Flame-Retardant Mechanism and Mechanical Properties of Wet-Spun Poly(acrylonitrile-co-vinylidene chloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Wet Spinning of PANVDC Fibers with Flame Retardants
2.2.2. Preparation of PANVDC Films with Flame Retardants
2.2.3. Characterization
- Flame Retardant Mechanisms
- Thermal Properties
- Flame Retardancy
- Morphology and Mechanical Properties of Fibers
3. Results and Discussions
3.1. Preparation of PANVDC Fiber with Flame Retardants
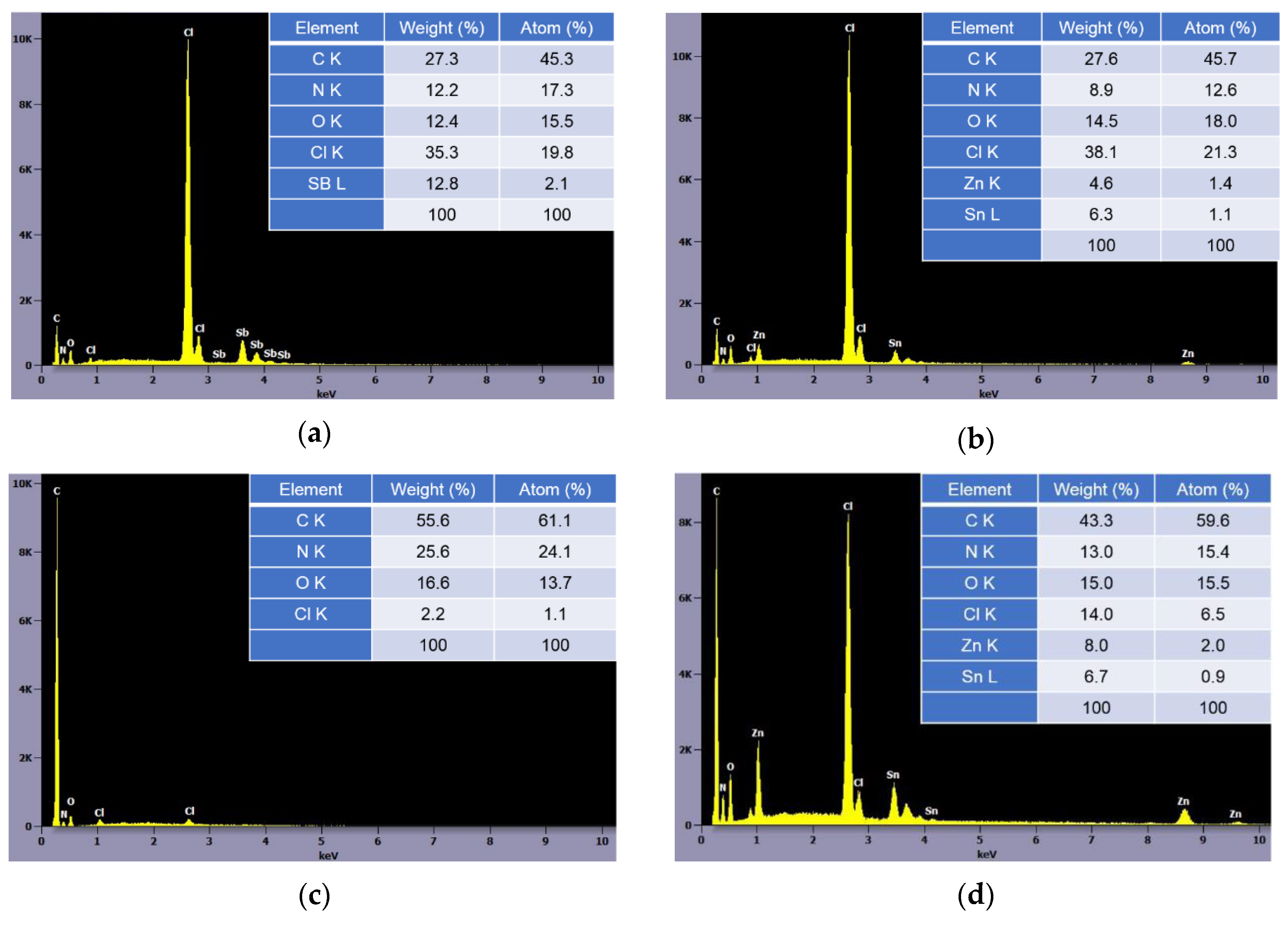
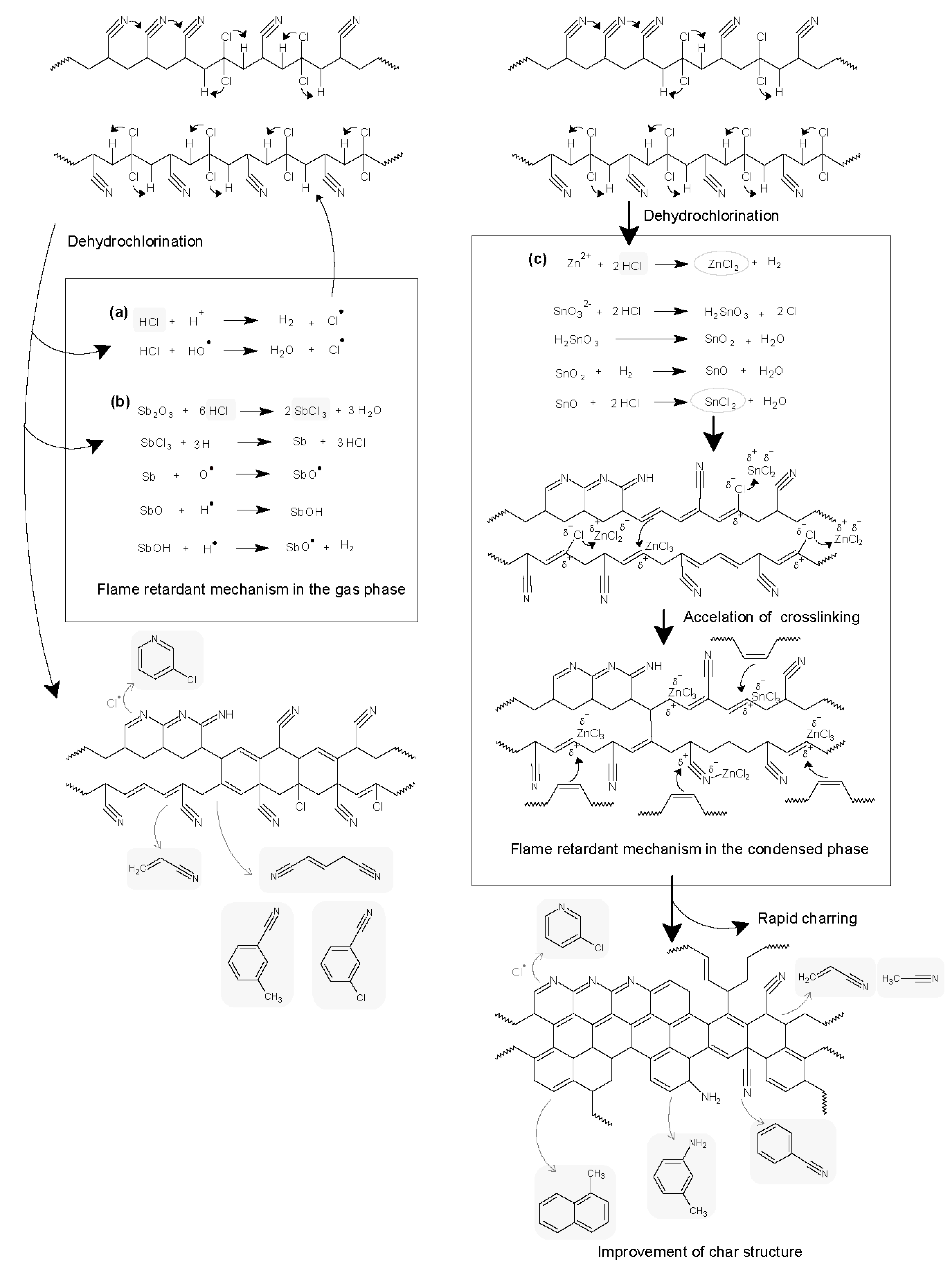
3.2. Flame Retardant Mechanisms
3.3. Thermal Properties
3.4. Flame Retardancy of PANVDC Containing ATO and ZHS
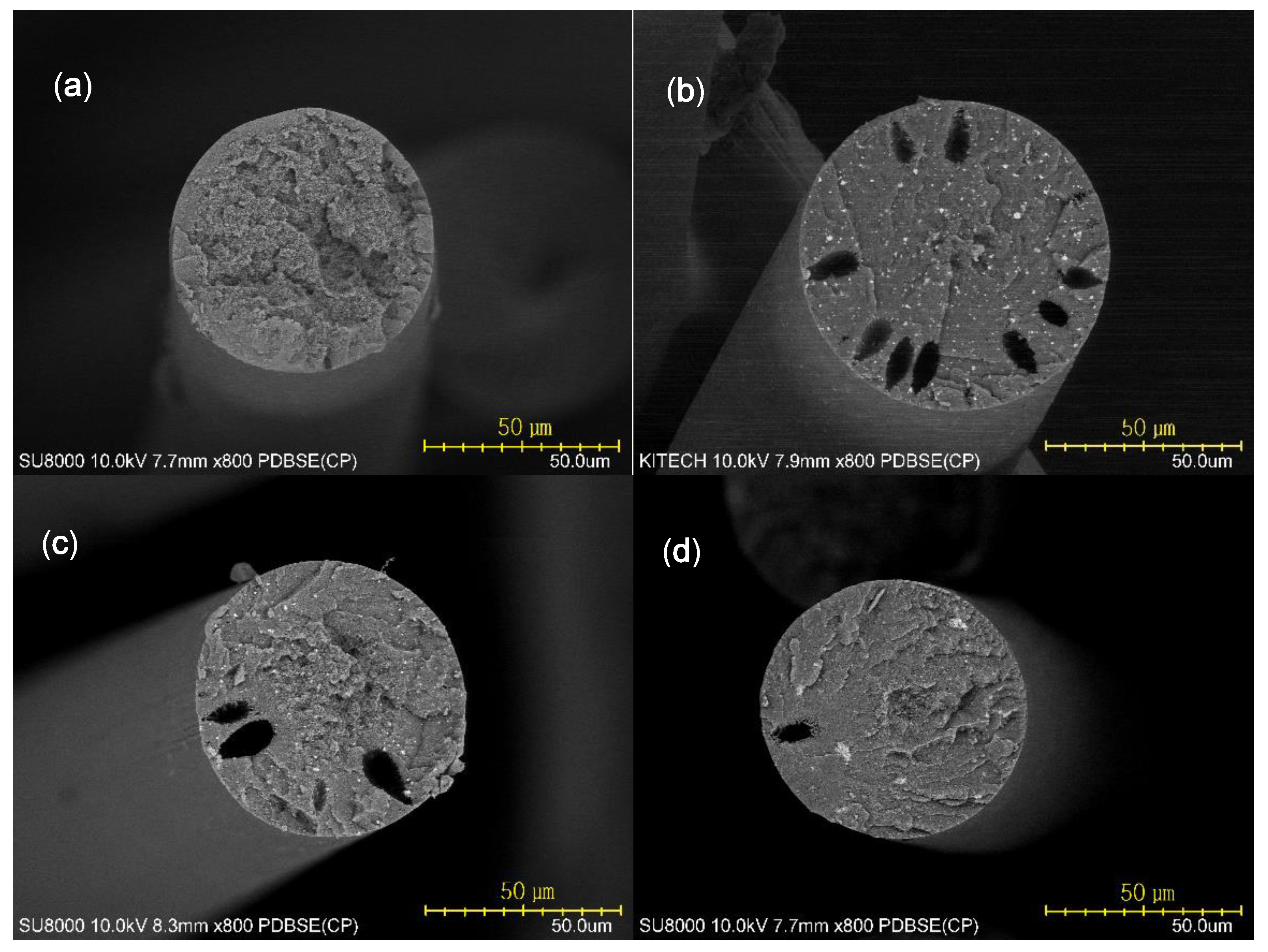
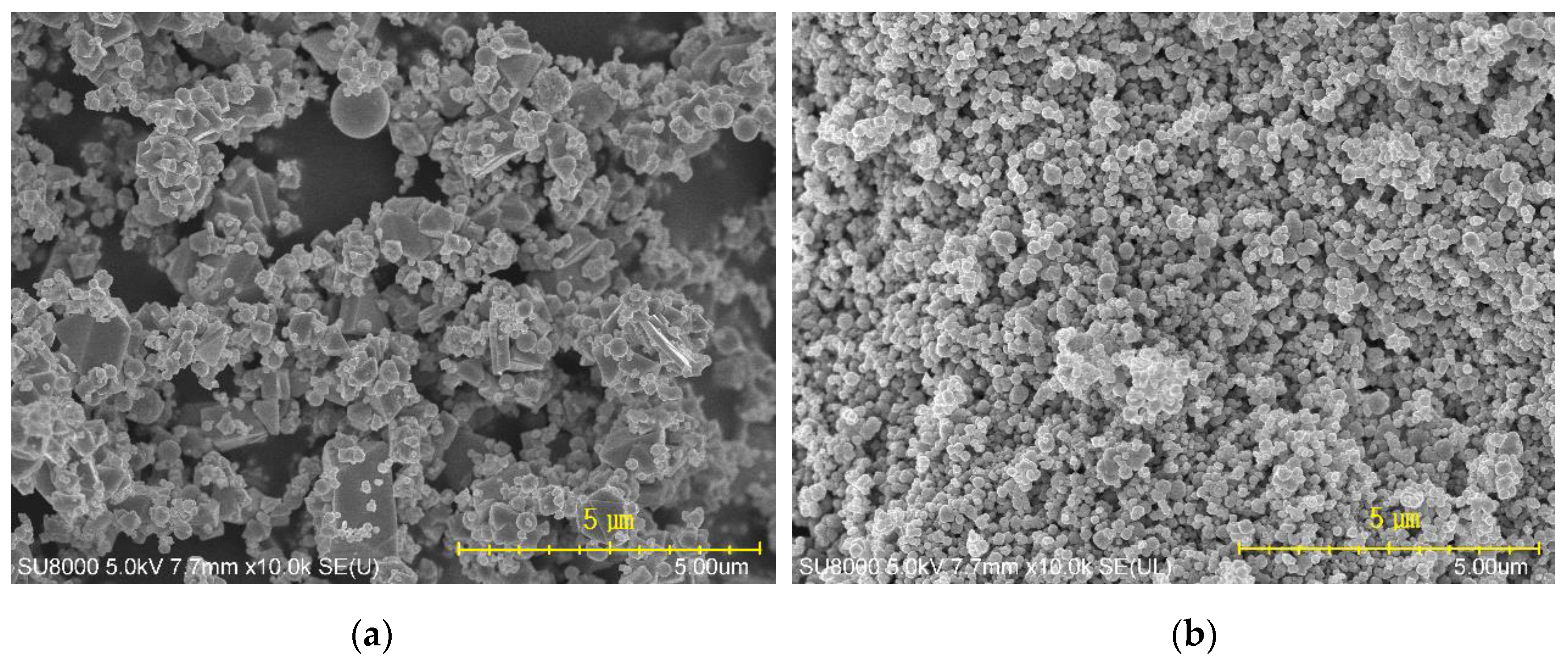
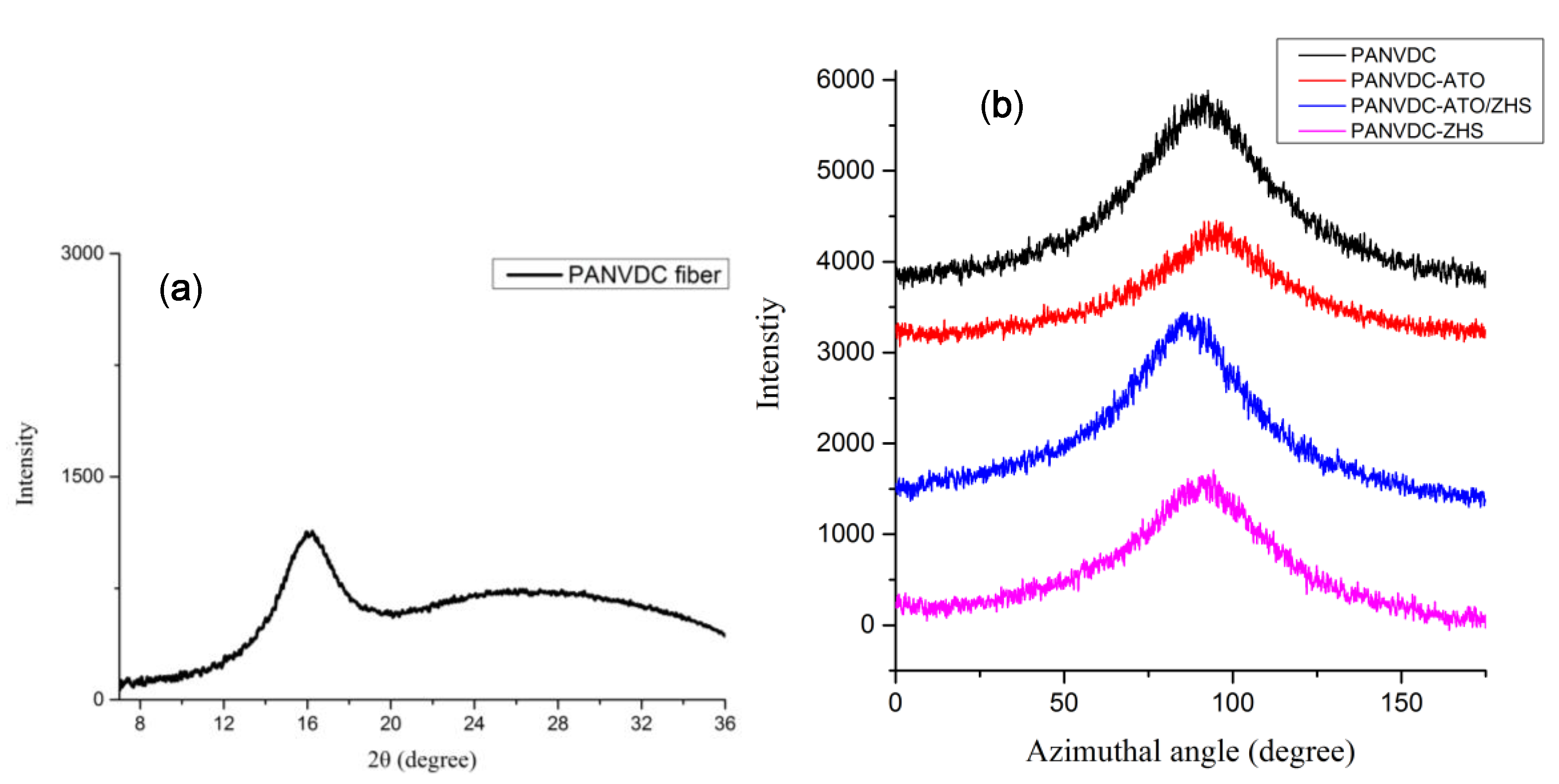
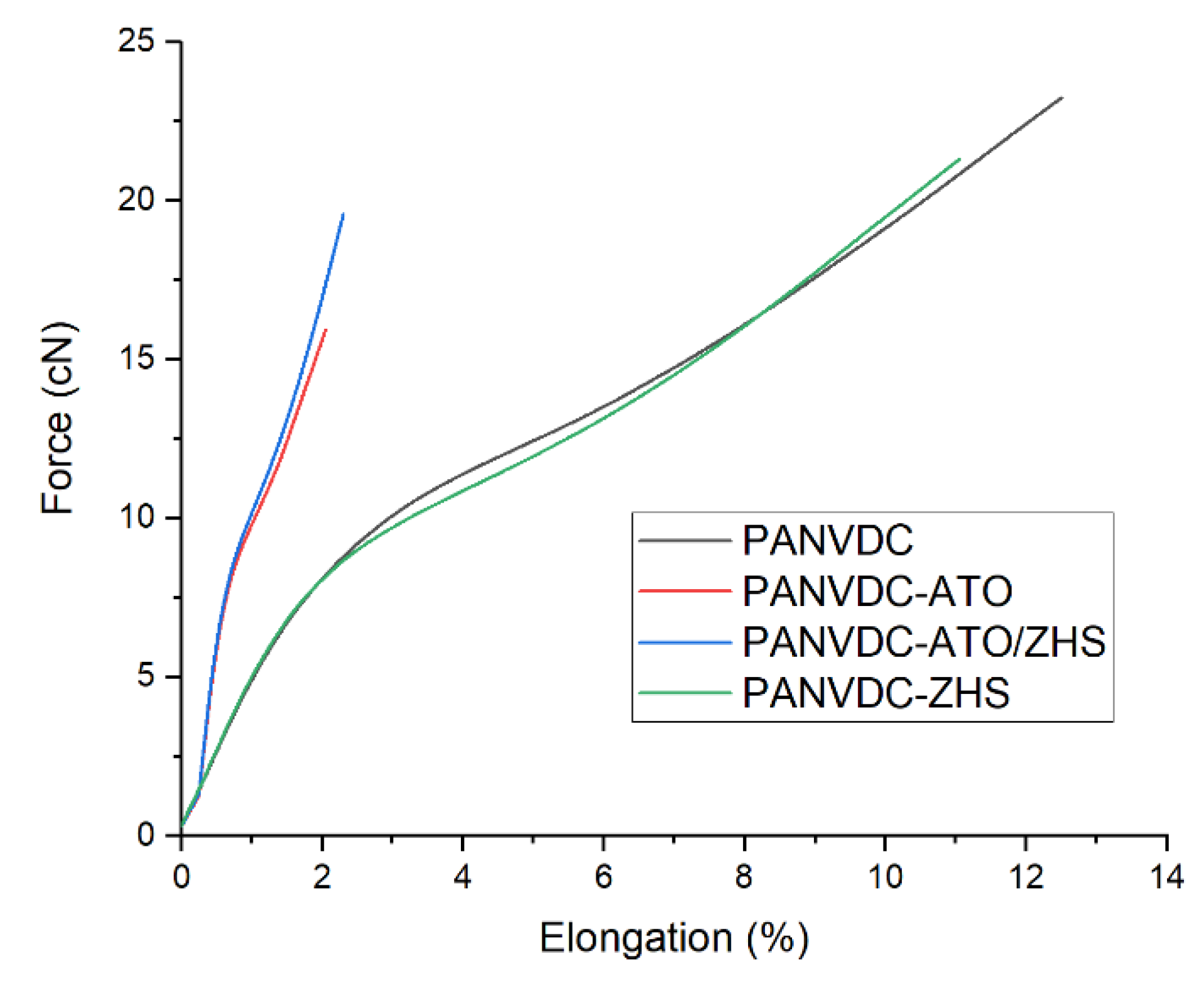
3.5. Morphology and Mechanical Properties of Fibers
4. Conclusions
- Flame retardant mechanism of the PANVDC-ATO fiber: ATO played a role in the original flame retardant mechanism of PANVDC, which resulted mainly from a halogen unit in the gas phase.
- Flame retardant mechanism of the PANVDC-ZHS fiber: ZHS reacted with the halogen unit and then with the AN unit to promote cyclization and carbonization, contributing to a stable char structure and flame retardancy.
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, J.-S. The effect of flame-retardants on the properties of acrylic and modacrylic fibres. J. Mater. Sci. 1993, 28, 1161–1167. [Google Scholar] [CrossRef]
- Bajaj, P.; Agrawal, A.K.; Dhand, A.; Kasturia, N.; Hansraj. Flame retardation of acrylic fibers: An overview. J. Macromol. Sci. Part C Polym. Rev. 2000, 40, 309–337. [Google Scholar] [CrossRef]
- Grand, A.F.; Wilkie, C.A. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Duquesne, S.; Magniez, C.; Camino, G. Multifunctional Barriers for Flexible Structure; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Feng, G.; Jia, P.; Zhang, L.; Hu, L.; Zhang, M.; Zhou, Y.-H. Synthesis of a novel phosphorus-containing plasticizer based on castor oil and its application for flame retardancy of polyvinyl chloride. Korean J. Chem. Eng. 2015, 32, 1201–1206. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Smart, G.; Nazaré, S.; Kandola, B.; Price, D. Quantification of Zinc Hydroxystannate** and Stannate** Synergies in Halogen-containing Flame-retardant Polymeric Formulations. J. Fire Sci. 2009, 28, 217–248. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Qu, H.; Tian, C. Zinc hydroxystannate and zinc stannate as flame-retardant agents for flexible poly(vinyl chloride). J. Appl. Polym. Sci. 2005, 98, 1469–1475. [Google Scholar] [CrossRef]
- Qu, H.; Wu, W.; Zheng, Y.; Xie, J.; Xu, J. Synergistic effects of inorganic tin compounds and Sb2O3 on thermal properties and flame retardancy of flexible poly(vinyl chloride). Fire Saf. J. 2011, 46, 462–467. [Google Scholar] [CrossRef]
- Thomas, N.L. Zinc compounds as flame retardants and smoke suppressants for rigid PVC. Plast. Rubber Compos. 2003, 32, 413–419. [Google Scholar] [CrossRef]
- Tanaka, T.; Terakado, O.; Hirasawa, M. Flame retardancy in fabric consisting of cellulosic fiber and modacrylic fiber containing fine-grained MoO3 particles. Fire Mater. 2016, 40, 612–621. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, J.S.; Lim, D.; Jeong, W. Preparation and Characterization of Zinc Hydroxystannate Coated by Aluminum Phosphate and Its Application in Poly(acrylonitrile-co-vinylidene chloride). Polymers 2020, 12, 1365. [Google Scholar] [CrossRef]
- Tsai, J.-S. Selection of spinneret for modacrylic fibre Part II Effect of polymer content. J. Mater. Sci. Lett. 1993, 12, 802–804. [Google Scholar] [CrossRef]
- Troitzsch, J.H. Overview of flame retardants. Chim. Oggi 1998, 16, 18–24. [Google Scholar]
- Surianarayanan, M.; Vijayaraghavan, R.; Raghavan, K. Spectroscopic investigations of polyacrylonitrile thermal degradation. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 2503–2512. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Liu, X.; Huo, T.; Qin, Y. Preparation of durable flame retardant PAN fabrics based on amidoximation and phosphorylation. Appl. Surf. Sci. 2018, 428, 395–403. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.-C.; Zhang, C.-J.; Guo, Y.; Zhu, P.; Wang, D.-Y. Effect of manganese and cobalt ions on flame retardancy and thermal degradation of bio-based alginate films. J. Mater. Sci. 2016, 51, 1052–1065. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, J.; Cheng, B.; Fang, X.; Yuan, Y. Preparation and flame retardancy of 3-(hydroxyphenylphosphinyl)-propanoic acid esters of cellulose and their fibers. Cellulose 2015, 22, 229–244. [Google Scholar] [CrossRef]
- Ning, Y.; Guo, S. Flame-retardant and smoke-suppressant properties of zinc borate and aluminum trihydrate-filled rigid PVC. J. Appl. Polym. Sci. 2000, 77, 3119–3127. [Google Scholar] [CrossRef]
- Tian, C.; Wang, H.; Liu, X.; Ma, Z.; Guo, H.; Xu, J. Flame retardant flexible poly(vinyl chloride) compound for cable application. J. Appl. Polym. Sci. 2003, 89, 3137–3142. [Google Scholar] [CrossRef]
- Wang, M.Y.; Horrocks, A.; Horrocks, S.; Hall, M.; Pearson, J.; Clegg, S. Flame retardant textile back-coatings. Part 1: Antimony-halogen system interactions and the effect of replacement by phosphorus-containing agents. J. Fire Sci. 2000, 18, 265–294. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Gaan, S. Recent studies on the decomposition and strategies of smoke and toxicity suppression for polyurethane based materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q.; Lyon, R.E.; Hu, Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym. Degrad. Stab. 2010, 95, 108–115. [Google Scholar] [CrossRef]
- Lee, S.H.; Yi, G.R.; Lim, D.Y.; Jeong, W.Y.; Youk, J.H. Study on the Flame Retardant and Mechanical Properties of Wet-spun Poly(acrylonitrile-co-vinylchloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate. Fibers Polym. 2019, 20, 779–786. [Google Scholar] [CrossRef]






| ZHS Content (phr) | Tenacity (g/den) | Linear Density (den) | Elongation (%) | UL 94 Grade |
|---|---|---|---|---|
| 0 | 4.42 | 5.39 | 12.52 | - |
| 10 | 3.98 | 5.33 | 11.17 | - |
| 15 | 3.75 | 5.81 | 11.05 | V-0 |
| 20 | 3.46 | 5.64 | 10.63 | V-0 |
| Sample Code | Polymer | Flame Retardants |
|---|---|---|
| PANVDC | PANVDC | None |
| PANVDC-ATO | PANVDC | ATO (15 phr) |
| PANVDC-ATO/ZHS | PANVDC | ATO/ZHS(50:50) (15 phr) |
| PANVDC-ZHS | PANVDC | ZHS (15 phr) |
| PANVDC | PANVDC-ATO | ||
| Retention Time (min) | Compound Identified | Retention Time (min) | Compound Identified |
| 1.469 | hydrogen chloride * | 1.469 | hydrogen chloride * |
| 1.679 | acrylonitrile * | 1.679 | acrylonitrile * |
| 1.908 | methylacrylonitrile * | 1.908 | methylacrylonitrile * |
| 2.923 | 2,4-pentadienenitrile | 2.923 | 2,4-pentadienenitrile |
| 4.313 | cyanopentadiene | 4.313 | cyanopentadiene |
| 5.319~5.466 | chloropyridine isomers * | 5.319~5.466 | chloropyridine isomers * |
| 5.694 | 2-pentenedinitrile | 5.694 | 2-pentenedinitrile |
| 7.404 | 2-methylenepentanedinitrile * | 7.404 | 2-methylenepentanedinitrile * |
| 7.651 | 2-methylpentanedinitrile | 7.651 | 2-methylpentanedinitrile |
| 7.917 | 3-methylbenzonitrile | 7.917 | 3-methylbenzonitrile |
| 8.529 | 3-chlorobenzonitrile | 8.529 | 3-chlorobenzonitrile |
| - | - | 9.856 | Antimony compound |
| 10.551 | isophthalonitrile | 10.551 | isophthalonitrile |
| 13.935 | hexane-1,3-5-tricarbonitrile * | 13.935 | hexane-1,3-5-tricarbonitrile * |
| 14.282 | pentane-1,3,5-tricarbonitrile | 14.282 | pentane-1,3,5-tricarbonitrile |
| 15.389 | hexane-1,3-5-tricarbonitrile | 15.389 | hexane-1,3-5-tricarbonitrile |
| PANVDC-ATO/ZHS | PANVDC-ZHS | ||
| Retention Time (min) | Compound Identified | Retention Time (min) | Compound Identified |
| 1.469 | hydrogen chloride * | 1.469 | hydrogen chloride * |
| 1.615 | acetonitrile | 1.615 | acetonitrile |
| 1.67 | acrylonitrile * | 1.67 | acrylonitrile * |
| 1.899 | methylacrylonitrile * | 1.899 | methylacrylonitrile * |
| 2.923 | 2,4-pentadienenitrile | 2.923 | 2,4-pentadienenitrile |
| 5.319~5.466 | chloropyridine isomers * | 5.319~5.466 | chloropyridine isomers * |
| 6.225 | benzonitrile | 6.225 | benzonitrile |
| 7.386 | 2-methylenepentanedinitrile * | 7.386 | 2-methylenepentanedinitrile * |
| 7.642 | 2-methylpentanedinitrile | 7.642 | 2-methylpentanedinitrile |
| 7.908 | m-toluidine | 7.908 | m-toluidine |
| 9.856 | Antimony compound | - | - |
| 10.551 | isophthalonitrile | 10.551 | isophthalonitrile |
| 11.877 | 1-methylnaphthalene | 11.877 | 1-methylnaphthalene |
| 13.898 | hexane-1,3-5-tricarbonitrile | 13.898 | hexane-1,3-5-tricarbonitrile |
| 14.282 | pentane-1,3,5-tricarbonitrile | 14.282 | pentane-1,3,5-tricarbonitrile |
| 15.389 | hexane-1,3-5-tricarbonitrile | 15.389 | hexane-1,3-5-tricarbonitrile |
| Sample | First Stage | Second Stage | ||
|---|---|---|---|---|
| TMR1 * (°C) | Mass Loss (%) | TMR2 * (°C) | Mass Loss (%) | |
| PANVDC | 255 | 35 | 611 | 64 |
| PANVDC-ATO | 224 | 41 | 576 | 56 |
| PANVDC-ATO/ZHS | 188 | 36 | 605 | 63 |
| PANVDC-ZHS | 187 | 29 | 640 | 65 |
| Samples | LOI (%) |
|---|---|
| PANVDC | 26.4 |
| PANVDC-ATO | 29.0 |
| PANVDC-ATO/ZHS | 31.0 |
| PANVDC-ZHS | 33.5 |
| Samples | HRC (J/[gK]) | PHRR (W/g) | TPHRR (°C) | THR (kJ/g) |
|---|---|---|---|---|
| PANVDC | 90 | 90.4 | 279 | 2.6 |
| PANVDC-ATO | 116 | 115.4 | 272 | 2.1 |
| PANVDC-ATO/ZHS | 12 | 10.8 | 418 | 1.5 |
| PANVDC-ZHS | 11 | 9.8 | 433 | 1.6 |
| Sample | PANVDC | PANVDC-ATO | PANVDC-ATO/ZHS | PANVDC-ZHS |
|---|---|---|---|---|
| Crystal orientation (%) | 71.09 | 71.08 | 72.61 | 70.30 |
| Samples | Tenacity (g/den) | Linear Density (den) | Elongation (%) |
|---|---|---|---|
| PANVDC | 4.42 (±0.25) | 5.39 (±0.44) | 12.52 (±0.34) |
| PANVDC-ATO | 3.11 (±0.41) | 5.31 (±0.45) | 9.34 (±1.02) |
| PANVDC-ATO/ZHS | 3.73 (±0.52) | 5.39 (±0.42) | 10.69 (±0.52) |
| PANVDC-ZHS | 3.75 (±0.25) | 5.81 (±0.19) | 11.05 (±0.66) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Song, J.E.; Lim, D.; Ahn, H.; Jeong, W. Flame-Retardant Mechanism and Mechanical Properties of Wet-Spun Poly(acrylonitrile-co-vinylidene chloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate. Polymers 2020, 12, 2442. https://doi.org/10.3390/polym12112442
Kim JS, Song JE, Lim D, Ahn H, Jeong W. Flame-Retardant Mechanism and Mechanical Properties of Wet-Spun Poly(acrylonitrile-co-vinylidene chloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate. Polymers. 2020; 12(11):2442. https://doi.org/10.3390/polym12112442
Chicago/Turabian StyleKim, Ji Su, Ji Eun Song, Daeyoung Lim, Heejoon Ahn, and Wonyoung Jeong. 2020. "Flame-Retardant Mechanism and Mechanical Properties of Wet-Spun Poly(acrylonitrile-co-vinylidene chloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate" Polymers 12, no. 11: 2442. https://doi.org/10.3390/polym12112442
APA StyleKim, J. S., Song, J. E., Lim, D., Ahn, H., & Jeong, W. (2020). Flame-Retardant Mechanism and Mechanical Properties of Wet-Spun Poly(acrylonitrile-co-vinylidene chloride) Fibers with Antimony Trioxide and Zinc Hydroxystannate. Polymers, 12(11), 2442. https://doi.org/10.3390/polym12112442





