Polyvinyl Alcohol/Calcium Carbonate Nanocomposites as Efficient and Cost-Effective Cationic Dye Adsorbents
Abstract
:1. Introduction
2. Experimental
2.1. Materials
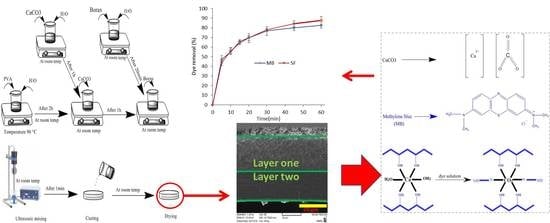
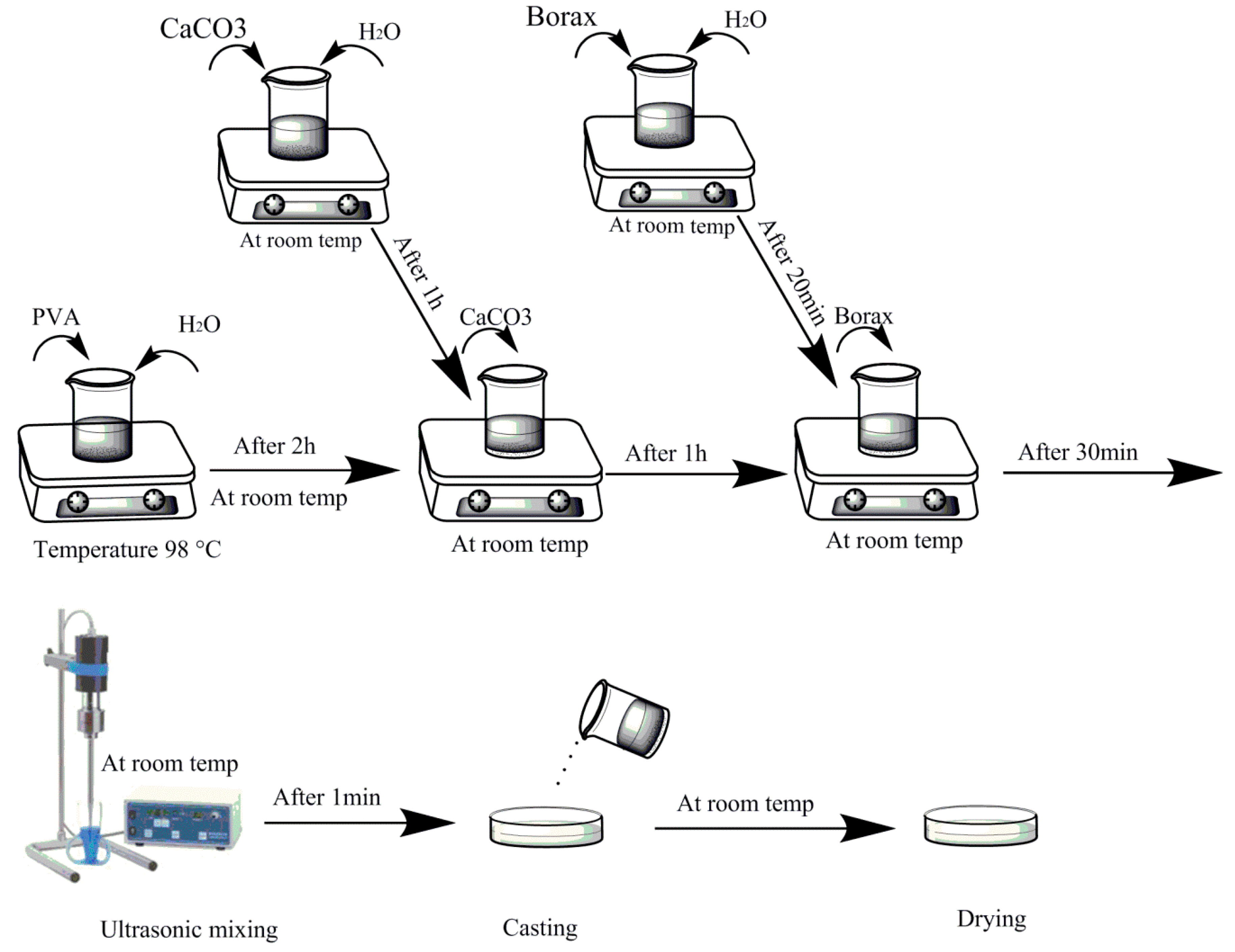
2.2. Fabrication of Nanocomposites
2.3. Characterization
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Dye Removal and Adsorption Efficiency
2.3.4. Dye Desorption
2.3.5. Swelling Measurements
3. Results and Discussion
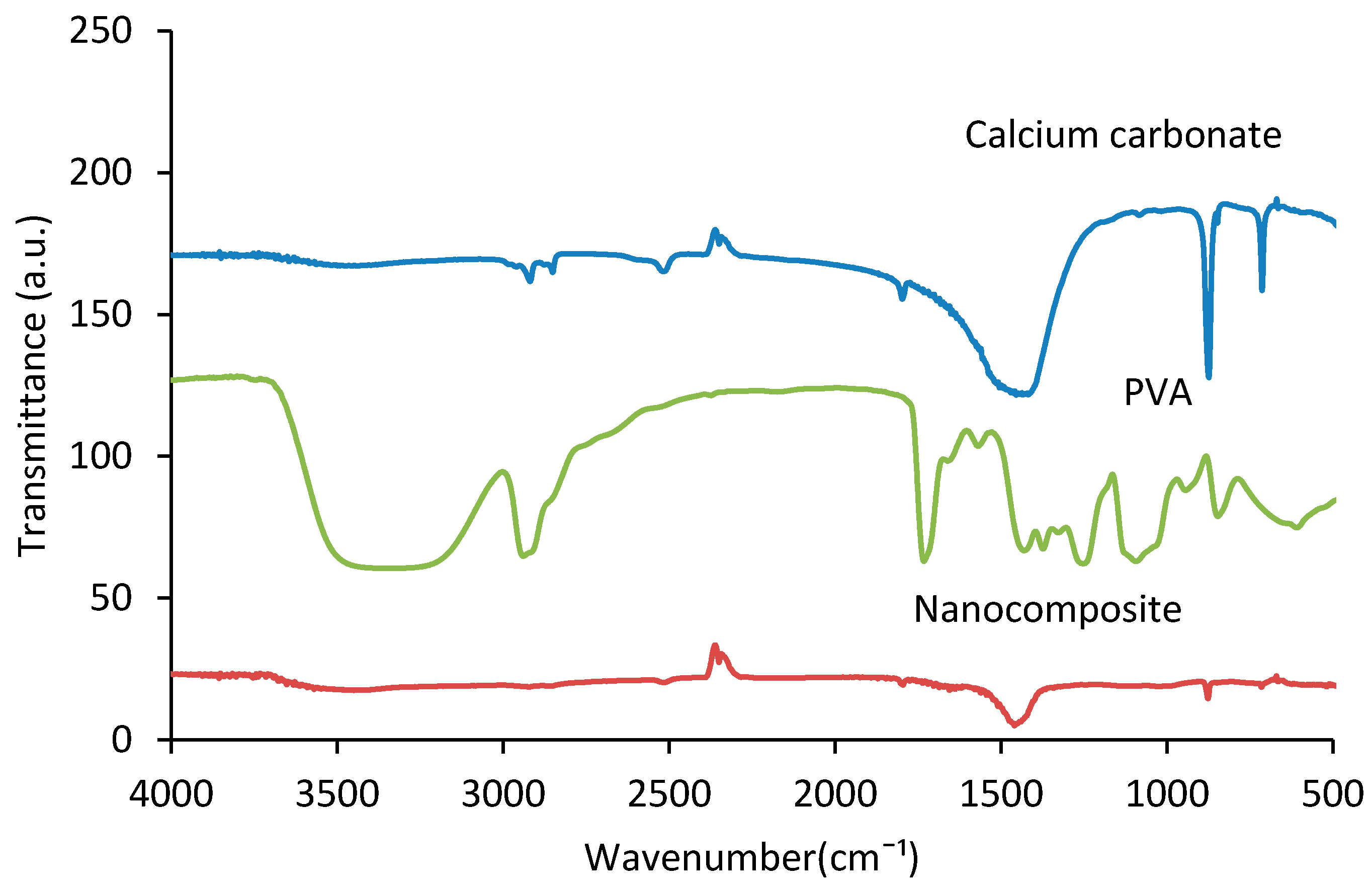
3.1. FTIR Spectroscopy
3.2. Microstructure
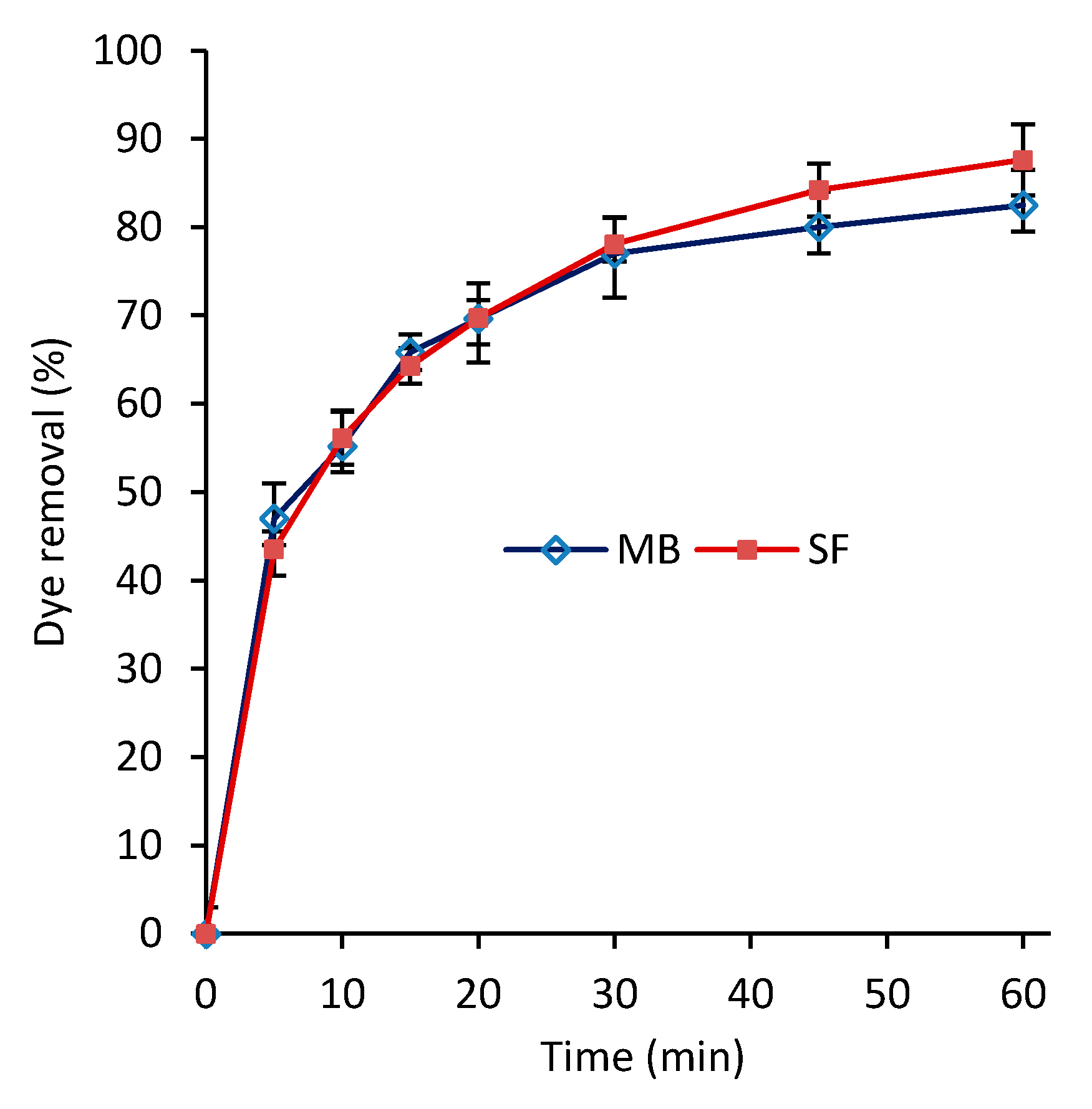
3.3. Dye Removal and Efficiency
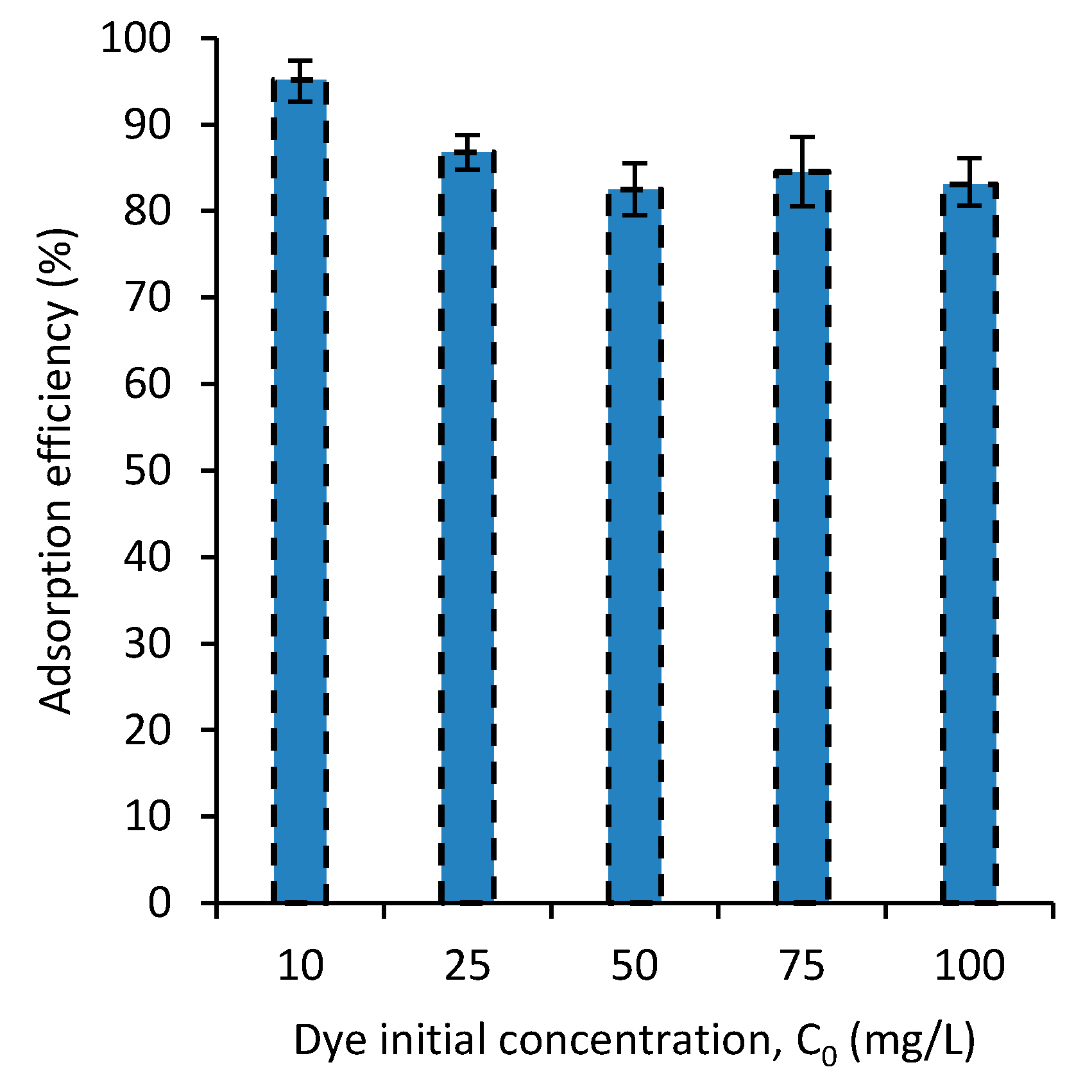
3.4. Dye Concentration Effect on Adsorption Efficiency
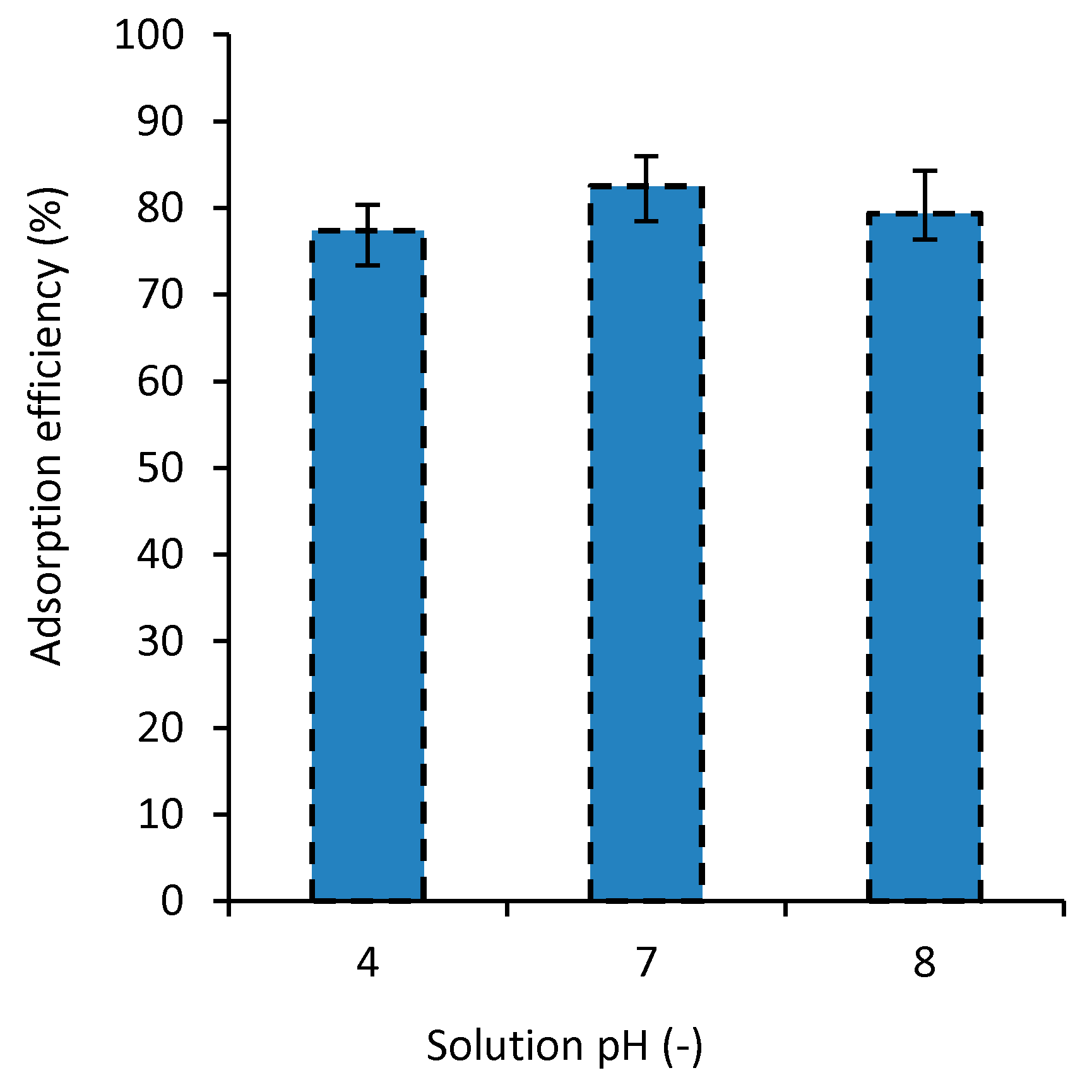
3.5. The Effect of Solution pH on Adsorption Efficiency
3.6. Swelling
3.7. Adsorption Mechanisms
3.8. Reusability
4. Modeling
4.1. Dye-Adsorption Kinetics
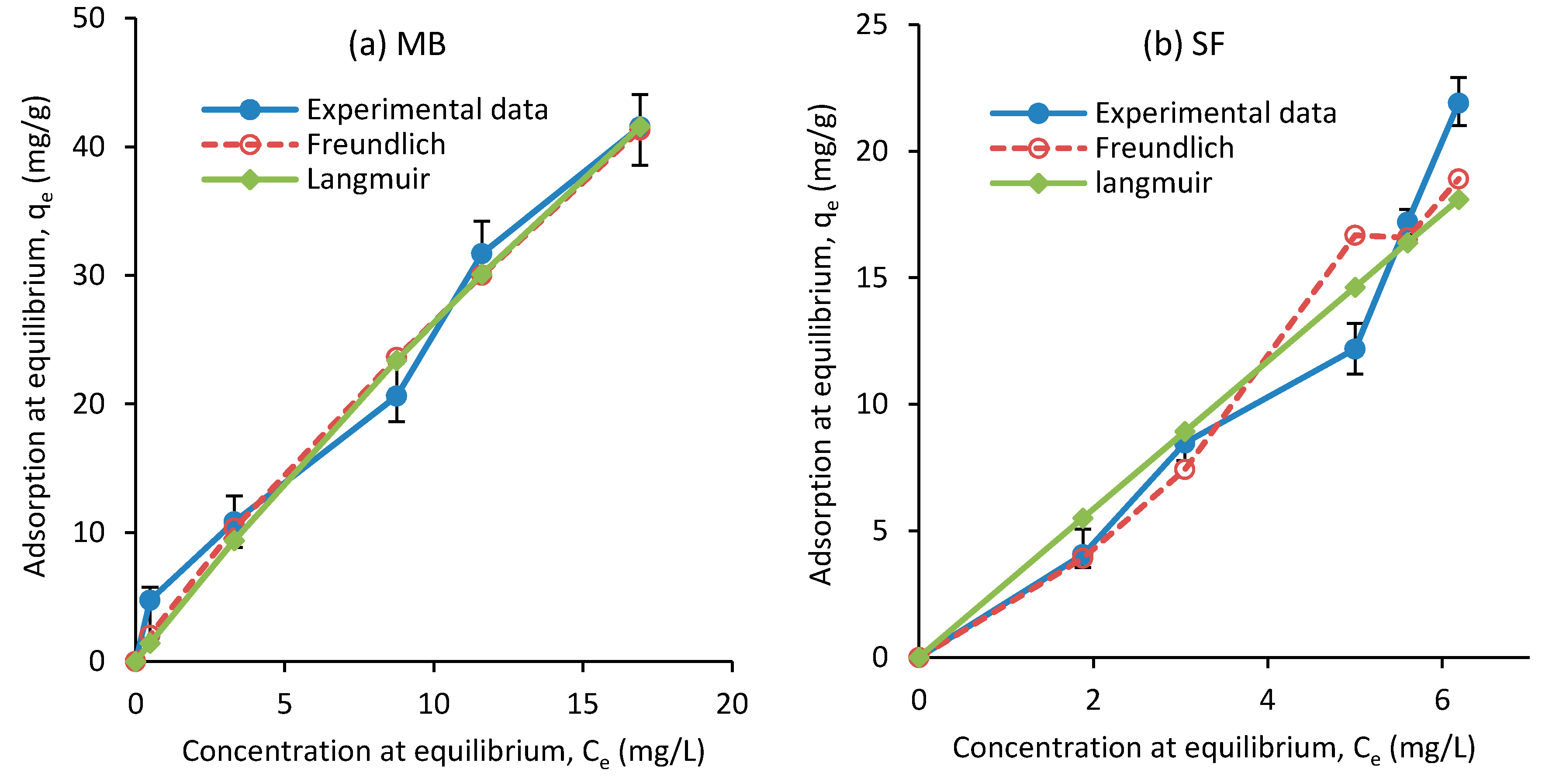
4.2. Isotherm Adsorption
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dos Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, N.D.; Novais, J.M.; Pinheiro, H.M. Effect of some operational parameters on textile dye biodegradation in a sequential batch reactor. J. Biotechnol. 2001, 89, 163–174. [Google Scholar] [CrossRef]
- Benefield, L.D.; Randall, C.W. Attached Growth Biological Treatment Processes, Biological Process Design for Waste Water Treatment; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1980; Volume 7632, pp. 410–412. [Google Scholar]
- Cengiz, S.; Tanrikulu, F.; Aksu, S. An alternative source of adsorbent for the removal of dyes from textile waters: Posidonia oceanica (L.). Chem. Eng. J. 2012, 189, 32–40. [Google Scholar] [CrossRef]
- Gong, J.L.; Wang, B.; Zeng, G.M.; Yang, C.P.; Niu, C.G.; Niu, Q.Y.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef]
- Fan, W.; Zheng, S. Reaction-induced microphase separation in thermosetting blends of epoxy resin with poly(methyl methacrylate)-block-polystyrene block copolymers: Effect of topologies of block copolymers on morphological structures. Polymer 2008, 49, 3157–3167. [Google Scholar] [CrossRef]
- Haacke, G.; Longordo, E.; Andrawes, F.; Campbell, B.H. Interactions between light stabilizers and pigment particles in polymeric coatings. Prog. Org. Coat. 1998, 34, 75–83. [Google Scholar] [CrossRef]
- He, D.; Susanto, H. Mathias Ulbricht. Photo-irradiation for preparation, modification and stimulation of polymeric membranes. Prog. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Mohan, D.; Chander, S. Removal and recovery of metal ions from acid mine drainage using lignite—A low cost sorbent. J. Hazard. Mater. 2006, 137, 1545–1553. [Google Scholar] [CrossRef]
- Mondali, M.; Yousefi, M. Prediction a range for elastic modulus of CNT reinforced polymer composites using analytical method. Modares Mech. Eng. 2014, 14, 52–60. [Google Scholar]
- Santhosh, C.; Nivetha, R.; Kollu, P.; Srivastava, V.; Sillanpaa, M.; Grace, A.N.; Bhatnagar, A. Removal of cationic and anionic heavy metals from water by 1D and 2D-carbon structures decorated with magnetic nanoparticles. Sci. Rep. 2017, 7, 14461–14472. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N. Adsorption of dyes from aqueous solutions on activated charcoal. J. Hazard. Mater. 2007, 139, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.S.; Özcan, A. Adsorption of acid dyes from aqueous solutions onto acid-activated bentonite. J. Colloid Interface Sci. 2004, 276, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Zhou, J.; Guo, S. Cellulose/chitin beads for adsorption of heavy metals in aqueous solution. Water Res. 2004, 38, 2643–2650. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Osman, N.A.A.; Hai, N.D.; Hassan, C.R.C. Adsorption of dyes using poly(vinyl alcohol) (PVA) and PVA-based polymer composite adsorbents—A review. J. Polym. Environ. 2020, 28, 775–793. [Google Scholar] [CrossRef]
- Papancea, A.; Patachia, S. Characterization of dyes loaded polyvinyl alcohol (PVA) based hydrogels through cielab method. Environ. Eng. Manag. J. 2015, 14, 361–371. [Google Scholar]
- Ghourbanpour, J.; Sabzi, M.; Shafagh, N. Effective dye adsorption behavior of poly(vinyl alcohol)/chitin nanofiber/Fe (III) complex. Int. J. Biol. Macromol. 2019, 137, 296–306. [Google Scholar] [CrossRef]
- Li, C.; She, M.; She, X.; Dai, J.; Kong, L. Functionalization of polyvinyl alcohol hydrogels with graphene oxide for potential dye removal. J. Appl. Polym. Sci. 2014, 13, 39–87. [Google Scholar] [CrossRef]
- Cole, M.; Eggleston, G.; Wang, Y.J. Understanding the causes of calcium carbonate crystal growth and inhibition during the carbonatation refining of raw sugars. Food Chem. 2019, 275, 24–31. [Google Scholar] [CrossRef]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J. Environ. Chem. Eng. 2014, 2, 2156–2161. [Google Scholar] [CrossRef]
- Subramanian, M.N. Plastics Additives and Testing; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Baláž, M.; Bujňáková, Z.; Baláž, P.; Zorkovská, A.; Danková, Z.; Briančin, J. Adsorption of cadmium(II) on waste biomaterial. J. Colloid Interface Sci. 2015, 454, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.A.; El Sayed, A.S.; Zayed, A.M. Removal of oil spills from salt water by magnesium, calcium carbonates and oxides. J. Appl. Sci. Environ. Manag. 2005, 8, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.H.; Gao, H.W. Turning calcium carbonate into a cost-effective wastewater-sorbing material by occluding waste dye. Environ. Sci. Pollut. Res. 2010, 17, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol−gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Sawhney, A.P.S.; Condon, B.; Singh, K.V.; Pang, S.S.; Li, G.; Hui, D. Modern Applications of nanotechnology in textiles. Text. Res. J. 2008, 78, 731–739. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Fayazi, M.; Afzali, D.; Taher, M.A.; Mostafavi, A.; Gupta, V.K. Removal of safranin dye from aqueous solution using magnetic mesoporous clay: Optimization study. J. Mol. Liq. 2015, 212, 675–685. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.C.; Hanafiah, M.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Taleb, M.F.A.; El-Mohdy, H.A.; El-Rehim, H.A. Radiation preparation of PVA/CMC copolymers and their application in removal of dyes. J. Hazard. Mater. 2009, 168, 68–75. [Google Scholar] [CrossRef]
- Peppas, N.A. Biomedical Applications of Hydrogels Handbook; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Shalumon, K.T.; Binulal, N.S.; Selvamurugan, N.; Nair, S.V.; Menon, D.; Furuike, T.; Jayakumar, R. Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr. Polym. 2009, 77, 863–869. [Google Scholar] [CrossRef]
- Han, X.; Chen, S.; Hu, X. Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating. Desalination 2009, 240, 21–26. [Google Scholar] [CrossRef]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. J. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar]
- Cai, G.B.; Chen, S.F.; Liu, L.; Jiang, J.; Yao, H.B.; Xu, A.W.; Yu, S.H. 1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2017, 12, 234–241. [Google Scholar] [CrossRef]
- Brecevic, L.; Nielsen, A.E. Solubility of amorphous calcium carbonate. J. Cryst. Growth 1989, 98, 504–510. [Google Scholar] [CrossRef]
- Shafiu Kamba, A.; Ismail, M.; Ibrahim, T.; Azmi, T.; Zakaria, Z.A.B. Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockle shell powder (Anadara granosa). J. Nanomater. 2013, 52, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Reig, F.B.; Adelantado, J.V.G.; Moreno, M.C.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. J. Elsevier Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- El-Latif, M.A.; El-Kady, M.; Ibrahim, M.; Ossman, M. Alginate/polyvinyl alcohol—Kaolin composite for removal of methylene blue from aqueous solution in a batch stirred tank reactor. J. Am. Sci. 2010, 6, 280–292. [Google Scholar]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Garg, V.K.; Amita, M.; Kumar, R.; Gupta, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dye. Pigment. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Mahdavinia, G.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M. Modified chitosan 4. Super absorbent hydrogels from poly(acrylic acid-co-acrylamide) grafted chitosan with salt- and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Huang, X.; Gao, B.; Yue, Q.; Zhang, Y.; Sun, S. Compound bioflocculant used as a coagulation aid in synthetic dye wastewater treatment: The effect of solution pH. Sep. Purif. Technol. 2015, 154, 108–114. [Google Scholar] [CrossRef]
- Deniz, F.; Karaman, S. Removal of basic red 46 dye from aqueous solution by pine tree leaves. Chem. Eng. J. 2011, 170, 67–74. [Google Scholar] [CrossRef]
- Gürses, A.; Doğar, Ç.; Yalçın, M.; Açıkyıldız, M.; Bayrak, R.; Karaca, S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. J. Hazard. Mater. 2006, 131, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef]
- Vahidhabanu, S.; Karuppasamy, D.; Adeogun, A.I.; Babu, B.R. Impregnation of zinc oxide modified clay over alginate beads: A novel material for the effective removal of congo red from wastewater. RSC Adv. 2017, 7, 5669–5678. [Google Scholar] [CrossRef] [Green Version]
- Moussavi, G.; Khosravi, R. The removal of cationic dyes from aqueous solutions by adsorption onto pistachio hull waste. Chem. Eng. Res. Des. 2011, 89, 2182–2189. [Google Scholar] [CrossRef]
- Shen, C.; Shen, Y.; Wen, Y.; Wang, H.; Liu, W. Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe (III) hydrogel. Water Res. 2011, 45, 5200–5210. [Google Scholar] [CrossRef]
- Marrakchi, F.; Khanday, W.A.; Asif, M.; Hameed, B.H. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol. 2016, 93, 1231–1239. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Bressloff, P.C.; Newby, J.M. Stochastic models of intracellular transport. Rev. Mod. Phys. 2013, 85, 135. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Malik, P. Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2004, 113, 81–88. [Google Scholar] [CrossRef] [PubMed]
- AlmasiNahnaji, A.; Mahdavian, L. Investigation of the removal of chromium (VI) by Nanocomposites Chitosan-tragacanth solution from aqueous solution. J. Environ. Health Eng. 2016, 3, 129–142. [Google Scholar]
- Nagaiah, C.; Rüdiger, S.; Warnecke, G.; Falcke, M. Adaptive space and time numerical simulation of reaction–diffusion models for intracellular calcium dynamics. Appl. Math. Comput. 2012, 218, 10194–10210. [Google Scholar] [CrossRef]
- Tharaneedhar, V.; Kumar, P.S.; Saravanan, A.; Ravikumar, C.; Jaikumar, V. Prediction and interpretation of adsorption parameters for the sequestration of methylene blue dye from aqueous solution using microwave assisted corncob activated carbon. Sustain. Mater. Technol. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Yusuf, M.; Elfghi, F.M.; Mallak, S.K. Kinetic studies of Safranin-O removal from Aqueous Solutions using Pineapple Peels. Iran. J. Energy Environ. 2015, 6, 173–180. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Sahraei, R.; Pour, Z.S.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hajjati, S.; Mahmudi, Z.; Tyagi, I.; Agarwal, S.; Maity, A.; Gupta, V.K. Modeling of competitive ultrasonic assisted removal of the dyes–Methylene blue Safranin. Chem. Eng. J. 2015, 268, 29–37. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Bakar, N.F.A.; Him, N.R.N.; Lau, W.J. Adsorption Kinetics of Methylene Blue Dyes onto Magnetic Graphene Oxide. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mohamed, A.S. Adsorption Removal of Safranin Dye Contaminants from Water Using Various Types of Natural Zeolite. Silicon 2018, 11, 1635–1647. [Google Scholar] [CrossRef]
- Sahu, M.K.; Patel, R.K. Removal of safranin-O dye from aqueous solution using modified red mud: Kinetic and equilibrium studies. RSC Adv. 2015, 5, 78491–78501. [Google Scholar] [CrossRef]





| Used Parameters in Equation (4) | Used Parameters in Equation (5) | Used Parameters in Equation (6) |
|---|---|---|
| : time | : time | : time |
| : Pseudo-first-order kinetic coefficient | : Pseudo-second-order kinetic coefficient | : intraparticle diffusion coefficient |
| (mg/g): adsorption efficiency or equilibrium adsorption | (mg/g): adsorption efficiency or equilibrium adsorption | : intercept for boundary layer thickness |
| Concentration | Experimental | Pseudo First-Order Kinetic Model | Pseudo Second-Order Kinetic Model | Intra-Particle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/g) | (mg/g) | (min−1) | R2 | (mg/g) | (min−1) | R2 | R2 | ||
| 10 | 4.76 ± 0.12 | 4.49 | 0.18 | 0.98 | 4.70 | 34.81 | 0.99 | 2.44 | 0.35 | 0.91 |
| 25 | 10.85 ± 0.16 | 10.54 | 0.11 | 0.98 | 10.94 | 252.80 | 0.99 | 2.99 | 1.23 | 0.94 |
| 50 | 20.63 ± 0.63 | 19.65 | 0.14 | 0.97 | 20.46 | 2132.01 | 0.99 | 8.19 | 1.91 | 0.94 |
| 75 | 31.70 ± 0.88 | 30.88 | 0.13 | 0.98 | 32.11 | 7685.08 | 0.99 | 11.60 | 3.23 | 0.91 |
| 100 | 41.55 ± 0.95 | 29.23 | 0.18 | 0.97 | 41.07 | 23,260.09 | 0.99 | 21.12 | 3.16 | 0.64 |
| Concentration | Experimental | Pseudo First-Order Kinetic Model | Pseudo Second-Order Kinetic Model | Intraparticle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/g) | (mg/g) | (min−1) | R2 | (mg/g) | (min−1) | R2 | R2 | ||
| 10 | 4.06 ± 0.12 | 3.87 | 0.12 | 0.99 | 4.04 | 13.36 | 0.99 | 2.35 | 0.03 | 0.76 |
| 20 | 8.47 ± 0.26 | 8.14 | 0.14 | 0.98 | 8.50 | 148.41 | 0.99 | 5.39 | 0.06 | 0.76 |
| 30 | 12.18 ± 0.32 | 11.55 | 0.15 | 0.98 | 12.08 | 459.92 | 0.99 | 7.91 | 0.08 | 0.77 |
| 40 | 17.19 ± 0.43 | 16.43 | 0.13 | 0.98 | 17.12 | 1187.32 | 0.99 | 10.75 | 0.13 | 0.80 |
| 50 | 21.90 ± 0.65 | 20.76 | 0.11 | 0.97 | 21.56 | 2022.21 | 0.99 | 12.43 | 0.18 | 0.85 |
| Used Parameters in Equation (7) | Used Parameters in Equation (8) |
|---|---|
| : equilibrium concentration | : equilibrium concentration |
| : Langmuir constant | : Freundlich constant indicating adsorption capacity at unit concentration |
| (mg/g): equilibrium adsorption or the maximum absorption capacity | (mg/g): Freundlich constant indicating adsorption intensity |
| Dye | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| kL (L/mg) | Qm (mg/g) | R2 | RL | kf ((mg/g)(L/mg)) | 1/nf | R2 | |
| MB | 0.0118 | 249.9 | 0.97 | 0.83 | 3.795 | 0.85 | 0.97 |
| SF | 0.00035 | 81.83 | 0.81 | 0.99 | 1.075 | 1.32 | 0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahani, D.; Nazari, A.; Ghourbanpour, J.; Ameli, A. Polyvinyl Alcohol/Calcium Carbonate Nanocomposites as Efficient and Cost-Effective Cationic Dye Adsorbents. Polymers 2020, 12, 2179. https://doi.org/10.3390/polym12102179
Jahani D, Nazari A, Ghourbanpour J, Ameli A. Polyvinyl Alcohol/Calcium Carbonate Nanocomposites as Efficient and Cost-Effective Cationic Dye Adsorbents. Polymers. 2020; 12(10):2179. https://doi.org/10.3390/polym12102179
Chicago/Turabian StyleJahani, Davoud, Amin Nazari, Jaber Ghourbanpour, and Amir Ameli. 2020. "Polyvinyl Alcohol/Calcium Carbonate Nanocomposites as Efficient and Cost-Effective Cationic Dye Adsorbents" Polymers 12, no. 10: 2179. https://doi.org/10.3390/polym12102179
APA StyleJahani, D., Nazari, A., Ghourbanpour, J., & Ameli, A. (2020). Polyvinyl Alcohol/Calcium Carbonate Nanocomposites as Efficient and Cost-Effective Cationic Dye Adsorbents. Polymers, 12(10), 2179. https://doi.org/10.3390/polym12102179