Thermal Properties of Binary Filler Hybrid Composite with Graphene Oxide and Pyrolyzed Silicon-Coated Boron Nitride
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Graphene Oxide (GO)
2.3. Surface-Modification of Boron Nitride (BN)
2.4. Fabrication of the GO/P-BN/Epoxy Composite
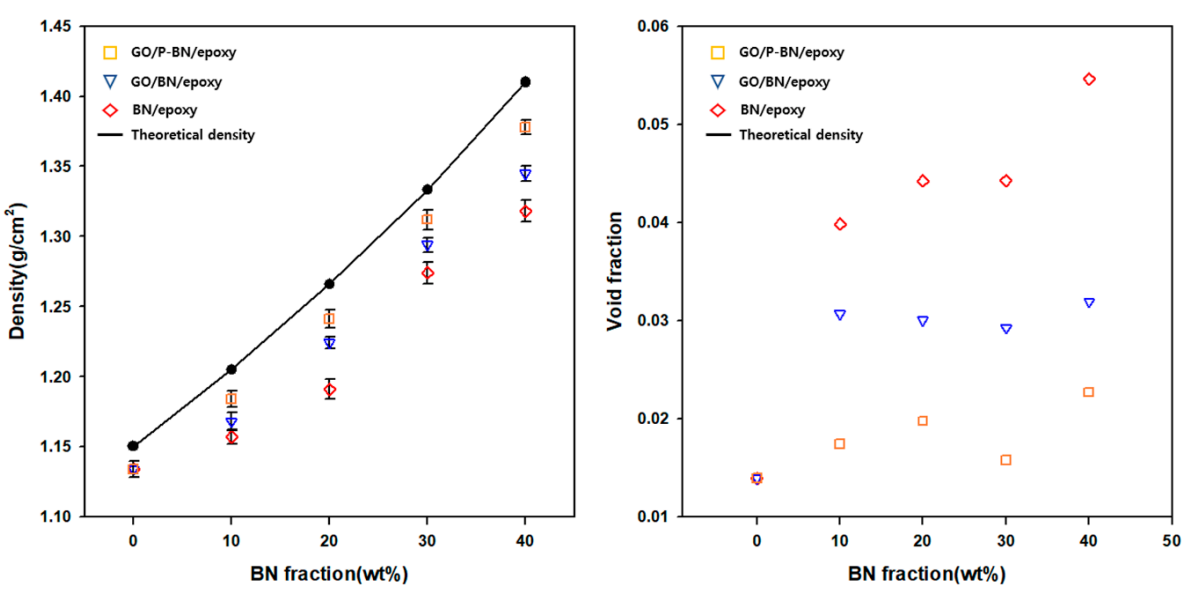
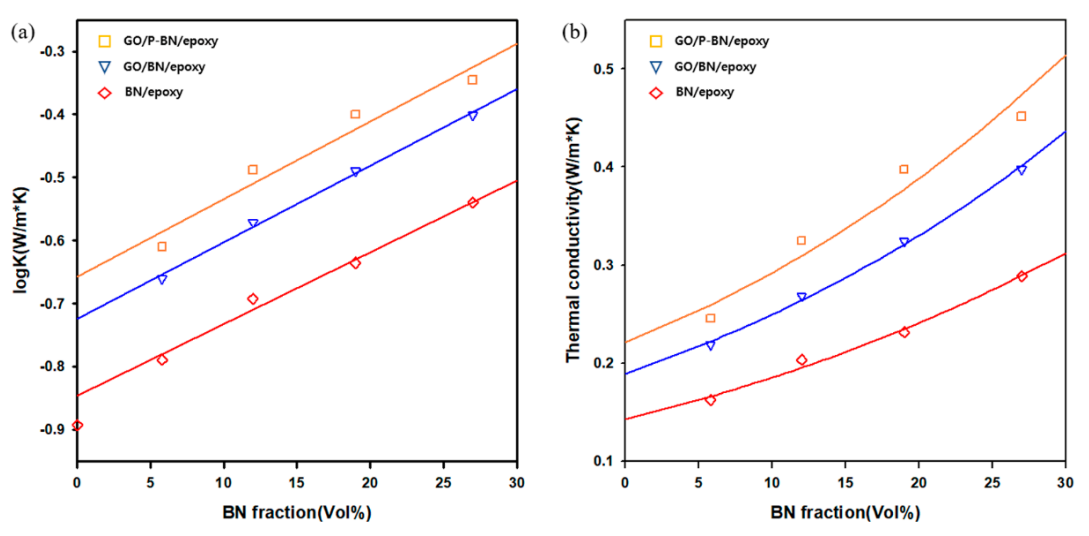
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leung, S.N. Thermally conductive polymer composites and nanocomposites: Processing-structure-property relationships. Compos. Part B Eng. 2018, 150, 78–92. [Google Scholar] [CrossRef]
- Mehra, N.; Mu, L.; Ji, T.; Li, Y.; Zhu, J. Moisture driven thermal conduction in polymer and polymer blends. Compos. Sci. Technol. 2017, 151, 115–123. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Singh, P.; Mahajan, R.L. Role of oxygen functional groups for improved performance of graphene-silicone composites as a thermal interface material. Carbon 2019, 145, 131–139. [Google Scholar] [CrossRef]
- Teng, C.; Su, L.; Chen, J.; Wang, J. Flexible, thermally conductive layered composite films from massively exfoliated boron nitride nanosheets. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105498. [Google Scholar] [CrossRef]
- Han, J.; Du, G.; Gao, W.; Bai, H. An anisotropically high thermal conductive boron nitride/epoxy composite based on nacre-mimetic 3D network. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zloczower, I. Epoxy composites with carbon nanotubes and graphene nanoplatelets-Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Loos, M.; Yang, J.; Feke, D.; Manas-Zloczower, I. Effect of block-copolymer dispersants on properties of carbon nanotube/epoxy systems. Compos. Sci. Technol. 2012, 72, 482–488. [Google Scholar] [CrossRef]
- Xie, X.-L.; Tang, C.-Y.; Zhou, X.-P.; Li, R.K.-Y.; Yu, Z.-Z.; Zhang, Q.-X.; Mai, Y.-W. Enhanced interfacial adhesion between PPO and glass beads in composites by surface modification of glass beads via in situ polymerization and copolymerization. Chem. Mater. 2004, 16, 133–138. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Feng, Y.; Li, S.; Zhou, X.; Xie, X. Enhanced thermal conductivity and ideal dielectric properties of epoxy composites containing polymer modified hexagonal boron nitride. Compos. Part A Appl. Sci. Manuf. 2018, 107, 657–664. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Kuang, W.; Tang, Z.; Guo, B. Coating polyrhodanine onto boron nitride nanosheets for thermally conductive elastomer composites. Compos. Part A Appl. Sci. Manuf. 2017, 94, 77–85. [Google Scholar] [CrossRef]
- Kim, K.; Wie, J.; Kim, J. Synergistic interaction of P and N co-doping EDTA with controllable active EDTA-cobalt sites as efficient electrocatalyst for oxygen reduction reaction. J. Ind. Eng. Chem. 2020, 83, 252–259. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Kleebe, H.-J.; Müller, M.M.; Fasel, C.; Yazdi, M.B.; Gurlo, A.; Riedel, R. Nanoporous silicon oxycarbonitride ceramics derived from polysilazanes in situ modified with nickel nanoparticles. Chem. Mater. 2011, 23, 4112–4123. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.Q.; Motz, G. Polymeric and ceramic silicon-based coatings-A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Kim, K.; Ju, H.; Kim, J. Pyrolysis behavior of polysilazane and polysilazane-coated-boron nitride for high thermal conductive composite. Compos. Sci. Technol. 2017, 141, 1–7. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- McGrail, B.T.; Rodier, B.J.; Pentzer, E.B. Rapid functionalization of graphene oxide in water. Chem. Mater. 2014, 26, 5806–5811. [Google Scholar] [CrossRef]
- Wie, J.; Kim, K.; Kim, J. High thermal conductivity composites obtained by novel surface treatment of boron nitride. Ceram. Int. 2020, 46, 17614–17620. [Google Scholar] [CrossRef]
- Bauer, F.; Decker, U.; Dierdorf, A.; Ernst, H.; Heller, R.; Liebe, H.; Mehnert, R. Preparation of moisture curable polysilazane coatings. Prog. Org. Coat. 2005, 53, 183–190. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long-cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef] [Green Version]
- Socher, R.; Krause, B.; Muller, M.T.; Boldt, R.; Pötschke, P. The influence of matrix viscosity on MWCNT dispersion and electrical properties in different thermoplastic nanocomposites. Polymers 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Oh, H.; Kim, Y.; Wie, J.; Kim, K.; Kim, J. Tailoring of Si-C-N-O ceramic-coated reduced graphene oxide by oil/water-solution process for high thermal conductive epoxy composite with electrical insulation. Compos. Sci. Technol. 2020, 197, 108257. [Google Scholar] [CrossRef]
- Bisht, A.; Dasgupta, K.; Lahiri, D. Investigating the role of 3D network of carbon nanofillers in improving the mechanical properties of carbon fiber epoxy laminated composite. Compos. Part A Appl. Sci. Manuf. 2019, 126, 105601. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly anisotropic thermal conductivity of free-standing reduced graphene oxide films annealed at high temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J. Fabrication of polymethyl methacrylate composites with silanized boron nitride by in-situ polymerization for high thermal conductivity. Compos. Sci. Technol. 2019, 172, 153–162. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Wu, H.; Guo, S. Enhanced thermally conductivity and mechanical properties of polyethylene (PE)/boron nitride (BN) composites through multistage stretching extrusion. Compos. Sci. Technol. 2013, 89, 24–28. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.-Z.; Bai, S.; Wang, Q. High-performance thermal and electrical conductive composites from multilayer plastic packaging waste and expanded graphite. J. Mater. Chem. C 2018, 6, 11209–11218. [Google Scholar] [CrossRef]
- Goldin, N.; Dodiuk, H.; Lewitus, D. Enhanced thermal conductivity of photopolymerizable composites using surface modified hexagonal boron nitride fillers. Compos. Sci. Technol. 2017, 152, 36–45. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Sultan, M.; Alothman, O.Y.; Abdullah, L.C. Thermomechanical and dynamic mechanical properties of bamboo/woven kenaf mat reinforced epoxy hybrid composites. Compos. Part B Eng. 2019, 163, 165–174. [Google Scholar] [CrossRef]
- Joseph, S.; Appukuttan, S.P.; Kenny, J.M.; Puglia, D.; Thomas, S.; Joseph, K. Dynamic mechanical properties of oil palm microfibril-reinforced natural rubber composites. J. Appl. Polym. Sci. 2010, 117, 1298–1308. [Google Scholar] [CrossRef]
- Zhang, X.; Alloul, O.; He, Q.; Zhu, J.; Verde, M.J.; Li, Y.; Wei, S.; Guo, Z. Strengthened magnetic epoxy nanocomposites with protruding nanoparticles on the graphene nanosheets. Polymers 2013, 54, 3594–3604. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wie, J.; Kim, J. Thermal Properties of Binary Filler Hybrid Composite with Graphene Oxide and Pyrolyzed Silicon-Coated Boron Nitride. Polymers 2020, 12, 2553. https://doi.org/10.3390/polym12112553
Wie J, Kim J. Thermal Properties of Binary Filler Hybrid Composite with Graphene Oxide and Pyrolyzed Silicon-Coated Boron Nitride. Polymers. 2020; 12(11):2553. https://doi.org/10.3390/polym12112553
Chicago/Turabian StyleWie, Jaehyun, and Jooheon Kim. 2020. "Thermal Properties of Binary Filler Hybrid Composite with Graphene Oxide and Pyrolyzed Silicon-Coated Boron Nitride" Polymers 12, no. 11: 2553. https://doi.org/10.3390/polym12112553




