Synthesis of a Novel Spirocyclic Inflatable Flame Retardant and Its Application in Epoxy Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PIPC
2.3. Preparation of the EP/PIPC Composites
2.4. Characterization
3. Results
3.1. Characterization of the Structure of and Thermal Stability PIPC
3.2. Analysis on Morphology and Dispersion of EP/PIPC Composites
3.3. Analysis on Thermal Properties of EP/PIPC Composites
3.4. Analysis on Fire Safety of EP/PIPC Composites
3.5. Analysis on the Flame-Retardant Mechanism of Condensed Phase for EP/PIPC Composites
3.6. Analysis of Gas Phase Flame Retardant Mechanism of EP/PIPC Composites
3.7. Analysis of Flame Retardant Mechanism of EP/PIPC Composites
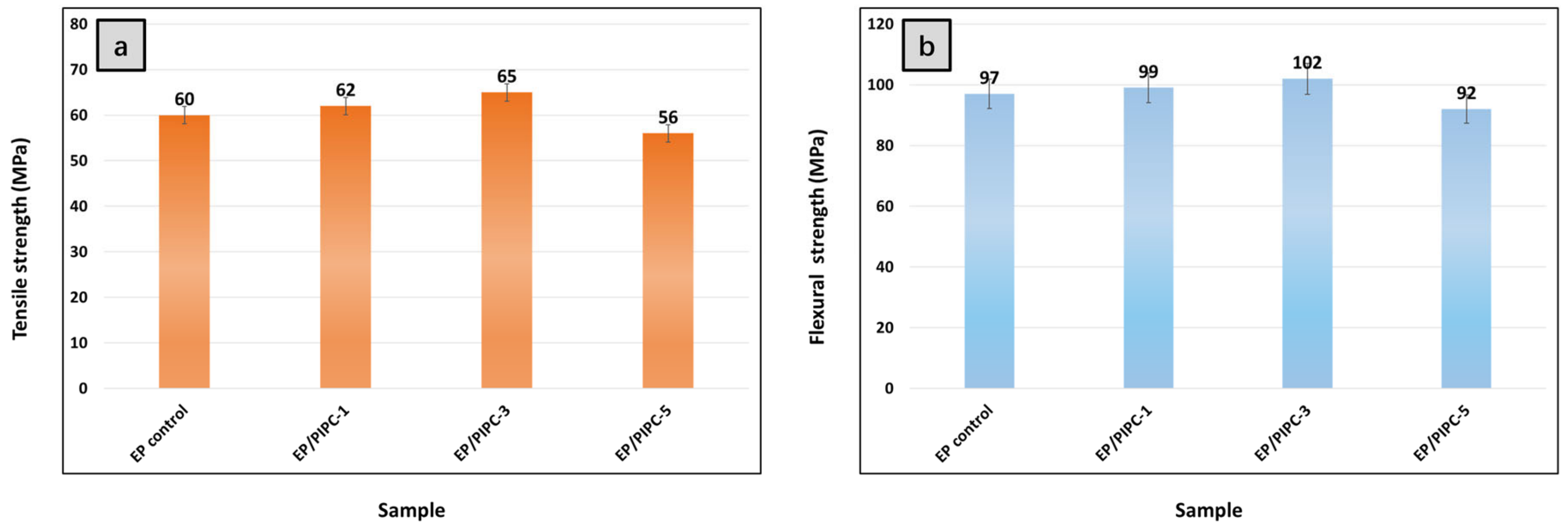
3.8. Mechanical Properties of EP/PIPC Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Thermal Properties and Fracture Toughness of Epoxy Nanocomposites Loaded with Hyperbranched-Polymers-Based Core/Shell Nanoparticles. Nanomaterials 2019, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Wang, Y.; Ruan, F.; Liu, J.; Li, N.; Li, X. Effects of a Macromolecule Spirocyclic Inflatable Flame Retardant on the Thermal and Flame Retardant Properties of Epoxy Resin. Polymers 2020, 12, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Bajpai, M. Development of Siliconized Epoxy Resins and Their Application as Anticorrosive Coatings. Adv. Chem. Eng. Sci. 2011, 1, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotta, A.; Ambrogi, V.; Cerruti, P.; Mija, A. Green approaches in the synthesis of furan-based diepoxy monomers. RSC Adv. 2018, 8, 16330–16335. [Google Scholar] [CrossRef] [Green Version]
- Gérard, C.; Fontaine, G.; Bourbigot, S. New Trends in Reaction and Resistance to Fire of Fire-retardant Epoxies. Materials 2010, 3, 4476–4499. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Wang, D.-Y. Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J. Mater. Chem. A 2018, 6, 6376–6386. [Google Scholar] [CrossRef]
- Zuo, J.; Su, Y.; Liu, S.; Sheng, Q. Preparation and properties of FR-PP with phosphorus-containing intumescent flame retardant. J. Polym. Res. 2010, 18, 1125–1129. [Google Scholar] [CrossRef]
- Vladimir, Y.; Arnis, A.; Dzintra, V.; Irina, S. Polyurethane Coatings Based on Linseed Oil Phosphate Ester Polyols with Intumescent Flame Retardants. Fire Mater. 2019, 43, 92–100. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Saheb, S.; Tambe, P.; Malathi, M. Influence of Halloysite Nanotubes and Intumescent Flame Retardant on Mechanical and Thermal Properties of 80/20 (wt/wt) PP/ABS Blend and Their Composites in The Presence of Dual Compatibilizer. J. Thermoplast. Compos. Mater. 2018, 31, 202–222. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.; Chen, X.; Guo, J.; Zhou, D.; Chen, S.; Wang, M.; Li, L. Novel Intumescent Flame Retardant Masterbatch Prepared through Different Processes and Its Application in EPDM/PP Thermoplastic Elastomer: Thermal Stability, Flame Retardancy, and Mechanical Properties. Polymers 2018, 11, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, T.-H.; Leu, T.-S.; Sun, Y.-M.; Shieh, J.-Y. Thermal degradation kinetics and flame retardancy of phosphorus-containing dicyclopentadiene epoxy resins. Polym. Degrad. Stab. 2006, 91, 2347–2356. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of aryl phosphates in combination with boehmite in bisphenol A polycarbonate/acrylonitrile–butadiene–styrene blends. Polym. Degrad. Stab. 2008, 93, 657–667. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Shi, Y.; Song, L.; Ma, C.; Hu, Y. The influence of highly dispersed Cu2O-anchored MoS2 hybrids on reducing smoke toxicity and fire hazards for rigid polyurethane foam. J. Hazard. Mater. 2020, 382, 121028. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z.; Jin, Y.; Lu, F. A novel intumescent flame retardant: Synthesis and application in ABS copolymer. Polym. Degrad. Stab. 2007, 92, 720–726. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymers 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Zhang, P.; He, Y.; Tian, S.; Fan, H.; Chen, Y.; Yan, J. Flame retardancy, mechanical, and thermal properties of waterborne polyurethane conjugated with a novel phosphorous-nitrogen intumescent flame retardant. Polym. Compos. 2015, 38, 452–462. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, C.; Zhou, Y.; Wang, H.; Yan, X.; He, Q.; Guo, J.; Guo, Z. Enhanced flame retardancy of cotton fabrics with a novel intumescent flame-retardant finishing system. Fibers Polym. 2015, 16, 388–396. [Google Scholar] [CrossRef]
- Wu, G.M.; Schartel, B.; Bahr, H.; Kleemeier, M.; Yu, D.; Hartwig, A. Experimental and quantitative assessment of flame retardancy by the shielding effect in layered silicate epoxy nanocomposites. Combust. Flame 2012, 159, 3616–3623. [Google Scholar] [CrossRef]
- Matusinovic, Z.; Wilkie, C.A. Fire retardancy and morphology of layered double hydroxide nanocomposites: A review. J. Mater. Chem. 2012, 22, 18701–18704. [Google Scholar] [CrossRef]
- Sun, W.; He, Q.; Luo, Y. Synthesis and properties of cinnamic acid series organic UV ray absorbents–interleaved layered double hydroxides. Mater. Lett. 2007, 61, 1881–1884. [Google Scholar] [CrossRef]
- Kuan, C.F.; Chen, W.J.; Li, Y.L.; Chen, C.H.; Kuan, H.C.; Chiang, C.L. Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol–gel method. J. Phys. Chem. Solids 2010, 71, 539–543. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, L.; Shen, Y.; Xu, L.; Shen, Y. Study on Preparation and Functional Finishing of TiO2 Supported Nano ZnO. J. Nanosci. Nanotechnol. 2018, 18, 7703–7712. [Google Scholar] [CrossRef]
- Camino, G.; Tartaglione, G.; Frache, A.; Manferti, C.; Costa, G. Thermal and combustion behaviour of layered silicate–epoxy nanocomposites. Polym. Degrad. Stab. 2005, 90, 354–362. [Google Scholar] [CrossRef]
- Gao, Y.S.; Wu, J.W.; Zhang, Z.; Jin, R.; Zhang, X.; Yan, X.R.; Umar, A.; Guo, Z.H.; Wang, Q. Synthesis of polypropylene/Mg3Al-X (X = C, N, Cl−, S) LDH nanocomposites using a solvent mixing method: Thermal and melt rheological properties. J. Mater. Chem. A 2013, 1, 9928–9934. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Cai, J.; Heng, H.-M.; Hu, X.-P.; Xu, Q.-K.; Miao, F. A facile method for the preparation of novel fire-retardant layered double hydroxide and its application as nanofiller in UP. Polym. Degrad. Stab. 2016, 126, 47–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.; Liew, K.M.; Song, L.; Wang, C.; Hu, Y. Highly Effective P–P Synergy of a Novel DOPO-Based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Wang, Y.; Yan, H.; Zhang, X.; Xu, B. Highly efficient flame retardant, flexible, and strong adhesive intumescent coating on polypropylene using hyperbranched polyamide. Chem. Eng. J. 2017, 324, 237–250. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Khelifa, F.; Rose, G.; Brohez, S.; Delvosalle, C.; Dubois, P. Cellulose/phosphorus combinations for sustainable fire retarded polylactide. Eur. Polym. J. 2016, 74, 218–228. [Google Scholar] [CrossRef]
- Xiang, H.; Sun, C.; Jiang, D.; Zhang, Q.; Dong, C.; Liu, L. A novel halogen-free intumescent flame retardant containing phosphorus and nitrogen and its application in polypropylene systems. J. Vinyl Addit. Technol. 2010, 16, 261–271. [Google Scholar] [CrossRef]
- Li, L.; Cai, Z. Flame-Retardant Performance of Transparent and Tensile-Strength-Enhanced Epoxy Resins. Polymers 2020, 12, 317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, H.; Zhang, Y.; Zhang, Y.-M.; Kan, W.; Pan, M. Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils. Polymers 2020, 12, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.; Chen, L.; Lu, F.; Song, P.; Dai, J.; Meng, L. Mechanically Robust, Flame-Retardant Poly(lactic acid) Biocomposites via Combining Cellulose Nanofibers and Ammonium Polyphosphate. ACS Omega 2018, 3, 5615–5626. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tawiah, B.; Shi, Y.; Cai, S.; Rao, X.; Liu, C.; Yang, Y.; Yang, F.; Yu, B.; Liang, Y.; et al. Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion. Polymers 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zhang, W.; Yang, R. The characterization of DOPO/MMT nanocompound and its effect on flame retardancy of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2017, 98, 124–135. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Ni, A.; Chen, H.; Shen, P. Synthesis of a Novel Phosphorous-Nitrogen Based Charring Agent and Its Application in Flame-retardant HDPE/IFR Composites. Polymers 2019, 11, 1062. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, X.; Yang, R. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym. Degrad. Stab. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- Zhao, W. Study on Synthesis of Polymeric Phosphorus-based Flame Retardant and Flame Retardant Mechanism of Epoxy Resin; Beijing Institute of Technology: Beijing, China, 2016. [Google Scholar]
- Cheng, J.; Ma, D.; Li, S.; Qu, W.; Wang, D. Preparation of Zeolitic Imidazolate Frameworks and Their Application as Flame Retardant and Smoke Suppression Agent for Rigid Polyurethane Foams. Polymers 2020, 12, 347. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Kim, J. Thermal stabilities and flame retardancies of nitrogen–phosphorus flame retardants based on bisphosphoramidates. Polym. Degrad. Stab. 2008, 93, 1037–1043. [Google Scholar] [CrossRef]
- Qian, X.D.; Song, L.; Yu, B.; Wang, B.B.; Yuan, B.H.; Shi, Y.Q.; Hu, Y.; Yuen, R.K.K. Novel organic–inorganic flame retardants containing exfoliated graphene: Preparation and their performance on the flame retardancy of epoxy resins. J. Mater. Chem. A 2013, 1, 6822–6830. [Google Scholar] [CrossRef]




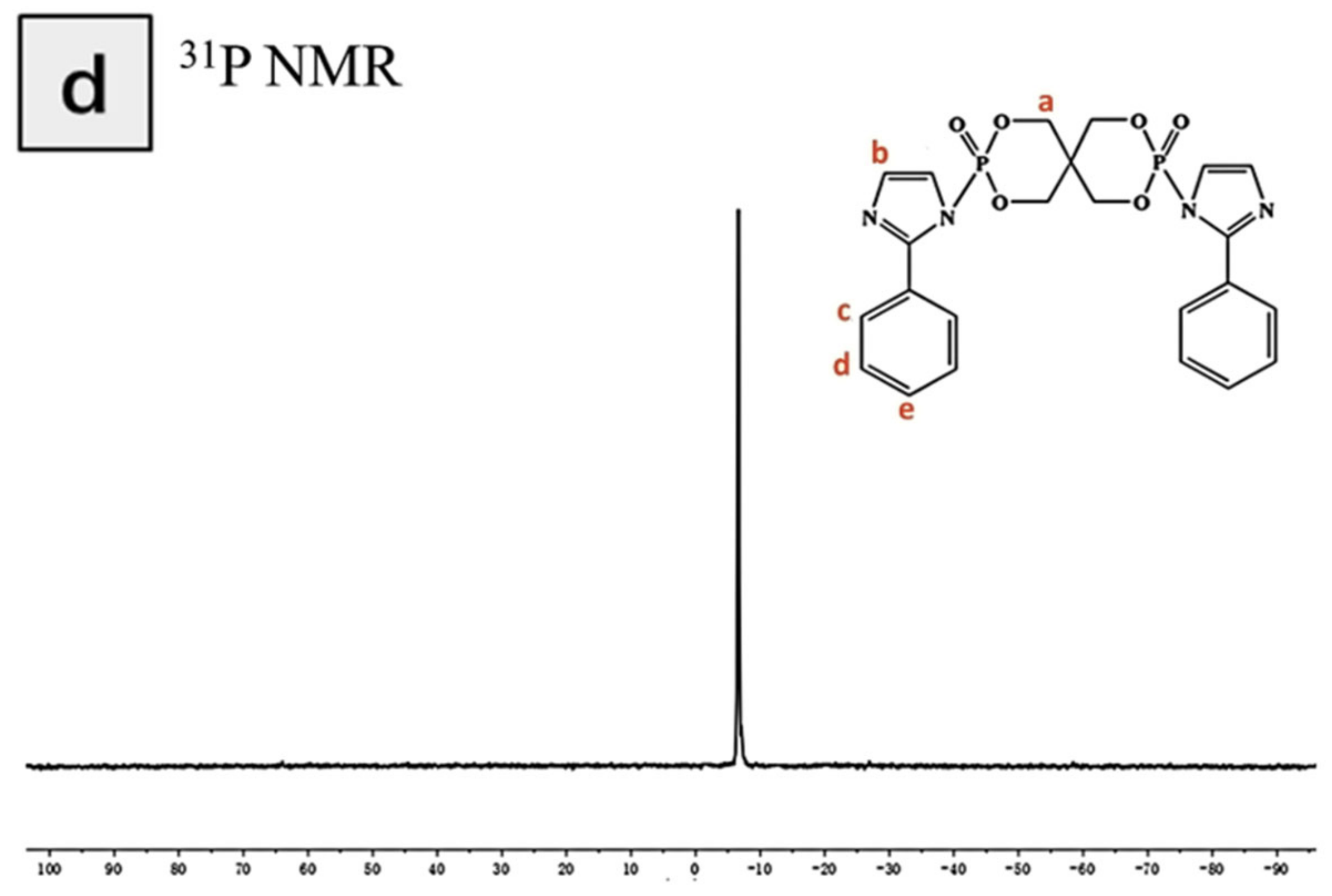

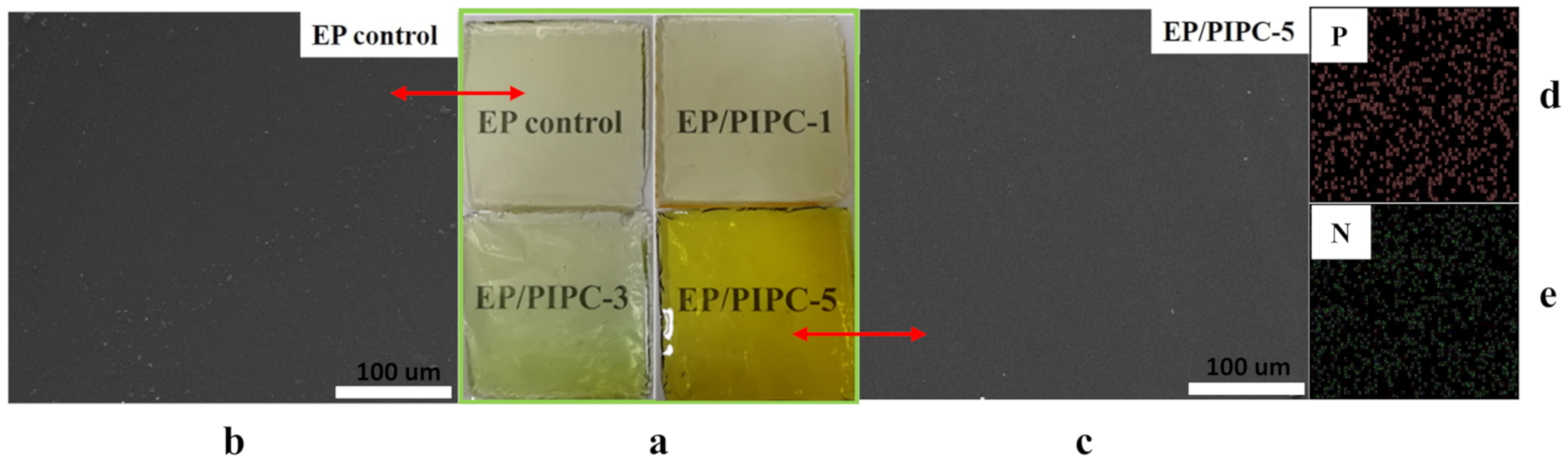











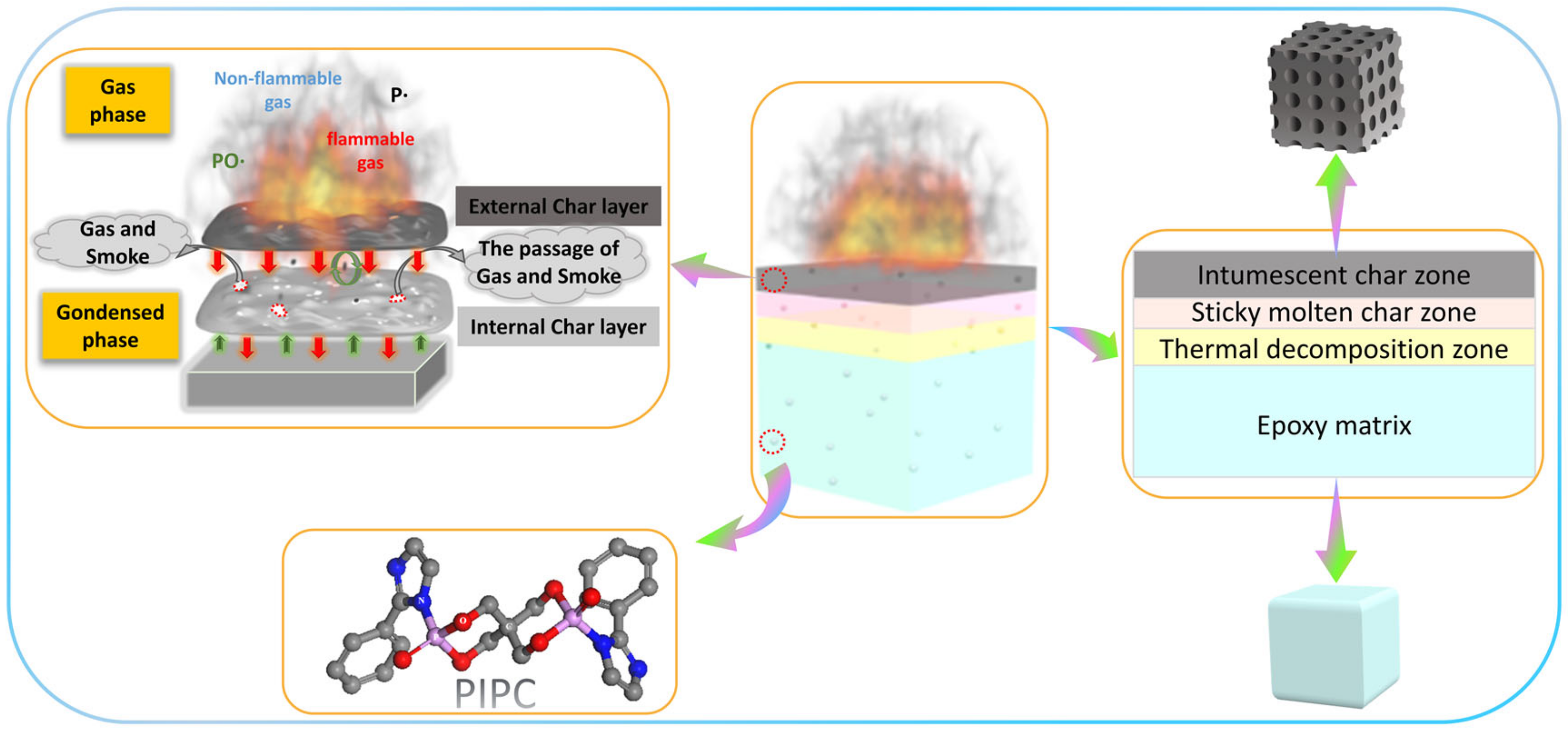
| Samples | T5%(°C) | Tmax(°C) | Residues at 600 °C | Residues at 800 °C |
|---|---|---|---|---|
| SPDPC | 228.6 | 271.1 & 340.8 & 496.8 | 28.0 | 25.7 |
| PIPC | 278.6 | 316.6 & 397.0 | 43.9 | 42.1 |
| Samples | T5%(°C) | Tmax(°C) | Residues at 600 °C | Residues at 800 °C |
|---|---|---|---|---|
| EP control | 384 | 399 | 17.7 | 15.6 |
| EP/PIPC-1 | 359 | 397 | 18.7 | 17.1 |
| EP/PIPC-3 | 344 | 393 | 20.0 | 18.6 |
| EP/PIPC-5 | 333 | 391 | 20.1 | 19.9 |
| Samples | EP Control | EP/PIPC–1 | EP/PIPC–3 | EP/PIPC–5 |
|---|---|---|---|---|
| PHRR (kW/m2) | 1023 | 883 | 711 | 602 |
| THR (MJ/m2) | 67.0 | 65.8 | 57.3 | 52.5 |
| TSR (m2/m2) | 2928 | 2651 | 2574 | 2562 |
| Mean CO2Y (kg/kg) | 1.96 | 1.82 | 1.61 | 1.40 |
| Mean COY (kg/kg) | 0.08 | 0.07 | 0.07 | 0.06 |
| Wavenumber (cm−1) | Structure |
|---|---|
| 3735, 3650 | –OH in water or phenolic compounds |
| 3038, 829 | C–H in aromatic compounds |
| 3540, 3340 | N–H in amine compounds |
| 2970 | C–H in hydrocarbons |
| 1744 | C=O in carbonyl compounds |
| 1605, 1510, 1338 | C=C in aromatic compounds |
| 1257, 1178 | Ester/ether compounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Wang, Y.; Ruan, F.; Yang, W.; Liu, J. Synthesis of a Novel Spirocyclic Inflatable Flame Retardant and Its Application in Epoxy Composites. Polymers 2020, 12, 2534. https://doi.org/10.3390/polym12112534
Song K, Wang Y, Ruan F, Yang W, Liu J. Synthesis of a Novel Spirocyclic Inflatable Flame Retardant and Its Application in Epoxy Composites. Polymers. 2020; 12(11):2534. https://doi.org/10.3390/polym12112534
Chicago/Turabian StyleSong, Kunpeng, Yinjie Wang, Fang Ruan, Weiwei Yang, and Jiping Liu. 2020. "Synthesis of a Novel Spirocyclic Inflatable Flame Retardant and Its Application in Epoxy Composites" Polymers 12, no. 11: 2534. https://doi.org/10.3390/polym12112534
APA StyleSong, K., Wang, Y., Ruan, F., Yang, W., & Liu, J. (2020). Synthesis of a Novel Spirocyclic Inflatable Flame Retardant and Its Application in Epoxy Composites. Polymers, 12(11), 2534. https://doi.org/10.3390/polym12112534






