Ultrafine and High-Strength Silk Fibers Secreted by Bimolter Silkworms
Abstract
:1. Introduction
2. Materials and Methods
2.1. AJH Induction of Silkworm Bimolters
2.2. Chitin Staining of Silk Glands
2.3. Morphology Observations
2.4. Filament Size Testing
2.5. Cleanliness Test of the Silk
2.6. ATR-FTIR Analysis
2.7. XRD Analysis
2.8. Mechanical Testing
3. Results
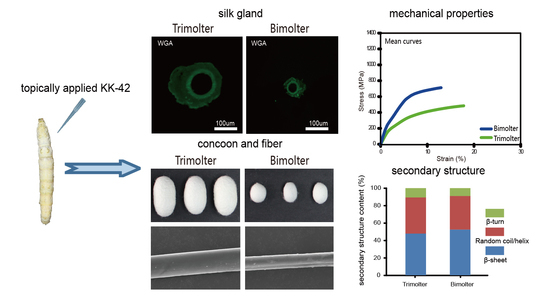
3.1. KK-42-Induced Bimolters and Changes in the Cocoon
3.2. Morphological Characteristics of the Cocoon and Silk
3.3. Quality Characteristics of Silk
3.4. Chitin Staining of the Silk Glands
3.5. Effect of Bimolter Induction on the Secondary Structure of Silk Fibers
3.6. Crystalline Structure Characteristics of Silk Fibers
3.7. Mechanical Properties of the Silk Fiber
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Srivastava, Y.; Marquez, M.; Thorsen, T. Microfluidic electrospinning of biphasic nanofibers with Janus morphology. Biomicrofluidics 2009, 3, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Wang, J.; Wang, Y.; Li, L.; Guo, X.; Zhou, S. An implantable active-targeting micelle-in-nanofiber device for efficient and safe cancer therapy. ACS Nano 2015, 9, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yang, G.; Li, X.; Chen, B.; Fan, J.; Hou, X.; Zhou, S. Flexible polymer ultra-fine fiber with extreme toughness. ACS Appl. Mater. Interfaces 2018, 10, 14276–14280. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Kuwano, E.; ETO, M. Anti-juvenile hormone activity of 1-citronellyl-5-phenylimidazole in the 3rd instar silkworm, Bombyx mori L. (Lepidoptera: Bombycidae). Appl. Entomol. Zool. 1984, 19, 212–220. [Google Scholar] [CrossRef]
- Zhuang, D. The effect of trimolter by treatment with anti-juvenile hormone (AJH) analogue (YA20) and it’s quality of cocoon filament in silkworm (Bombyx mori). Acta Seric. Sin. 1992, 18, 93–99. (In Chinese) [Google Scholar]
- Niu, R.H.; Wu, Z.P.; Yin, P.F.; Wang, G.H.; Tan, J.Z. Analysis of cocoon and silk quality of trimolter silkworms induced by anti-juvenile hormone. Adv. Mater. Res. 2013, 796, 57–61. [Google Scholar] [CrossRef]
- Hou, L.X.; Han, Y.P.; Wei, D.Y.; Dong, Y.J. The functional application and production of superfine fiber. Shandong Text. Econ. 2007, 3, 84–87. [Google Scholar] [CrossRef]
- Lin, Y.N.; Shu, H.G.; Men, W.; Zhang, M.F. A preliminary report on producing ultrafine silk filament by using anti-juvenile hormone to induce bimolter. Acta Seric. Sin. 2009, 35, 179–181. [Google Scholar] [CrossRef]
- Guo, K.; Dong, Z.; Zhang, Y.; Wang, D.; Tang, M.; Zhang, X.; Xia, Q.; Zhao, P. Improved strength of silk fibers in Bombyx mori trimolters induced by an anti-juvenile hormone compound. Biochim. Et Biophys. Acta (BBA) - Gen. Subj. 2018, 1862, 1148–1156. [Google Scholar] [CrossRef]
- Tsukada, M.; Obo, M.; Kato, H.; Freddi, G.; Zanetti, F. Structure and dyeability of Bombyx mori silk fibers with different filament sizes. J. Appl. Polym. Sci. 1996, 60, 1619–1627. [Google Scholar] [CrossRef]
- Tan, E.P.; Ng, S.Y.; Lim, C.T. Tensile testing of a single ultrafine polymeric fiber. Biomaterials 2005, 26, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Guan, J.; Vollrath, F. Spider silk: Super material or thin fibre? Adv. Mater. 2013, 25, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Vollrath, F.J.N. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Koh, L.D.; Li, D.; Ji, B.; Zhang, Y.; Yeo, J.; Guan, G.; Han, M.Y.; Zhang, Y.W. Peptide-graphene interactions enhance the mechanical properties of silk fibroin. ACS Appl. Mater. Interfaces 2015, 7, 21787–21796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.; Zhang, M.; Jian, M.; Zhang, Y. Feeding single-walled carbon nanotubes or graphene to silkworms for reinforced silk fibers. Nano Lett. 2016, 16, 6695–6700. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Xu, J.; Dong, Q.; Yu, Y.; Niu, B.L.; Ji, D.F.; Li, M.W.; Huang, Y.P.; Chen, X.; Tan, A.J. Mass spider silk production through targeted gene replacement in Bombyx mori. Proc. Natl. Acad. Sci. USA 2018, 115, 8757–8762. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Amsden, J.J.; Strikwerda, A.C.; Fan, K.; Kaplan, D.L.; Zhang, X.; Averitt, R.D.; Omenetto, F. Metamaterial silk composites at terahertz frequencies. Adv. Mater. 2010, 22, 3527–3531. [Google Scholar] [CrossRef]
- Qin, W.; Quan, C.; Yang, Y.; Shao, Z. Effect of various dissolution systems on the molecular weight of regenerated silk fibroin. Biomacromolecules 2013, 14, 285–289. [Google Scholar] [CrossRef]
- Xu, S.; Lin, Y.; Huang, J.; Li, Z.; Xu, X.; Zhang, L. Construction of high strength hollow fibers by self-assembly of a stiff polysaccharide with short branches in water. J. Mater. Chem. A 2013, 1, 4198–4206. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, C.; Zhang, L.; Li, W.; Hu, W.; Ma, S.; Xia, Q. A simple method for the cross-section area determination of single profiled fibers and its application. Microsc. Microanal. 2018, 24, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.J.; Tan, J.Z.; Wang, G.H. Study on quality and property of fine-fenier raw silk. Adv. Mater. Res. 2013, 796, 152–155. [Google Scholar] [CrossRef]
- Ito, H.; Muraoka, Y.; Yamazaki, T.; Imamura, T.; Mori, H.; Ichida, M.; Sumida, M.; Matsubara, F. Structure and chemical composition of silk proteins in relation to silkworm diet. Text. Res. J. 1995, 65, 755–759. [Google Scholar] [CrossRef]
- Švarcová, S.; Čermáková, Z.; Hradilová, J.; BezdiČka, P.; Hradil, D. Non-destructive micro-analytical differentiation of copper pigments in paint layers of works of art using laboratory-based techniques. Spectrochim. Acta Part A 2014, 132, 514–525. [Google Scholar] [CrossRef]
- Ling, S.; Qi, Z.; Knight, D.P.; Shao, Z.; Chen, X. Synchrotron FTIR microspectroscopy of single natural silk fibers. Biomacromolecules 2011, 12, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Fang, G.; Huang, Y.; Tang, Y.; Qi, Z.; Yao, J.; Shao, Z.; Chen, X. Insights into silk formation process: Correlation of mechanical properties and structural evolution during artificial spinning of silk fibers. ACS Biomater. Sci. Eng. 2016, 2, 1992–2000. [Google Scholar] [CrossRef]
- Li, M.; Wei, T.; Kuga, S.; Nishiyama, Y. Controlling molecular conformation of regenerated wild silk fibroin by aqueous ethanol treatment. Polym. Adv. Technol. 2003, 14, 694–698. [Google Scholar] [CrossRef]
- Fu, C.; Porter, D.; Chen, X.; Vollrath, F.; Shao, Z. Understanding the mechanical properties of Antheraea Pernyi silk—from primary structure to condensed structure of the protein. Adv. Funct. Mater. 2011, 21, 729–737. [Google Scholar] [CrossRef]
- Chung, D.E.; Kim, H.H.; Kim, M.K.; Lee, K.H.; Park, Y.H.; Um, I.C. Effects of different Bombyx mori silkworm varieties on the structural characteristics and properties of silk. Int. J. Biol. Macromol. 2015, 79, 943–951. [Google Scholar] [CrossRef]
- Fang, G.; Sapru, S.; Behera, S.; Yao, J.; Shao, Z.; Kundu, S.C.; Chen, X. Exploration of the tight structural–mechanical relationship in mulberry and non-mulberry silkworm silks. J. Mater. Chem. B 2016, 4, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Zheng, Z.; Yao, J.; Min, C.; Tang, Y.; Zhong, J.; Qi, Z.; Zhao, L.; Shao, Z.; Xin, C. Tough protein–carbon nanotube hybrid fibers comparable to natural spider silks. J. Mater. Chem. B 2015, 3, 3940–3947. [Google Scholar] [CrossRef]
- Guo, Y.P.; Wang, H.J.; Guo, Y.J.; Guo, L.H.; Chu, L.F.; Guo, C.X. Fabrication and characterization of hierarchical ZSM-5 zeolites by using organosilanes as additives. Chem. Eng. J. 2011, 166, 391–400. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Zhang, Q.; Guan, Y.; Cai, J.; You, R.; Li, X. Effect of degumming methods on the degradation behavior of silk fibroin biomaterials. J Fibers Polymers 2019, 20, 45–50. [Google Scholar] [CrossRef]
- Chen, X.; Shao, Z.; Marinkovic, N.S.; Miller, L.M.; Zhou, P.; Chance, M.R. Conformation transition kinetics of regenerated Bombyx mori silk fibroin membrane monitored by time-resolved FTIR spectroscopy. Biophys. Chem. 2001, 89, 25–34. [Google Scholar] [CrossRef]
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2006, 82, 223–233. [Google Scholar] [CrossRef]
- Fang, G.; Tang, Y.; Qi, Z.; Yao, J.; Shao, Z.; Xin, C. Precise correlation of macroscopic mechanical properties and microscopic structures of animal silks—using Antheraea pernyi silkworm silk as an example. J. Mater. Chem. B 2017, 5, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Miyazawa, M. Molecular orientation behavior of silk sericin film as revealed by ATR infrared spectroscopy. Biomacromolecules 2005, 6, 2049–2057. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Zhou, X.; Huang, H. Low pressure-induced secondary structure transitions of regenerated silk fibroin in its wet film studied by time-resolved infrared spectroscopy. Proteins Struct. Funct. Genet. 2018, 86, 621–628. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, X.; Liu, C.; Dong, Z.; Wang, F.; Wang, X.; Hu, W.; Zhang, X.; Zhao, P.; Xia, Q. Structural and mechanical properties of silk from different instars of Bombyx mori. Biomacromolecules 2019, 20, 1203–1216. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D.M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986, 130, 290–311. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xie, W.; Gao, Q.; Wang, D.; Gao, F.; Li, S.; Zhao, L. In situ biomineralization by silkworm feeding with ion precursors for the improved mechanical properties of silk fiber. Int. J. Biol. Macromol. 2018, 109, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Qin, Z.; Li, C.; Huang, W.; Kaplan, D.L.; Buehler, M.J. Polymorphic regenerated silk fibers assembled through bioinspired spinning. Nat. Commun. 2017, 8, 1387. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.; Huang, P.; Caughey, W.S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, Y.; Ren, J.; Tang, Y.; Qi, Z.; Zhou, X.; Chen, X.; Shao, Z.; Chen, M.; Kaplan, D.L.; et al. Understanding secondary structures of silk materials via micro- and nano-infrared spectroscopies. ACS Biomater. Sci. Eng. 2019, 5, 3161–3183. [Google Scholar] [CrossRef]
- Chou, P.Y.; Fasman, G.D. β-Turns in proteins. J. Mol. Biol. 1977, 115, 135–175. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, H.; Hu, X. Morphology and structure of electrospun mats from regenerated silk fibroin aqueous solutions with adjusting pH. Int. J. Biol. Macromol. 2007, 41, 469–474. [Google Scholar] [CrossRef]
- Wang, J.T.; Li, L.L.; Zhang, M.Y.; Liu, S.L.; Jiang, L.H.; Shen, Q. Directly obtaining high strength silk fiber from silkworm by feeding carbon nanotubes. Mater. Sci. Eng. C 2014, 34, 417–421. [Google Scholar] [CrossRef]
- Williams, C.M. The juvenile hormone of insects. Nature 1956, 178, 212–213. [Google Scholar] [CrossRef]
- Dubrovsky, E.B. Hormonal cross talk in insect development. Trends Endocrinol. Metab. 2005, 16, 6–11. [Google Scholar] [CrossRef]
- Asano, S.; Kuwano, E.; Eto, M. Precocious metamorphosis induced by an anti-juvenile hormone compound applied to 3rd instar silkworm larvae, Bombyx mori L. (Lepidoptera:Bombycidae). Appl. Entomol. Zool. 1986, 21, 305–312. [Google Scholar] [CrossRef]
- Cheng, D.; Peng, J.; Meng, M.; Wei, L.; Kang, L.; Qian, W.; Xia, Q. Microarray analysis of the juvenile hormone response in larval integument of the silkworm, Bombyx mori. Int. J. Genom. 2014, 2014, 426025. [Google Scholar] [CrossRef]
- Bowers, W.S. How anti-juvenile hormones work. BioScience 1981, 21, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Stall, G. Anti juvenile hormone agents. Annu. Rev. Entomol. 1986, 31, 391–429. [Google Scholar] [CrossRef]
- Kuwano, E.; Takeya, R.; Eto, M. Synthesis and anti-juvenile hormone activity of I-substituted- 5-[(E)-2,6-dimethyl-l,5-heptadienyl]imidazoles. Agric. Biol. Chem. 1985, 49, 483–486. [Google Scholar] [CrossRef]
- Lu, X. Studies on the superfine silk production of induced trimolting silkworm by SM-1. Acta Seric. Sin. 1987, 13, 71–76. Available online: http://www.chinaagrisci.com/CN/ (accessed on 26 October 2020). (In Chinese).
- Yamashita, O.; Kadono-Okuda, K.; Kuwano, E.; Eto, M. An imidazole compound as a potent anti-ecdysteroid in an insect. Agric. Biol. Chem. 1987, 51, 2295–2297. [Google Scholar] [CrossRef]
- Magoshi, J.; Magoshi, Y.; Nakamura, S. Low energy and super spinning of silkworm (silk):multiple spinning of Bombyx mori. Sen’i Gakkaishi 1997, 53, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Vollrath, F.; Knight, D.P. Structure and function of the silk production pathway in the spider Nephila edulis. Int. J. Biol. Macromol. 1999, 24, 243–249. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, H.; Shao, H.; Hu, X. Effect of shearing on formation of silk fibers from regenerated Bombyx mori silk fibroin aqueous solution. Int. J. Biol. Macromol. 2006, 38, 284–288. [Google Scholar] [CrossRef]
- Eisoldt, L.; Hardy, J.G.; Heim, M.; Scheibel, T.R. The role of salt and shear on the storage and assembly of spider silk proteins. J. Struct. Biol. 2010, 170, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Nova, A.; Keten, S.; Pugno, N.M.; Redaelli, A.; Buehler, M.J. Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils. Nano Lett. 2010, 10, 2626–2634. [Google Scholar] [CrossRef] [Green Version]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Koh, L.D.; Li, D.; Ji, B.; Han, M.Y.; Zhang, Y.W. On the strength of beta-sheet crystallites of Bombyx mori silk fibroin. J. R. Soc. Interface 2014, 11, 20140305. [Google Scholar] [CrossRef] [Green Version]
- Du, N.; Yang, Z.; Liu, X.Y.; Li, Y.; Xu, H.Y. Structural origin of the strain-hardening of spider silk. Adv. Funct. Mater. 2011, 21, 772–778. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Adamcik, J.; Jeong, J.S.; Lashuel, H.A.; Mezzenga, R.; Dietler, G. Influence of the beta-sheet content on the mechanical properties of aggregates during amyloid fibrillization. Angew. Chem. Int. Ed. Engl. 2015, 54, 2462–2466. [Google Scholar] [CrossRef]
- Yazawa, K.; Malay, A.D.; Ifuku, N.; Ishii, T.; Masunaga, H.; Hikima, T.; Numata, K. Combination of amorphous silk fiber spinning and postspinning crystallization for tough regenerated silk fibers. Biomacromolecules 2018, 19, 2227–2237. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Liu, L.; Chen, G.; Tan, J. Induction, development and regulation of trimolters: Great progress in the domesticated silkworm (Bombyx mori L.). Afr. J. Biotechnol. 2013, 12, 1171–1177. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Zhang, X.; Dong, Z.; Ni, Y.; Chen, Y.; Zhang, Y.; Li, H.; Xia, Q.; Zhao, P. Ultrafine and High-Strength Silk Fibers Secreted by Bimolter Silkworms. Polymers 2020, 12, 2537. https://doi.org/10.3390/polym12112537
Guo K, Zhang X, Dong Z, Ni Y, Chen Y, Zhang Y, Li H, Xia Q, Zhao P. Ultrafine and High-Strength Silk Fibers Secreted by Bimolter Silkworms. Polymers. 2020; 12(11):2537. https://doi.org/10.3390/polym12112537
Chicago/Turabian StyleGuo, Kaiyu, Xiaolu Zhang, Zhaoming Dong, Yuhui Ni, Yuqing Chen, Yan Zhang, Haoyun Li, Qingyou Xia, and Ping Zhao. 2020. "Ultrafine and High-Strength Silk Fibers Secreted by Bimolter Silkworms" Polymers 12, no. 11: 2537. https://doi.org/10.3390/polym12112537
APA StyleGuo, K., Zhang, X., Dong, Z., Ni, Y., Chen, Y., Zhang, Y., Li, H., Xia, Q., & Zhao, P. (2020). Ultrafine and High-Strength Silk Fibers Secreted by Bimolter Silkworms. Polymers, 12(11), 2537. https://doi.org/10.3390/polym12112537