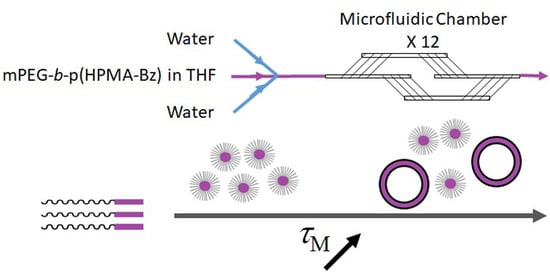
Tuning Size and Morphology of mPEG-b-p(HPMA-Bz) Copolymer Self-Assemblies Using Microfluidics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
Laminar Chaotic Mixing Microfluidic System
2.3. Methods
2.3.1. Dynamic Light Scattering (DLS) Analysis
2.3.2. Asymmetric Flow Field-Flow Fractionation Connected to Multi-Angle Laser Light Scattering Detector (AF4-MALLS)
2.3.3. Cryo-Transmission Electron Microscopy (Cryo-TEM) Analysis
2.3.4. Polymer Synthesis
2.3.5. Preparation of Nanoparticles Based on mPEG-b-p(HPMA-Bz) Using Microfluidics
3. Results and Discussion
3.1. Synthesis of mPEG-b-p(HPMA-Bz) Block Copolymers
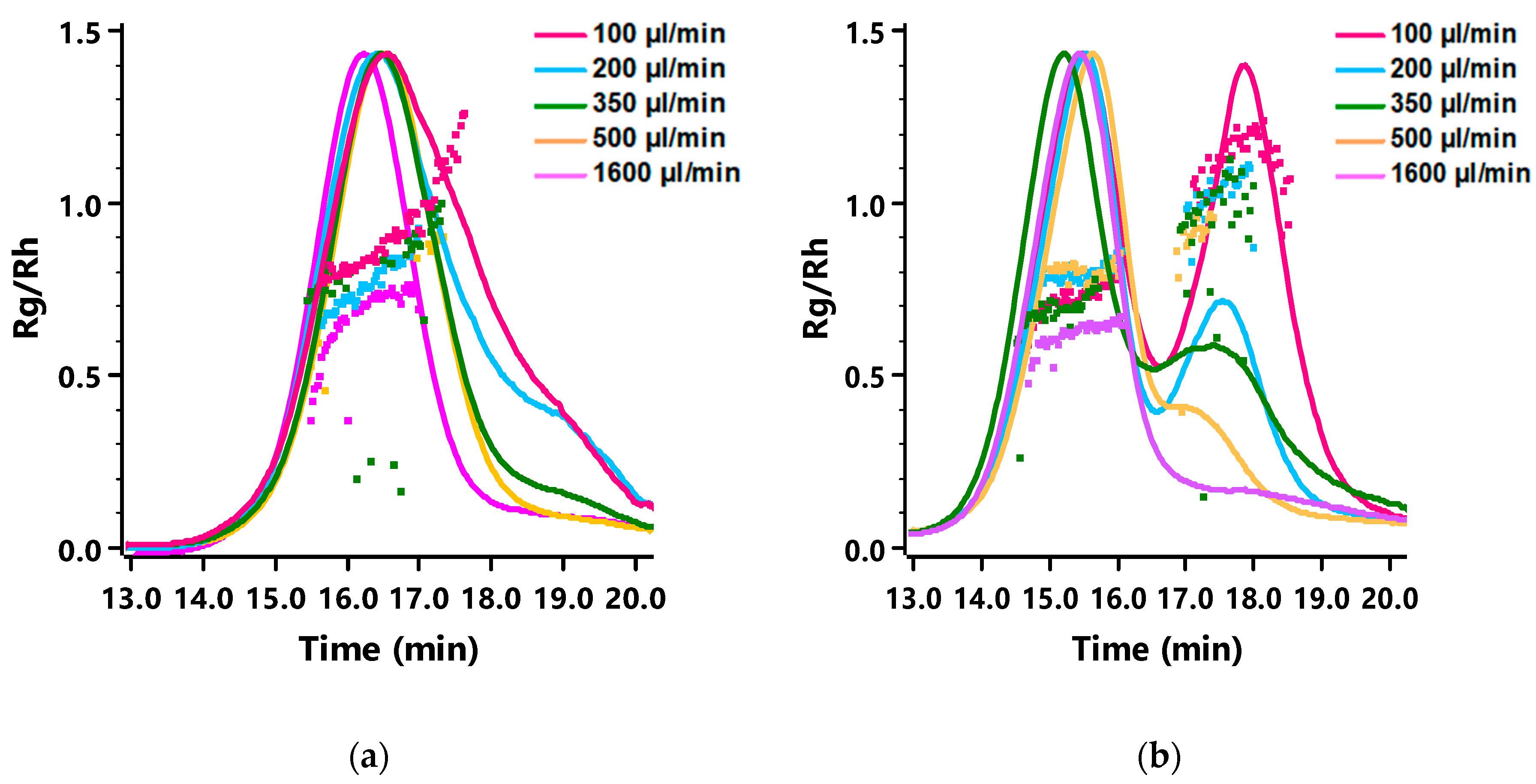
3.2. The Effect of Mixing Time on the Size and Morphology of mPEG-b-p(HPMA-Bz) Nanoparticles
3.3. Morphology of mPEG-b-p(HPMA-Bz) nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Lammers, T.; Hennink, W.E. Cancer nanomedicines: Oversold or underappreciated? Expert Opin. Drug Deliv. 2017, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-installed nanocarriers toward precision therapy. Adv. Mater. 2020, 32, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.A.M.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef]
- Sun, Q.; Ojha, T.; Kiessling, F.; Lammers, T.; Shi, Y. Enhancing tumor penetration of nanomedicines. Biomacromolecules 2017, 18, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Liu, J.; Zhang, W. Particle morphology: An important factor affecting drug delivery by nanocarriers into solid tumors. Expert Opin. Drug Deliv. 2018, 15, 379–395. [Google Scholar] [CrossRef]
- Pijpers, I.A.B.; Abdelmohsen, L.K.E.A.; Xia, Y.; Cao, S.; Williams, D.S.; Meng, F.; Hest, J.C.M.; Zhong, Z. Adaptive polymersome and micelle morphologies in anticancer nanomedicine: From design rationale to fabrication and proof-of-concept studies. Adv. Ther. 2018, 1, 1800068. [Google Scholar] [CrossRef]
- Qin, S.Y.; Cheng, Y.J.; Jiang, Z.W.; Ma, Y.H.; Zhang, A.Q. Morphology control of self-deliverable nanodrug with enhanced anticancer efficiency. Colloids Surf. B Biointerfaces 2018, 165, 345–354. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Thioune, O.; Fessi, H.; Devissaguet, J.P.; Puisieux, F. Preparation of pseudolatex by nanoprecipitation: Influence of the solvent nature on intrinsic viscosity and interaction constant. Int. J. Pharm. 1997, 146, 233–238. [Google Scholar] [CrossRef]
- Pagels, R.F.; Edelstein, J.; Tang, C.; Prud’homme, R.K. Controlling and predicting nanoparticle formation by block copolymer directed rapid precipitations. Nano Lett. 2018, 18, 1139–1144. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Rytting, E.; Lebhardt, T.; Wang, X.; Kissel, T. Preparation of nanoparticles by solvent displacement for drug delivery: A shift in the “ouzo region” upon drug loading. Eur. J. Pharm. Sci. 2010, 41, 244–253. [Google Scholar] [CrossRef]
- Zhang, C.; Pansare, V.J.; Prud’Homme, R.K.; Priestley, R.D. Flash nanoprecipitation of polystyrene nanoparticles. Soft Matter 2012, 8, 86–93. [Google Scholar] [CrossRef]
- Aubry, J.; Ganachaud, F.; Addad, J.P.C.; Cabane, B. Nanoprecipitation of polymethylmethacrylate by solvent shifting: 1. Boundaries. Langmuir 2009, 25, 1970–1979. [Google Scholar] [CrossRef]
- Nicolai, T.; Colombani, O.; Chassenieux, C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter 2010, 6, 3111–3118. [Google Scholar] [CrossRef]
- Campos-Villalobos, G.; Siperstein, F.R.; Charles, A.; Patti, A. Solvent-induced morphological transitions in methacrylate-based block-copolymer aggregates. J. Colloid Interface Sci. 2020, 572, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejeune, E.; Drechsler, M.; Jestin, J.; Müller, A.H.E.; Chassenieux, C.; Colombani, O. Amphiphilic diblock copolymers with a moderately hydrophobic block: Toward dynamic micelles. Macromolecules 2010, 43, 2667–2671. [Google Scholar] [CrossRef]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Wulff, J.E.; Moffitt, M.G. Microfluidic processing approach to controlling drug delivery properties of curcumin-loaded block copolymer nanoparticles. Mol. Pharm. 2018, 15, 4517–4528. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883. [Google Scholar] [CrossRef]
- Liu, D.; Cito, S.; Zhang, Y.; Wang, C.F.; Sikanen, T.M.; Santos, H.A. A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties. Adv. Mater. 2015, 27, 2298–2304. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, B.; Jin, M.; Yu, Y.; Zhou, G.; Shui, L. A review on self-assembly in microfluidic devices. J. Micromech. Microeng. 2017, 27, 113002. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J. Self-assembly of colloids based on microfluidics. Nanoscale 2019, 11, 16708–16722. [Google Scholar] [CrossRef]
- Wang, L.; Sánchez, S. Self-assembly via microfluidics. Lab Chip 2015, 15, 4383–4386. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, E.; Dorati, R.; Modena, T.; Conti, B.; Genta, I. Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int. J. Pharm. 2018, 536, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, S.; MacHado, A.; Lecommandoux, S.; Sandre, O.; Gu, F.; Colin, A. Controllable microfluidic production of drug-loaded PLGA nanoparticles using partially water-miscible mixed solvent microdroplets as a precursor. Sci. Rep. 2017, 7, 4794. [Google Scholar] [CrossRef]
- Ding, S.; Anton, N.; Vandamme, T.F.; Serra, C.A. Microfluidic nanoprecipitation systems for preparing pure drug or polymeric drug loaded nanoparticles: An overview. Expert Opin. Drug Deliv. 2016, 13, 1447–1460. [Google Scholar] [CrossRef]
- Martins, J.P.; Torrieri, G.; Santos, H.A. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin. Drug Deliv. 2018, 15, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2018, 128, 54–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, S.; Hasani-Sadrabadi, M.M.; Majedi, F.S.; Dashtimoghadam, E.; Tondar, M.; Jacob, K.I. Understanding biophysical behaviours of microfluidic-synthesized nanoparticles at nano-biointerface. Colloids Surf. B Biointerfaces 2016, 145, 802–811. [Google Scholar] [CrossRef]
- Bally, F.; Garg, D.K.; Serra, C.A.; Hoarau, Y.; Anton, N.; Brochon, C.; Parida, D.; Vandamme, T.; Hadziioannou, G. Improved size-tunable preparation of polymeric nanoparticles by microfluidic nanoprecipitation. Polymer 2012, 53, 5045–5051. [Google Scholar] [CrossRef] [Green Version]
- Keßler, S.; Drese, K.; Schmid, F. Simulating copolymeric nanoparticle assembly in the co-solvent method: How mixing rates control final particle sizes and morphologies. Polymer 2017, 126, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef]
- Lebleu, C.; Rodrigues, L.; Guigner, J.M.; Brûlet, A.; Garanger, E.; Lecommandoux, S. Self-assembly of PEG-b-PTMC copolymers: Micelles and polymersomes size control. Langmuir 2019, 35, 13364–13374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, M.; Bresseleers, J.; Varela-Moreira, A.; Sandre, O.; Meeuwissen, S.A.; Schiffelers, R.M.; Metselaar, J.M.; Van Nostrum, C.F.; Van Hest, J.C.M.; Hennink, W.E. Effect of formulation and processing parameters on the size of mPEG-b-p(HPMA-Bz) polymeric micelles. Langmuir 2018, 34, 15495–15506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundararajan, P.; Stroock, A.D. Transport phenomena in chaotic laminar flows. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 473–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Fer, G.; Portes, D.; Goudounet, G.; Guigner, J.M.; Garanger, E.; Lecommandoux, S. Design and self-assembly of PBLG-: B-ELP hybrid diblock copolymers based on synthetic and elastin-like polypeptides. Org. Biomol. Chem. 2017, 15, 10095–10104. [Google Scholar] [CrossRef]
- Bresseleers, J.; Bagheri, M.; Storm, G.; Metselaar, J.M.; Hennink, W.E.; Meeuwissen, S.A.; Van Hest, J.C.M. Scale-Up of the Manufacturing process to produce docetaxel-loaded mPEG-b-p(HPMA-Bz) block copolymer micelles for pharmaceutical applications. Org. Process Res. Dev. 2019, 23, 2707–2715. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Van Steenbergen, M.J.; Teunissen, E.A.; Novo, L.; Gradmann, S.; Baldus, M.; Van Nostrum, C.F.; Hennink, W.E. Π-Π Stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules 2013, 14, 1826–1837. [Google Scholar] [CrossRef]
- Micromixer Chip. Available online: https://www.dolomite-microfluidics.com/product/micromixer-chip/ (accessed on 26 May 2020).
- Zimm, B.H. Apparatus and methods for measurement and interpretation of the angular variation of light scattering; Preliminary results on polystyrene solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Theek, B.; Oude Blenke, E.; Pieters, E.H.E.; Fens, M.H.A.M.; Ehling, J.; Schiffelers, R.M.; Storm, G.; Van Nostrum, C.F.; et al. Complete regression of xenograft tumors upon targeted delivery of paclitaxel via Pi-Pi stacking stabilized polymeric micelles. ACS Nano 2015, 9, 3740–3752. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.; Cosgrove, T. A Small-Angle Neutron scattering study of adsorbed poly(ethylene oxide) on Laponite. Langmuir 2004, 20, 2298–2304. [Google Scholar] [CrossRef]
- Jain, S.; Bates, F.S. On the origins of morphological complexity in block copolymer surfactants. Science 2003, 300, 460–464. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Prud’homme, R.K. Controlling drug nanoparticle formation by rapid precipitation. Adv. Drug Deliv. Rev. 2011, 63, 417–426. [Google Scholar] [CrossRef]
- Kunz, D.; Thurn, A.; Burchard, W. Dynamic light scattering from spherical particles. Colloid Polym. Sci. 1983, 261, 635–644. [Google Scholar] [CrossRef]
- Ma, C.; Pan, P.; Shan, G.; Bao, Y.; Fujita, M.; Maeda, M. Core-Shell structure, biodegradation, and drug release behavior of poly(lactic acid)/poly(ethylene glycol) block copolymer micelles tuned by macromolecular stereostructure. Langmuir 2015, 31, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Zhao, Y.; Xie, Z.; Wu, C. Micellar formation of poly(caprolactone-block-ethylene oxide-block-caprolactone) and its enzymatic biodegradation in aqueous dispersion. Macromolecules 2003, 36, 8825–8829. [Google Scholar] [CrossRef]
- Nguyen, V.T.A.; De Pauw-Gillet, M.C.; Sandre, O.; Gauthier, M. Biocompatible polyion complex micelles synthesized from arborescent polymers. Langmuir 2016, 32, 13482–13492. [Google Scholar] [CrossRef]
- Giacomelli, C.; Schmidt, V.; Aissou, K.; Borsali, R. Block copolymer systems: From single chain to self-assembled nanostructures. Langmuir 2010, 26, 15734–15744. [Google Scholar] [CrossRef] [PubMed]
- Kale, T.S.; Klaikherd, A.; Popere, B.; Thayumanavan, S. Supramolecular assemblies of amphiphilic homopolymers. Langmuir 2009, 25, 9660–9670. [Google Scholar] [CrossRef]
- Abdelmohsen, L.K.E.A.; Rikken, R.S.M.; Christianen, P.C.M.; van Hest, J.C.M.; Wilson, D.A. Shape characterization of polymersome morphologies via light scattering techniques. Polymer 2016, 107, 445–449. [Google Scholar] [CrossRef]
- Chécot, F.; Brûlet, A.; Oberdisse, J.; Gnanou, Y.; Mondain-Monval, O.; Lecommandoux, S. Structure of polypeptide-based diblock copolymers in solution: Stimuli-responsive vesicles and micelles. Langmuir 2005, 21, 4308–4315. [Google Scholar] [CrossRef] [Green Version]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- He, X.; Schmid, F. Dynamics of spontaneous vesicle formation in dilute solutions of amphiphilic diblock copolymers. Macromolecules 2006, 39, 2654–2662. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]





| Polymer | M:MI | Mn | GPC | NHPMA-Bz | fPEG | ØPEG | ||
|---|---|---|---|---|---|---|---|---|
| Mn | Mw | Ð | ||||||
| A: mPEG5K-b-p(HPMA-Bz)17.1K | 200 | 22.1 | 15.8 | 20.7 | 1.31 | 69 | 23 | 24 |
| B: mPEG5K-b-p(HPMA-Bz)10.0K | 100 | 15.0 | 13.2 | 17.5 | 1.32 | 40 | 33 | 35 |
| C: mPEG5K-b-p(HPMA-Bz)5.2K | 50 | 10.2 | 10.8 | 14.0 | 1.30 | 21 | 49 | 50 |
| D: mPEG5K-b-p(HPMA-Bz)2.7K | 25 | 7.7 | 8.9 | 11.0 | 1.24 | 11 | 65 | 66 |
| Concentration (mg/mL) | Q (µL/min) | Rg (nm) | Rh (nm) | Rg/Rh | Mw(np) (103 kDa) | Nagg |
|---|---|---|---|---|---|---|
| 5 | 100 | 46 | 45 | 1.03 | 187 | 8500 |
| 5 | 200 | 35 | 39 | 0.90 | 142 | 6400 |
| 5 | 350 | 32 | 36 | 0.89 | 131 | 5900 |
| 5 | 500 | 30 | 37 | 0.82 | 150 | 6800 |
| 5 | 1600 | 21 | 26 | 0.81 | 36 | 1600 |
| 10 | 100 | 34 | 33 | 1.03 | 79 | 3600 |
| 10 | 200 | 24 | 30 | 0.82 | 64 | 2900 |
| 10 | 350 | 24 | 28 | 0.86 | 42 | 1900 |
| 10 | 500 | 22 | 28 | 0.78 | 39 | 1800 |
| 10 | 1600 | 17 | 25 | 0.69 | 26 | 1200 |
| 20 | 100 | 24 | 28 | 0.85 | 42 | 1900 |
| 20 | 200 | 22 | 26 | 0.82 | 34 | 1600 |
| 20 | 350 | 20 | 27 | 0.73 | 69 | 3100 |
| 20 | 500 | 21 | 28 | 0.76 | 36 | 1600 |
| 20 | 1600 | 20 | 25 | 0.78 | 34 | 1500 |
| Concentration (mg/mL) | Q (µL/min) | Peak 1 | Peak 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rg (nm) | Rh (nm) | Rg/Rh | Mw(np) (103 kDa) | Nagg | Rg (nm) | Rh (nm) | Rg/Rh | Mw(np) (103 kDa) | Nagg | ||
| 5 | 100 | 13 | 17 | 0.76 | 3.1 | 400 | 54 | 54 | 0.99 | 98 | 12,700 |
| 5 | 200 | 13 | 17 | 0.80 | 3.2 | 420 | 46 | 51 | 0.91 | 68 | 8800 |
| 5 | 350 | 13 | 17 | 0.77 | 3.3 | 430 | 34 | 40 | 0.85 | 91 | 11,800 |
| 5 | 500 | 12 | 17 | 0.68 | 3.5 | 450 | 32 | 39 | 0.82 | 171 | 22,200 |
| 5 | 1600 | 11 | 17 | 0.63 | 3.4 | 450 | - | - | - | - | - |
| 10 | 100 | 10 | 16 | 0.65 | 2.5 | 320 | 53 | 56 | 0.93 | 70 | 9000 |
| 10 | 200 | 11 | 16 | 0.67 | 2.9 | 380 | 47 | 76 | 0.62 | 11 | 1500 |
| 10 | 350 | 11 | 17 | 0.63 | 3.2 | 420 | 26 | 39 | 0.65 | 625 | 81,200 |
| 10 | 500 | 12 | 16 | 0.71 | 2.6 | 340 | - | 39 | - | - | - |
| 10 | 1600 | 13 | 17 | 0.77 | 2.8 | 360 | - | - | - | - | - |
| 20 | 100 | 12 | 16 | 0.75 | 2.3 | 300 | 59 | 54 | 1.09 | 147 | 19,100 |
| 20 | 200 | 13 | 16 | 0.81 | 2.3 | 300 | 47 | 45 | 1.04 | 145 | 18,780 |
| 20 | 350 | 11 | 16 | 0.71 | 2.5 | 320 | 48 | 45 | 1.06 | 66 | 8500 |
| 20 | 500 | 13 | 16 | 0.81 | 2.3 | 300 | 31 | 36 | 0.87 | 104 | 13,600 |
| 20 | 1600 | 11 | 16 | 0.75 | 2.5 | 320 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresseleers, J.; Bagheri, M.; Lebleu, C.; Lecommandoux, S.; Sandre, O.; Pijpers, I.A.B.; Mason, A.F.; Meeuwissen, S.; Nostrum, C.F.v.; Hennink, W.E.; et al. Tuning Size and Morphology of mPEG-b-p(HPMA-Bz) Copolymer Self-Assemblies Using Microfluidics. Polymers 2020, 12, 2572. https://doi.org/10.3390/polym12112572
Bresseleers J, Bagheri M, Lebleu C, Lecommandoux S, Sandre O, Pijpers IAB, Mason AF, Meeuwissen S, Nostrum CFv, Hennink WE, et al. Tuning Size and Morphology of mPEG-b-p(HPMA-Bz) Copolymer Self-Assemblies Using Microfluidics. Polymers. 2020; 12(11):2572. https://doi.org/10.3390/polym12112572
Chicago/Turabian StyleBresseleers, Jaleesa, Mahsa Bagheri, Coralie Lebleu, Sébastien Lecommandoux, Olivier Sandre, Imke A. B. Pijpers, Alexander F. Mason, Silvie Meeuwissen, Cornelus F. van Nostrum, Wim E. Hennink, and et al. 2020. "Tuning Size and Morphology of mPEG-b-p(HPMA-Bz) Copolymer Self-Assemblies Using Microfluidics" Polymers 12, no. 11: 2572. https://doi.org/10.3390/polym12112572
APA StyleBresseleers, J., Bagheri, M., Lebleu, C., Lecommandoux, S., Sandre, O., Pijpers, I. A. B., Mason, A. F., Meeuwissen, S., Nostrum, C. F. v., Hennink, W. E., & Hest, J. C. M. v. (2020). Tuning Size and Morphology of mPEG-b-p(HPMA-Bz) Copolymer Self-Assemblies Using Microfluidics. Polymers, 12(11), 2572. https://doi.org/10.3390/polym12112572