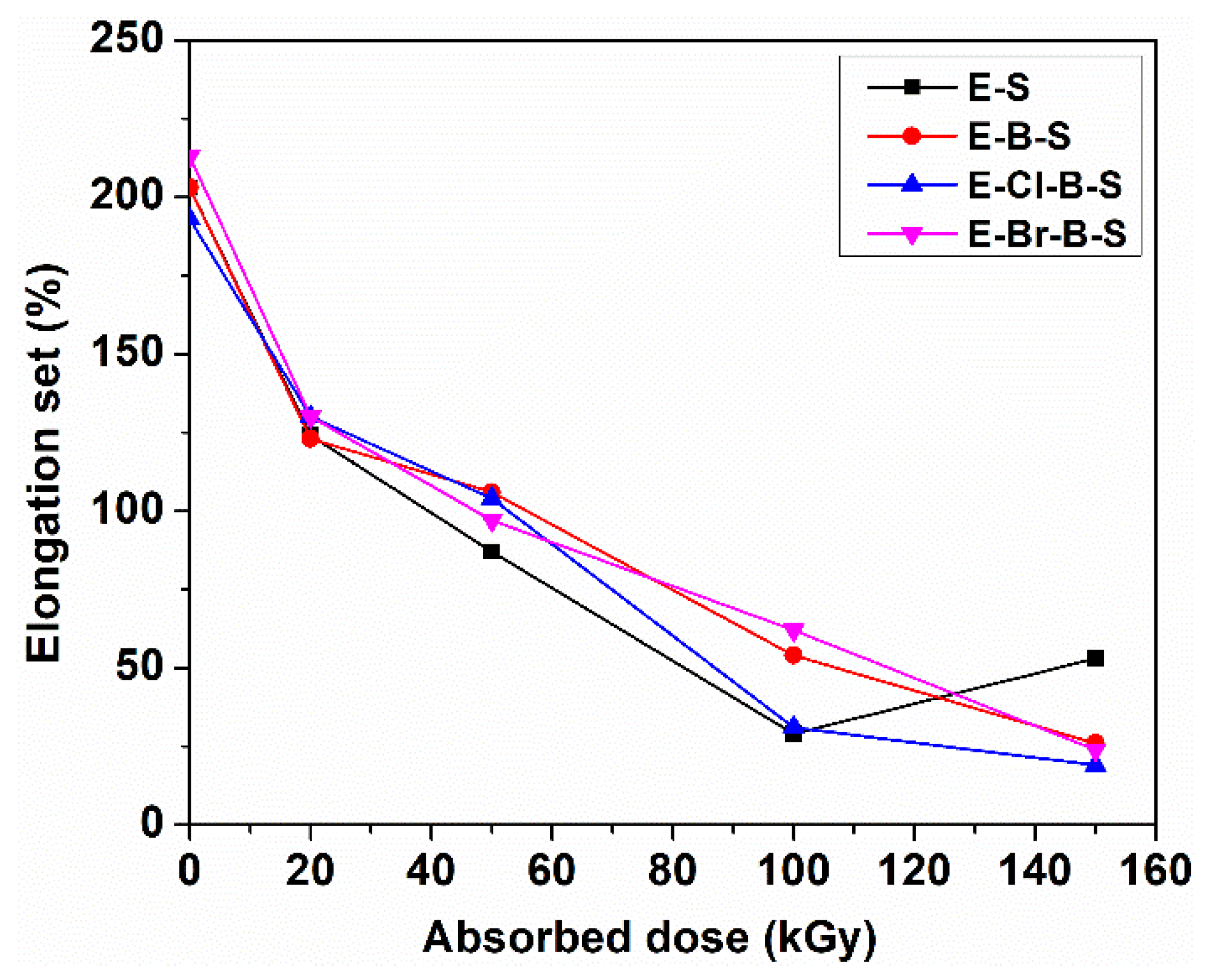
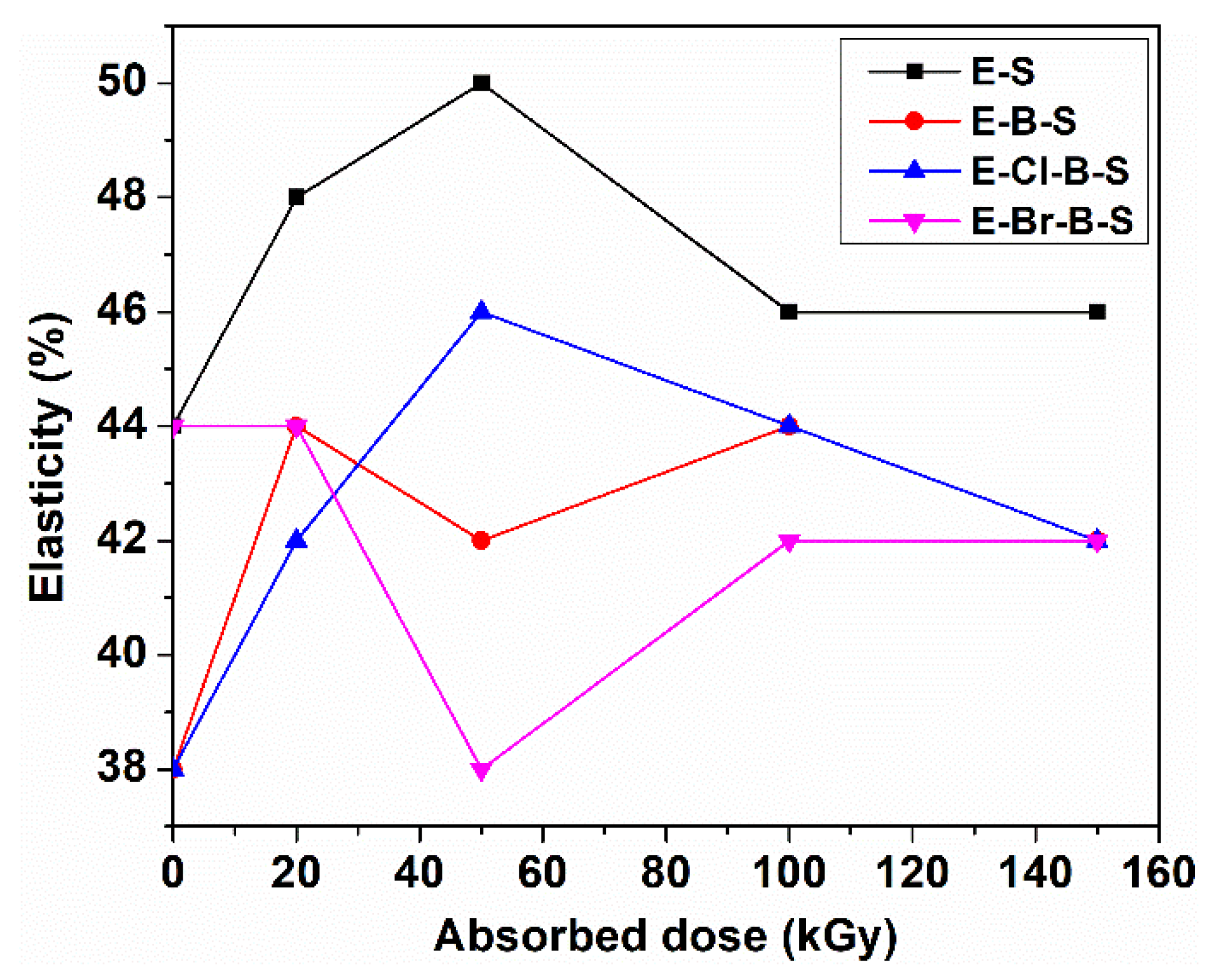
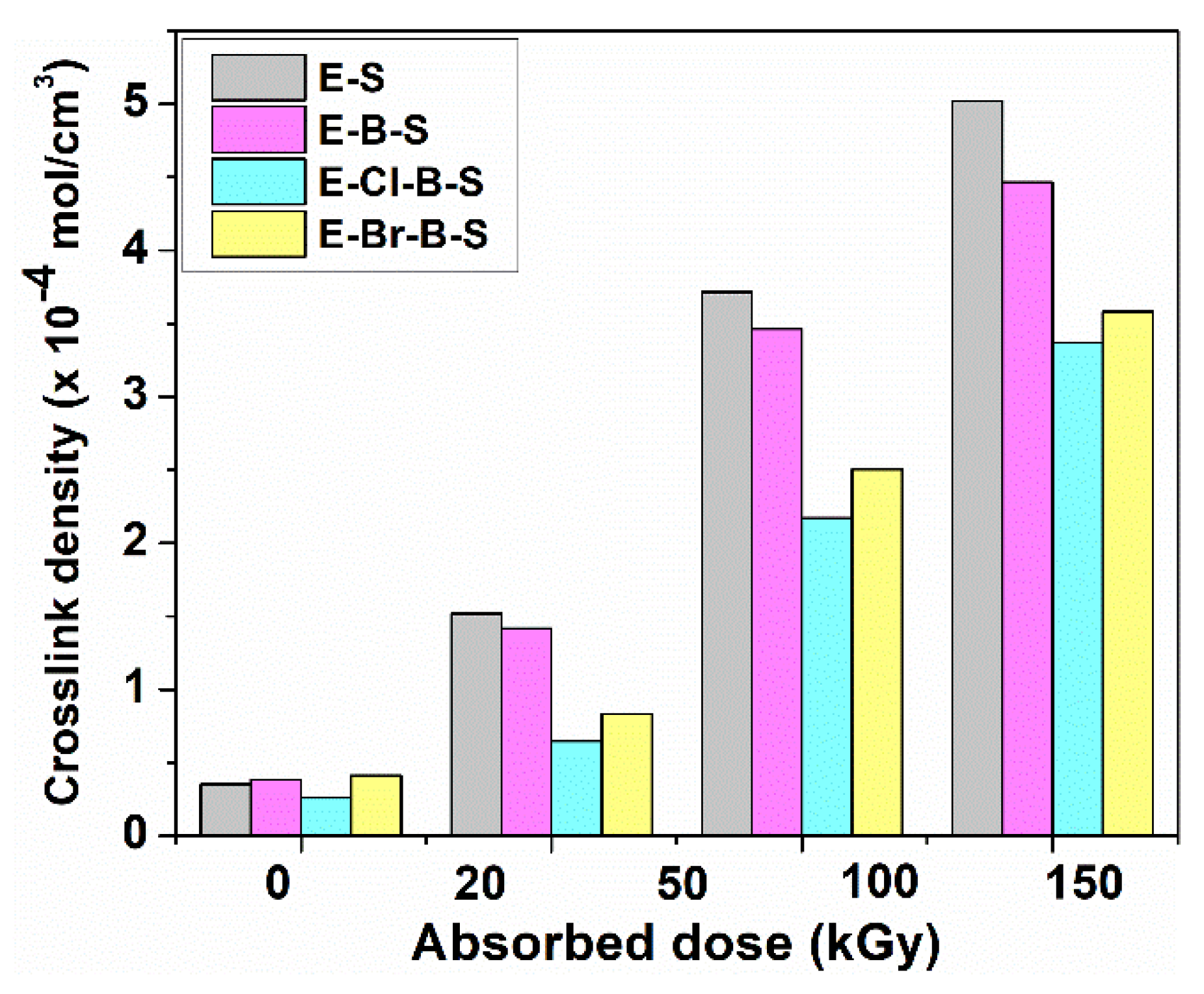
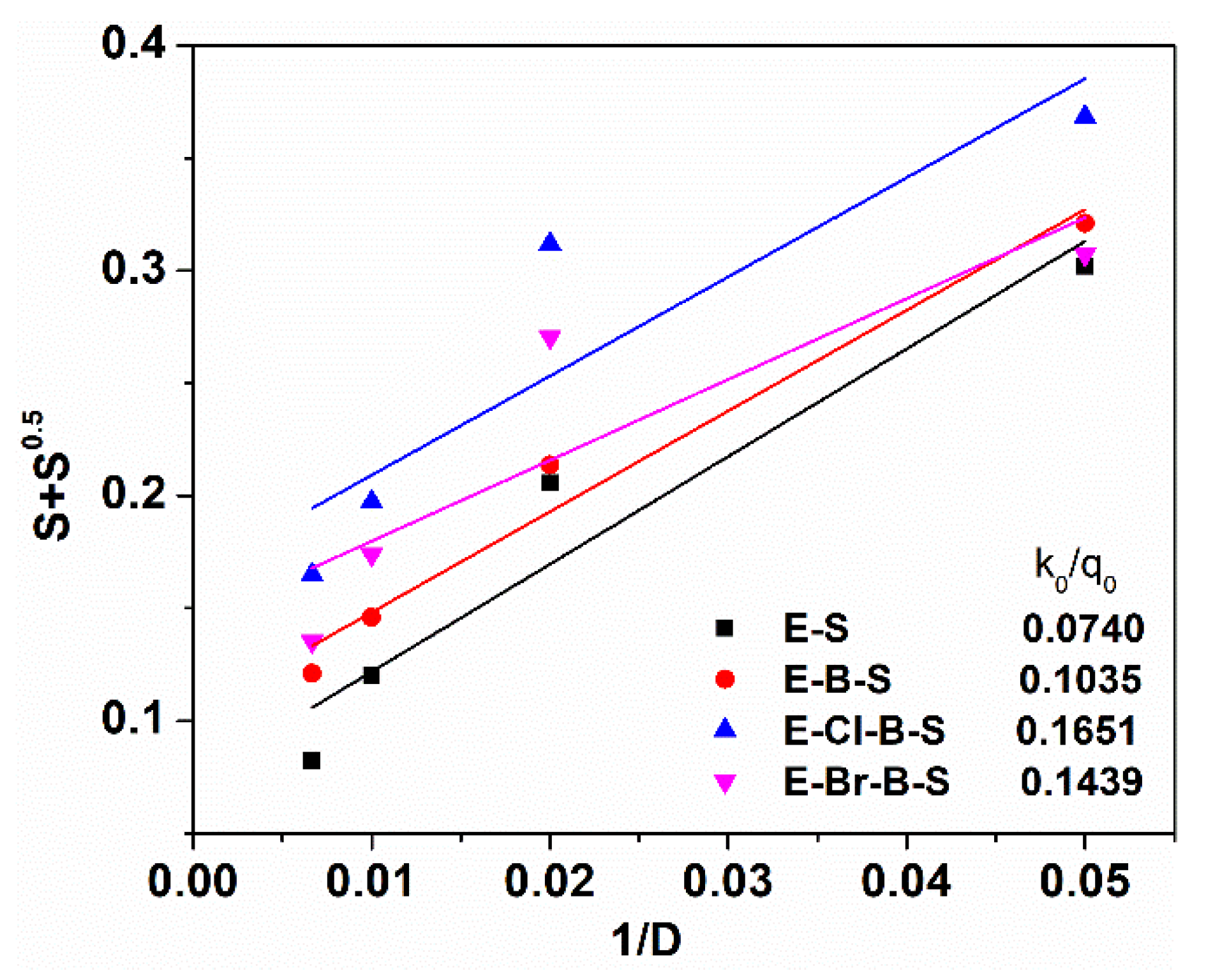
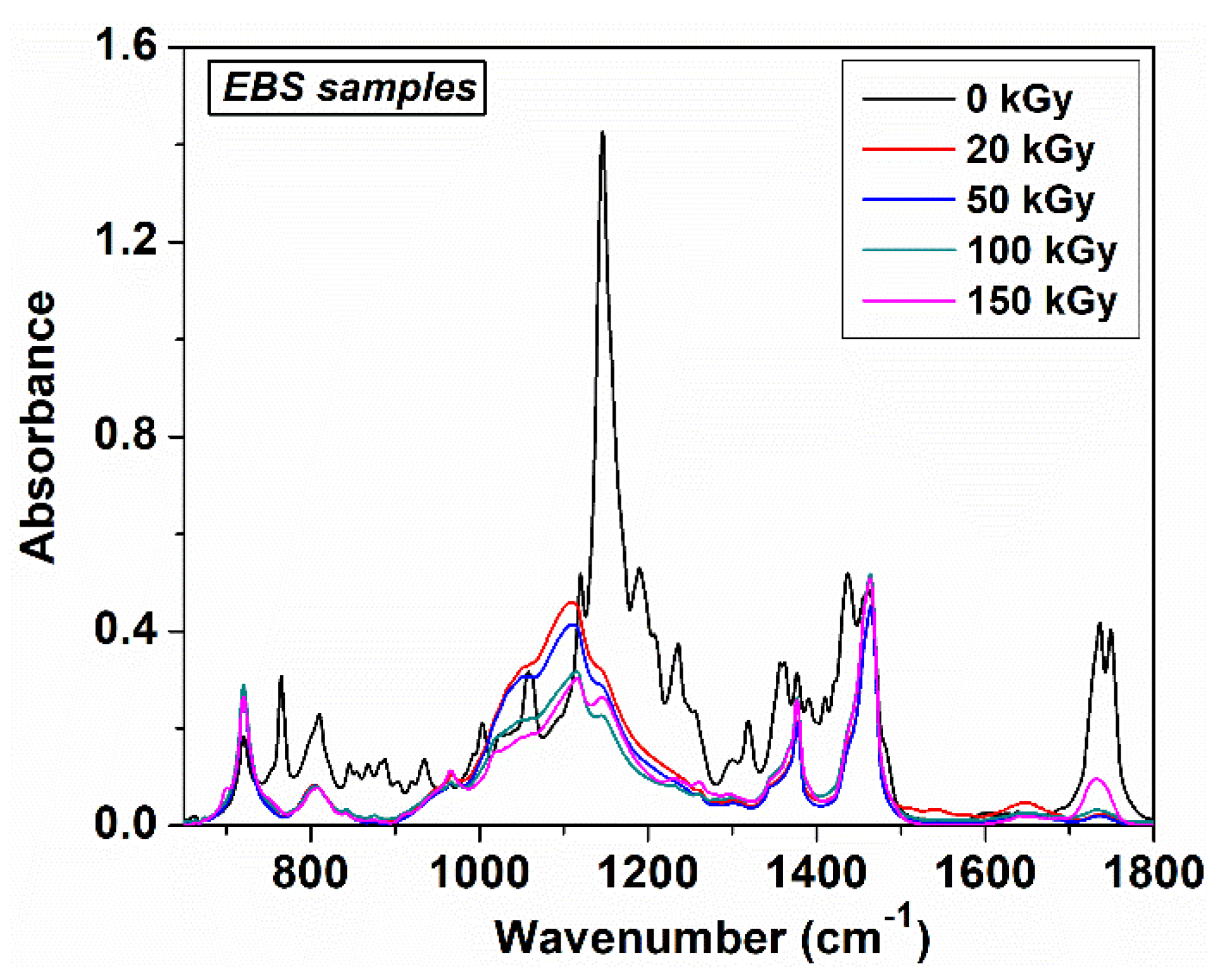
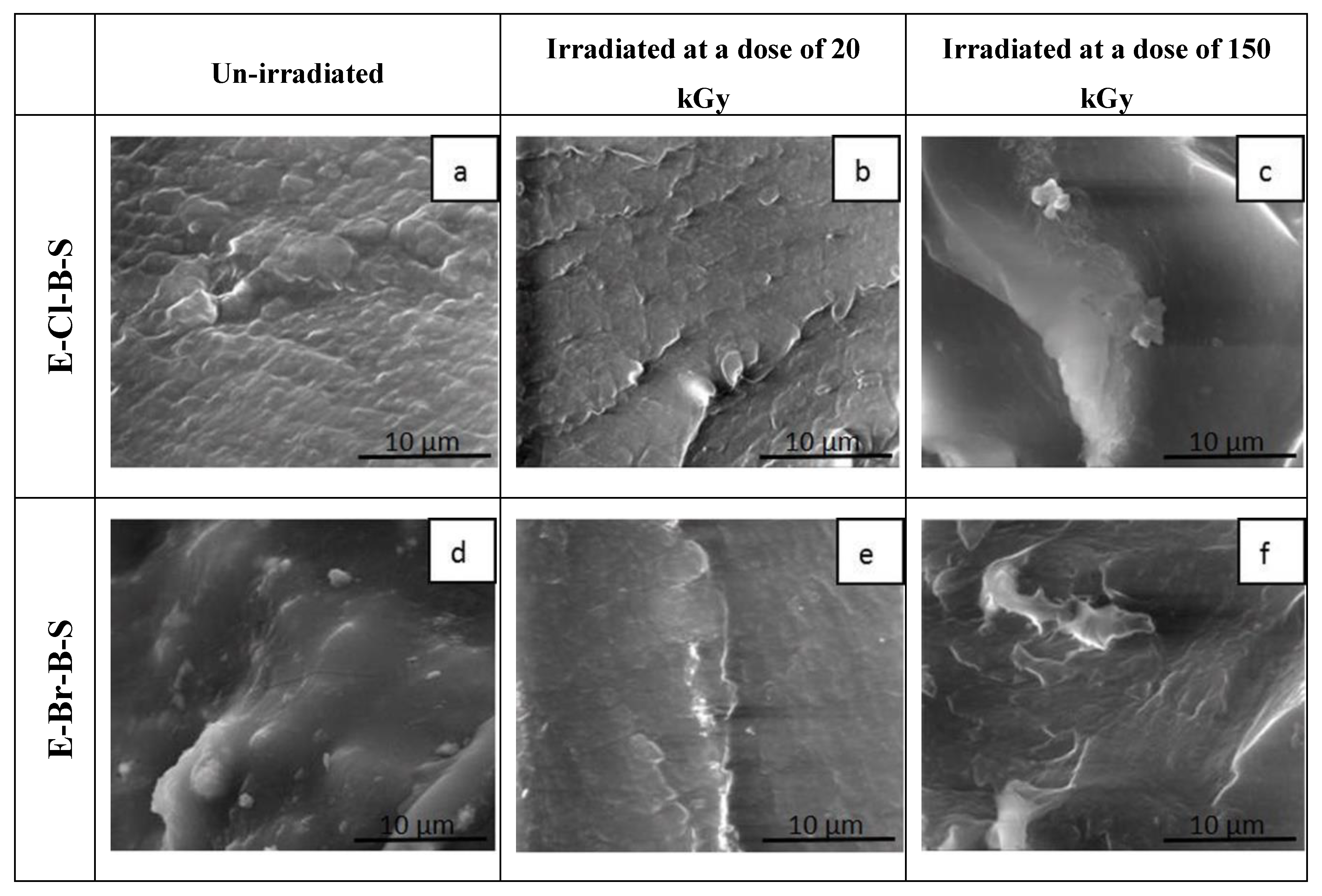
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Li, B.; Zhang, D.; Xie, L. External electric field induced structural transformation and decreased sensitivity of CL-20/EPDM composites. Chem. Phys. Lett. 2020, 757, 137875. [Google Scholar] [CrossRef]
- Airinei, A.; Homocianu, M.; Timpu, D.; Stelescu, D.M. New thermoplastic ionic elastomers based on MA-g-EPDM with advanced characteristics. In Thermoplastic Elastomers; El-Sonbati, A.Z., Ed.; Intech: Rijeka, Croatia, 2012; pp. 371–398. [Google Scholar]
- Stelescu, M.D.; Manaila, E.; Craciun, C. Vulcanization of ethylene-propylene termopolymer-based rubber mixtures by radiation processing. J. Appl. Polym. Sci. 2013, 128, 2325–2336. [Google Scholar] [CrossRef]
- El-Nemr, K.F.; Ali, M.A.M.; Hassan, M.M.; Hamed, H.E. Features of the structure and properties of radiation vulcanizates based on blends of polybutadiene and ethylene-propylene-diene rubber. J. Vinyl Addit. Technol. 2018, 25, E64–E72. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Li, L.Q.F.; Zhang, Q.; Wu, Y.P. Role of stearic acid in preparing EPDM/clay nanocomposites by melt compounding. Polym. J. 2007, 39, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Planes, E.; Chazeau, L.; Vigier, G.; Fourier, J. Evolution of EPDM networks aged by gamma irradiation-consequences on the mechanical properties. Polymer 2009, 50, 4028–4038. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.; Mathialagan, M. Comparative study on the effect of partial replacement of silica or calcium carbonate by bentonite on the properties of EPDM composites. Polym. Test. 2012, 31, 199–208. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, W.; Li, C.; Ye, L. Construction of multiple crosslinking networks in EPDM rubber: Synergistic reinforcing effect of graphene-zinc dimethacrylate on EPDM and improvement mechanism of sealing resilience. Compos. Part A Appl. Sci. Manuf. 2019, 121, 254–264. [Google Scholar] [CrossRef]
- Hajibabazadeh, S.; Palang, M.; Aghjeh, M.K.R. Effect of morphology development on mechanical properties and fracture behavior of PP/EPDM SiO2 blend-nanocomposites. Polym. Test. 2019, 73, 124–134. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deepraserktul, C. Reinforcement of natural rubber with silica carbon black hybrid filler. Polym. Test. 2007, 26, 367–377. [Google Scholar] [CrossRef]
- Lee, D.; Song, S.H. A study of silica reinforced rubber composites with eco-friendly processing aids for pneumatic tires. Macromol. Res. 2019, 27, 850–856. [Google Scholar] [CrossRef]
- Rajkumar, K.; Durivedi, C.; Thavamani, P.; Jeyanthi, P.; Pazhanisany, P. Effect of nanosilica on ethylene propylene diene monomer rubber nanocomposites. Int. J. Innov. Res. Dev. 2013, 2, 831–841. [Google Scholar]
- Planes, E.; Chazeau, L.; Vigier, G. Role of temperature during ageing under gamma irradiation of filled EPDM: Consequences on mechanical properties. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Acharya, H.; Srivastava, S.K. Mechanical, thermo-mechanical, thermal, and swelling properties of EPDM-organically modified mesoporous silica nanocomposites. Polym. Compos. 2017, 38, E371–E380. [Google Scholar] [CrossRef]
- Chatterjee, K.; Naskar, K. Study on characterization and properties of nanosilica-filled thermoplastic vulcanizates. Polym. Eng. Sci. 2008, 48, 1077–1084. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Georgescu, M.; Manaila, E. Aspects Regarding Crosslinking of a Natural Rubber Blend. In Proceedings of the 3rd International Conference on Advanced Materials and Systems, ICAMS 2010, Bucharest, Romania, 16–18 September 2010; pp. 313–318. [Google Scholar]
- Ning, N.; Ma, Q.; Zhang, Y.; Zhang, L.; Wu, H.; Tian, M. Enhanced thermo-oxidative aging resistance of EPDM at high temperature by using synergistic antioxidants. Polym. Degrad. Stab. 2014, 102, 1–8. [Google Scholar] [CrossRef]
- Jose, S.T.; Anand, A.K.; Joseph, R. EPDM/CIIR blends improved mechanical properties through procuring. Polym. Bull. 2009, 63, 135–146. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C.; Pamfil, D.; Doroftei, F. Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers 2018, 10, 1206. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Bbandyopadhyay, D. Rubber and Rubber-Like Materials. In Proceedings of the International Conference on Rubber and Rubber-like Materials, Jamshedpur, India, 6–8 November 1986; pp. 113–150. [Google Scholar]
- Vrowther, B.G.; Melley, R.E. Compouding EPDM rubbers for improved tack and adhesion. Rubber Ind. 1974, 8, 197–201. [Google Scholar]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Dumitrescu, M. New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci. World J. 2014, 2014, 684047. [Google Scholar] [CrossRef]
- Phetarporn, V.; Loykulnant, S.; Kongkaew, C.; Seubsai, A.; Prapainainar, P. Composite properties of graphene-based materials/natural rubber vulcanized using electron beam irradiation. Mater. Today Commun. 2019, 19, 413–424. [Google Scholar] [CrossRef]
- Aksüt, D.; Demeter, M.; Vancea, C.; Şen, M. Effect of radiation on vinyl-methyl-polysiloxane and phenyl-vinyl-methylpolysiloxane elastomers cured with different co-agents: Comparative study of mechanical and relaxation properties. Rad. Phys. Chem. 2019, 158, 87–93. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Zuga, N. The use of polyfunctional monomers in radical cure of chlorinated polyethylene. Polym. J. 2011, 43, 792–800. [Google Scholar] [CrossRef]
- Scaglinsi, S.R.; Cartoso, E.C.; Lungao, A.B. Radiation-induced degradation of butyl rubber vulcanized by three crosslinking systems. Radiat. Phys. Chem. 2012, 81, 991–994. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanism of crosslinked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Fifere, N.; Craciun, G.; Varganici, C.; Doroftei, F. Exploring the effect of electron beam irradiation on the properties of some EPDM-flax fiber composites. Polym. Compos. 2019, 40, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifhitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Della Volpe, C.; Siboni, S. Acid-base surface free energies of solids and the definition of scales in the Good-van Oss-Chaudhury theory. J. Adhes. Sci. Technol. 2000, 14, 235–272. [Google Scholar] [CrossRef]
- El-Nenic, K.F.; Mohamed, R.M. Sorbic acid as friendly curing agent for advanced properties of ethylene propylene diene monomer rubber using gamma radiation. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 711–719. [Google Scholar]
- Webb, R.N.; Shaffer, D.S.; Tsu, A.H. Butyl rubber. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NY, USA, 2014; Volume 5, pp. 356–381. [Google Scholar]
- Charlesby, A.; Pinner, S.H. Analysis of the solubility behavior of irradiation polyethylene and other polymers. Proc. R. Soc. London A 1959, 249, 367–386. [Google Scholar]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, M.; Jia, D.; Zhou, Y. Synthesis of natural rubber-g-maleic anhydride and its use as a compatibilizer in natural rubber/short nylon fiber composites. Chin. J. Polym. Sci. 2013, 31, 1127–1138. [Google Scholar] [CrossRef]
- Iacob, M.; Thomas, S.; Varughese, K.T. Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites. Compos. Sci. Technol. 2004, 64, 955–965. [Google Scholar]
- Zhou, Y.; Wang, S.; Zhang, Y.; Zhang, Y. Reinforcement effect of MMA on nano-CaCO3-filled EPDM vulcanizates and possible mechanism. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1226–1236. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Kalu, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mokkothu, T.H.; Luyt, A.S.; Messori, M. Reinforcement of EPDM rubber with in situ generated silica particles in presence of a coupling agent via a sol-gel route. Polym. Test. 2014, 33, 97–106. [Google Scholar] [CrossRef]
- Litvinov, V.M.; De, P.P. Spectroscopy of Rubbers and Rubbery Materials; RAPRA Technol. Ltd.: Shawbury, UK, 2002. [Google Scholar]
- Hagemeyer, H.J., Jr.; Edwards, M.B. Polyallomers: Synthesis and properties. J. Polym. Sci. Part C Polym. Symp. 1964, 4, 731–742. [Google Scholar] [CrossRef]
- Grochowicz, M. Investigation of the thermal behavior of 4-vinylpyridine-trimethylolpropane trimethacrylate copolymeric microspheres. J. Therm. Anal. Calorim. 2014, 118, 1602–1611. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.; Momen, G.; Ouellet-Plamondon, C.; David, E. Performance improvement of composites using modified fumed silica, titanium dioxide and grapheme additives. Polym Test. 2020, 84, 106281. [Google Scholar] [CrossRef]
- Fujimoto, K.; Nakade, S. Effects of termonomers on crosslinking rate and crosslinking structure of ethylene propylene terpolymers. J. Appl. Polym. Sci. 1969, 13, 1509–1522. [Google Scholar] [CrossRef]
- Carswell-Pomerantz, T.; Babanalbandi, A.; Dong, L.; Hill, D.J.T.; Perera, M.C.S.; Pomery, P.J.; Saadat, G.; Whittaker, A.K. Changes in molecular structure and properties of irradiated polymers at different compositions—ESR and NMR study. In Stability and Stabilization of Polymers under Irradiation; IAEA: Vienna, Austria, 1999; pp. 111–128. [Google Scholar]
- O’Donnell, J.H.; Whittaker, A.K. Observation by 13C NMR of H-crosslinks and methyl end groups due to main-chain scission in ethylene propylene copolymers after γ-irradiation. Polymer 1992, 33, 62–67. [Google Scholar] [CrossRef]
- Bremner, T.; Hill, D.J.T.; O’Donnell, J.H.; Perera, M.C.S.; Pomery, P.J. Mechanism of radiative degradation of polysobutylene. J. Polym. Sci. Polym. Chem. Ed. 1996, 34, 971–984. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C. Property correlations for composites based on ethylene propylene diene rubber reinforced with flax fibers. Polym. Test. 2017, 59, 75–83. [Google Scholar] [CrossRef]
- Celette, N.; Stevenson, I.; David, L.; Davemas, J.; Vigier, G.; Seytre, G. Irradiation effects on the relaxation behavior of EPDM elastomers. Polym. Int. 2004, 53, 495–505. [Google Scholar] [CrossRef]
- Varganici, C.D.; Ursache, O.; Gaina, C.; Gaina, V.; Simionescu, B.C. Studies of new hybrid materials prepared by both Diels-Alder and Michael addition reactions. J. Therm. Anal. Calorim. 2013, 111, 1561–1570. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Varganici, C.D. The thermal stability of some semi-interpenetrated polymer networks based on epoxy resin and aromatic polyurethane. J. Anal. Appl. Pyrolysis 2013, 100, 103–110. [Google Scholar] [CrossRef]
- Varganici, C.D.; Marangoci, N.; Rosu, L.; Barbu-Mic, C.; Rosu, D.; Pinteala, M.; Simionescu, B.C. TGA/DTA-FTIR-MS coupling analytical tool confirming inclusion complex occurred in supramolecular host-guest architectures. J. Anal. Appl. Pyrolysis 2015, 115, 132–142. [Google Scholar] [CrossRef]
- Fuke, C.A.; Mahanwar, P.A.; Chowdhury, S.R. Modified ethylene-propylene-diene elastomer (EPDM)-containing silicone rubber/ ethylene-propylene-diene elastomer (EPDM) blends: Effect of composition and electron beam crosslinking on mechanical, heat shrinkability, electrical and morphological properties. J. Appl. Polym. Sci. 2019, 136, 47787. [Google Scholar] [CrossRef]
- Rallini, M.; Kenny, J.M. Nanofillers in polymers. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–86. [Google Scholar]






| Ingredients (phr *) | Sample Code | |||
|---|---|---|---|---|
| E-S | E-B-S | E-Cl-B-S | E-Br-B-S | |
| EPDM (E) | 100 | 95 | 95 | 95 |
| TMPT | 3 | 3 | 3 | 3 |
| Irganox | 0.5 | 0.5 | 0.5 | 0.5 |
| Nanosilica | 2 | 2 | 2 | 2 |
| Butyl rubber (B) | - | 5 | - | - |
| Cl-butyl rubber (Cl-B) | - | - | 5 | - |
| Br-butyl rubber (Br-B) | - | - | - | 5 |
| Total | 105.5 | 105.5 | 105.5 | 105.5 |
| Sample | Absorbed Dose (kGy) | Vrf | Vro/Vrf |
|---|---|---|---|
| E-S | 20 | 0.1601 | 1.0295 |
| 50 | 0.2646 | 0.6228 | |
| 100 | 0.3518 | 0.4684 | |
| 150 | 0.3856 | 0.4273 | |
| E-B-S | 20 | 0.1609 | 1.0208 |
| 50 | 0.2571 | 0.6389 | |
| 100 | 0.3415 | 0.4810 | |
| 150 | 0.3708 | 0.4429 | |
| E-Cl-B-S | 20 | 0.1470 | 1.1886 |
| 50 | 0.2000 | 0.8738 | |
| 100 | 0.3013 | 0.5800 | |
| 150 | 0.3448 | 0.5068 | |
| E-Br-B-S | 20 | 0.1875 | 1.0590 |
| 50 | 0.2307 | 0.8607 | |
| 100 | 0.3261 | 0.6088 | |
| 150 | 0.3613 | 0.5496 |
| Sample. | Tg1 (°C) | Tm1 (°C) | ΔHm1 (J g−1) | Tg2 (°C) | Tm2 (°C) | ΔHm2 (J g−1) | Tcr (°C) | ΔHcr (J g−1) | Tcc (h1) (°C) | ΔHcc (J g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| E-S | −39 | 52 | 8.71 | −44 | 40 | 17.26 | 15 | −23.84 | 27 | −1.47 |
| E-S-150 | −37 | 54 | 8.27 | −42 | 45 | 16.18 | 22 | −21.22 | 31 | −2.37 |
| E-B-S | −40 | 53 | 9.07 | −41 | 36 | 13.69 | 16 | −13.41 | 25 | −2.51 |
| E-B-S-150 | −37 | 53 | 9.44 | −39 | 45 | 13.16 | 23 | −13.73 | 25 | −2.84 |
| E-Cl-B-S | −36 | 48 | 13.79 | −43 | 42 | 12.92 | 17 | −16.82 | – | – |
| E-Cl-B-S-150 | −31 | 53 | 5.17 | −40 | 47 | 7.25 | 22 | −8.96 | 28 | −1.16 |
| E-Br-B-S | −40 | 52 | 7.42 | −39 | 41 | 11.88 | 20 | −13.5 | 27 | −1.40 |
| E-Br-B-S-150 | −34 | 55 | 6.80 | −36 | 49 | 11.17 | 26 | −12.74 | 29 | −1.86 |
| Sample | T5% (°C) | Tmax (°C) | W (%) | T10% (°C) | T30% (°C) | T50% (°C) | R (%) |
|---|---|---|---|---|---|---|---|
| E-S | 438 | 473 | 96 | 448 | 461 | 470 | 2.85 |
| E-S-150 | 445 | 475 | 94 | 452 | 465 | 472 | 4.07 |
| E-B-S | 417 | 473 | 92 | 430 | 456 | 465 | 3.44 |
| E-B-S-150 | 420 | 474 | 94 | 432 | 458 | 468 | 3.88 |
| E-Cl-B-S | 413 | 470 | 96 | 431 | 456 | 468 | 3.31 |
| E-Cl-B-S-150 | 419 | 472 | 94 | 433 | 457 | 475 | 4.75 |
| E-Br-B-S | 412 | 471 | 94 | 431 | 455 | 466 | 4.36 |
| E-Br-B-S-150 | 416 | 472 | 93 | 433 | 457 | 467 | 4.96 |
| Sample | Contact Angle (Ɵ) Values | |||
|---|---|---|---|---|
| Water | Formamide | Diiodomethane | ||
| 0 kGy | E-S | 102.4 | 90.4 | 51.6 |
| E-B-S | 106.2 | 94.9 | 58.2 | |
| E-Cl-B-S | 101.1 | 92.3 | 73.5 | |
| E-Br-B-S | 98.9 | 92.2 | 61.3 | |
| 20 kGy | E-S | 109.6 | 97.1 | 67.7 |
| E-B-S | 111.6 | 104.2 | 77.7 | |
| E-Cl-B-S | 109.3 | 97.8 | 78.4 | |
| E-Br-B-S | 108.8 | 100.6 | 79.0 | |
| 150 kGy | E-S | 112.2 | 104.7 | 80.0 |
| E-B-S | 113.3 | 108.8 | 86.8 | |
| E-Cl-B-S | 111.9 | 106.6 | 88.6 | |
| E-Br-B-S | 110.4 | 105.8 | 87.2 | |
| Sample | γsvLW | γsv+ | γsv- | γsvAB | γlvTOT | P% | |
|---|---|---|---|---|---|---|---|
| 0 kGy | E-S | 33.38 | 2.6328 | 3.76 | 6.29 | 39.67 | 15.86 |
| E-B-S | 29.61 | 2.5962 | 3.15 | 5.72 | 35.33 | 16.18 | |
| E-Cl-B-S | 20.94 | 0.4338 | 5.04 | 2.96 | 23.90 | 12.37 | |
| E-Br-B-S | 27.83 | 2.0860 | 7.05 | 7.67 | 35.50 | 21.60 | |
| 20 kGy | E-S | 24.15 | 1.3893 | 2.02 | 3.35 | 27.50 | 12.17 |
| E-B-S | 18.69 | 1.5495 | 3.25 | 4.49 | 23.18 | 19.36 | |
| E-Cl-B-S | 18.35 | 0.3904 | 2.21 | 1.86 | 20.21 | 9.19 | |
| E-Br-B-S | 18.01 | 0.8119 | 3.48 | 3.36 | 21.37 | 15.73 | |
| 150 kGy | E-S | 17.50 | 1.2929 | 3.08 | 3.99 | 21.49 | 18.57 |
| E-B-S | 14.15 | 1.1605 | 3.83 | 4.22 | 18.37 | 22.95 | |
| E-Cl-B-S | 13.33 | 0.6330 | 3.75 | 3.08 | 16.41 | 18.76 | |
| E-Br-B-S | 14.00 | 0.7549 | 4.43 | 3.66 | 17.66 | 20.71 | |
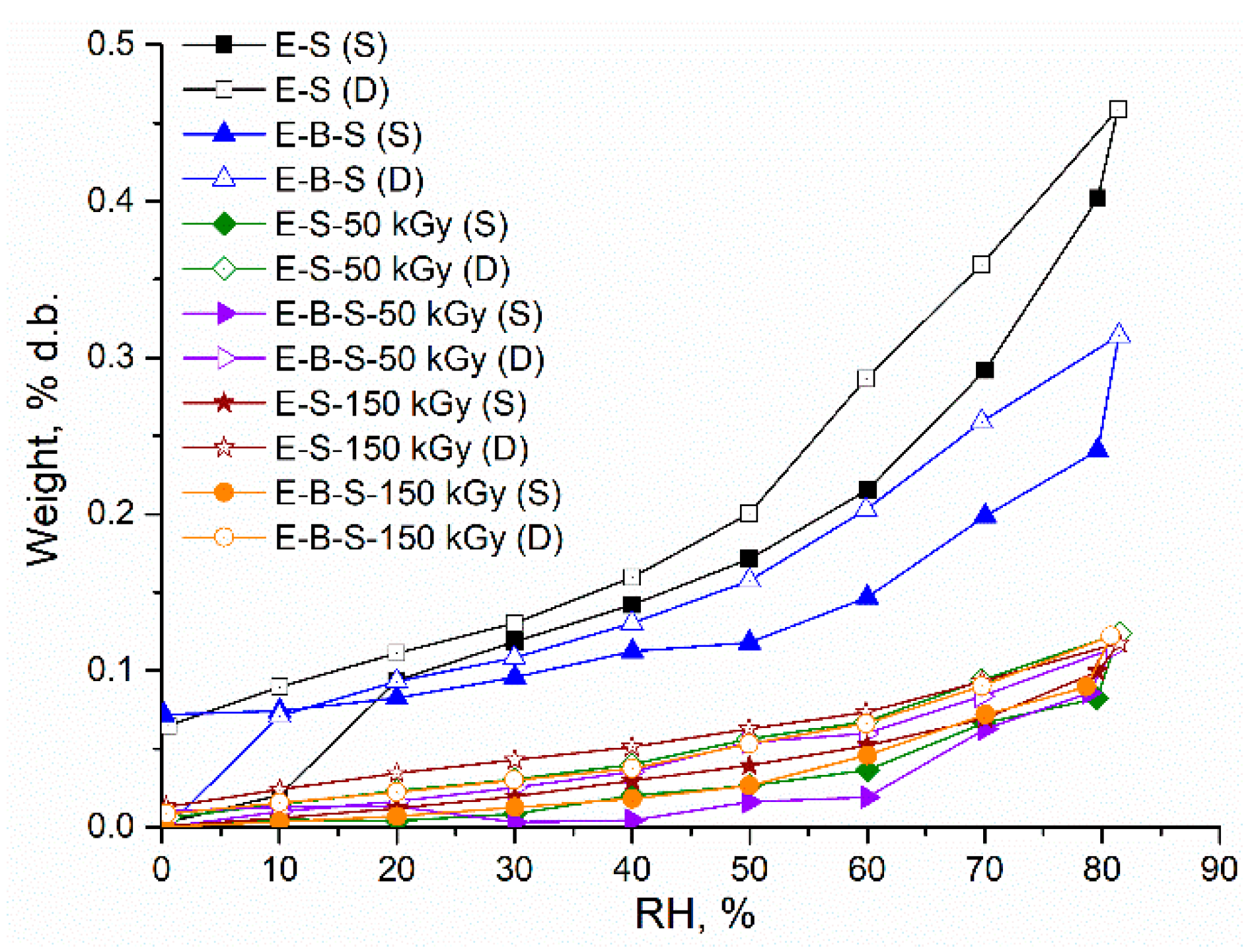
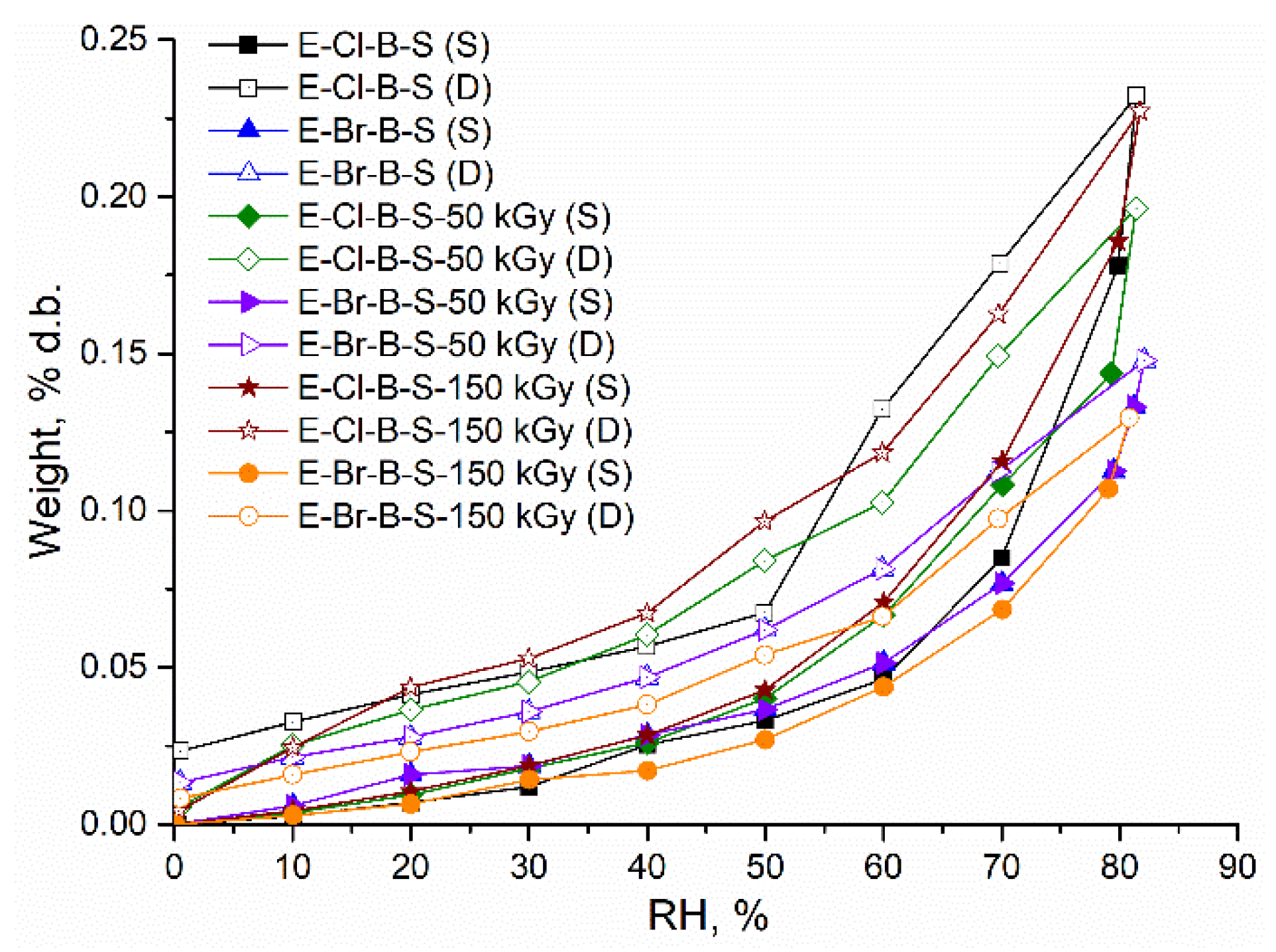
| Sample | Sorption Capacity (% d.b.) | rpm, (nm) | BET Data | |
|---|---|---|---|---|
| Area (m2 g−1) | Monolayer (g g−1) | |||
| E-S-0 * | 0.400 | 0.64 | 12.6 | 0.00350 |
| E-S-50 | 0.080 | 2.43 | 0.66 | 0.00019 |
| E-S-150 | 0.100 | 1.73 | 1.16 | 0.00033 |
| E-B-S-0 | 0.240 | 0.83 | 5.80 | 0.00160 |
| E-B-S-50 | 0.114 | 4.57 | 0.50 | 0.000001 |
| E-B-S-150 | 0.122 | 2.75 | 0.89 | 0.000254 |
| E-Cl-B-S-0 | 0.177 | 4.80 | 0.74 | 0.0021 |
| E-Cl-B-S-50 | 0.143 | 1.70 | 1.69 | 0.000482 |
| E-Cl-B-S-150 | 0.185 | 1.98 | 1.87 | 0.000535 |
| E-Br-B-S-0 | 0.120 | 2.64 | 0.912 | 0.00026 |
| E-Br-B-S-50 | 0.126 | 2.79 | 0.905 | 0.000301 |
| E-Br-B-S-150 | 0.107 | 2.41 | 0.890 | 0.000256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaila, E.; Airinei, A.; Stelescu, M.D.; Sonmez, M.; Alexandrescu, L.; Craciun, G.; Pamfil, D.; Fifere, N.; Varganici, C.-D.; Doroftei, F.; et al. Radiation Processing and Characterization of Some Ethylene-propylene-diene Terpolymer/Butyl (Halobutyl) Rubber/Nanosilica Composites. Polymers 2020, 12, 2431. https://doi.org/10.3390/polym12102431
Manaila E, Airinei A, Stelescu MD, Sonmez M, Alexandrescu L, Craciun G, Pamfil D, Fifere N, Varganici C-D, Doroftei F, et al. Radiation Processing and Characterization of Some Ethylene-propylene-diene Terpolymer/Butyl (Halobutyl) Rubber/Nanosilica Composites. Polymers. 2020; 12(10):2431. https://doi.org/10.3390/polym12102431
Chicago/Turabian StyleManaila, Elena, Anton Airinei, Maria Daniela Stelescu, Maria Sonmez, Laurentia Alexandrescu, Gabriela Craciun, Daniela Pamfil, Nicusor Fifere, Cristian-Dragos Varganici, Florica Doroftei, and et al. 2020. "Radiation Processing and Characterization of Some Ethylene-propylene-diene Terpolymer/Butyl (Halobutyl) Rubber/Nanosilica Composites" Polymers 12, no. 10: 2431. https://doi.org/10.3390/polym12102431
APA StyleManaila, E., Airinei, A., Stelescu, M. D., Sonmez, M., Alexandrescu, L., Craciun, G., Pamfil, D., Fifere, N., Varganici, C.-D., Doroftei, F., & Bele, A. (2020). Radiation Processing and Characterization of Some Ethylene-propylene-diene Terpolymer/Butyl (Halobutyl) Rubber/Nanosilica Composites. Polymers, 12(10), 2431. https://doi.org/10.3390/polym12102431








