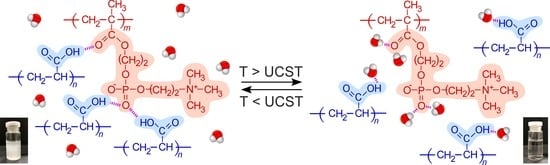
Thermo-Responsive Behavior of Mixed Aqueous Solution of Hydrophilic Polymer with Pendant Phosphorylcholine Group and Poly(Acrylic Acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PMPC
2.2. Preparation of PAAc
2.3. Measurements
3. Results and Discussion
3.1. Preparation and Characterization of the Polymers
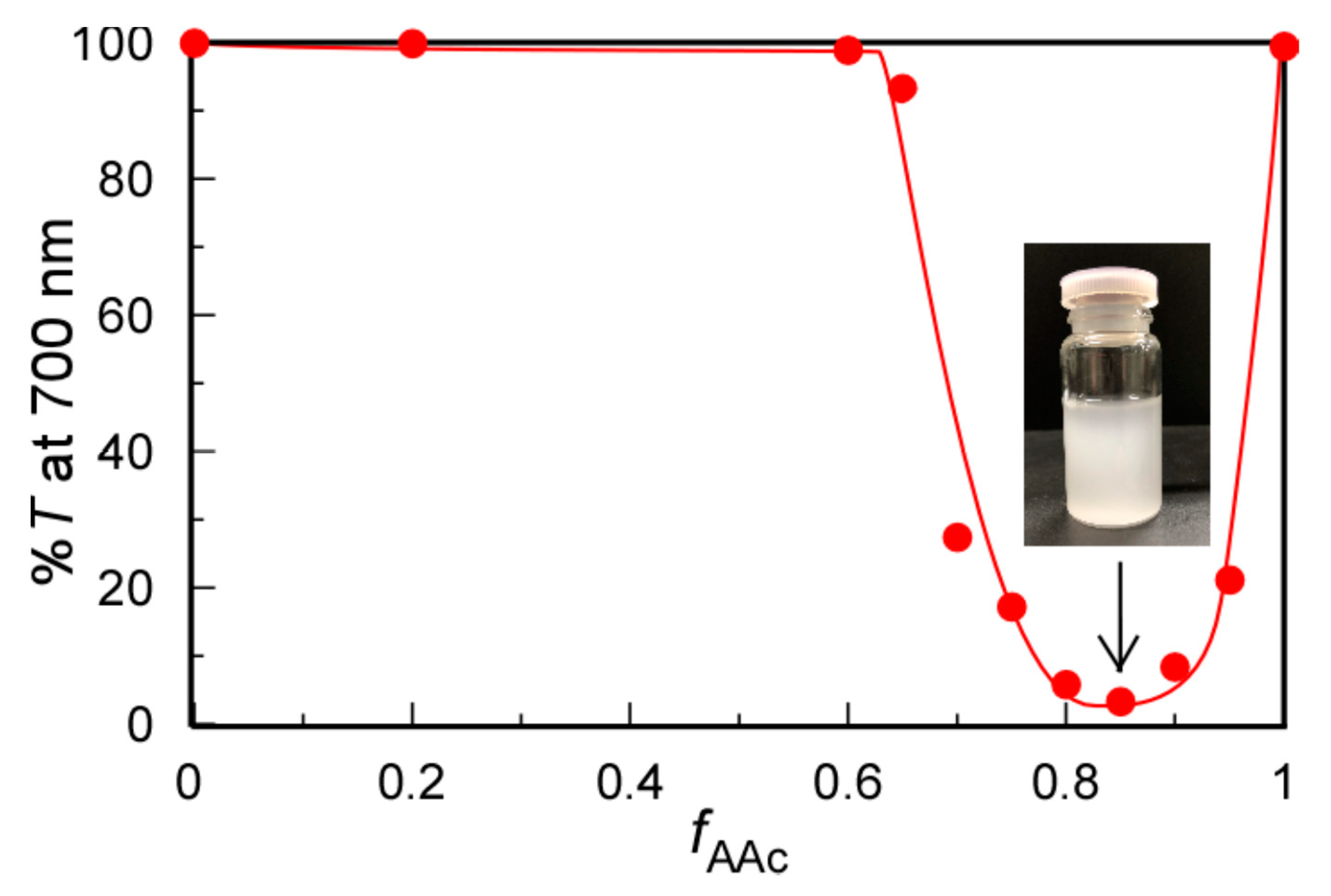
3.2. Mole Ratio Dependence on Solubility after Mixing Polymers
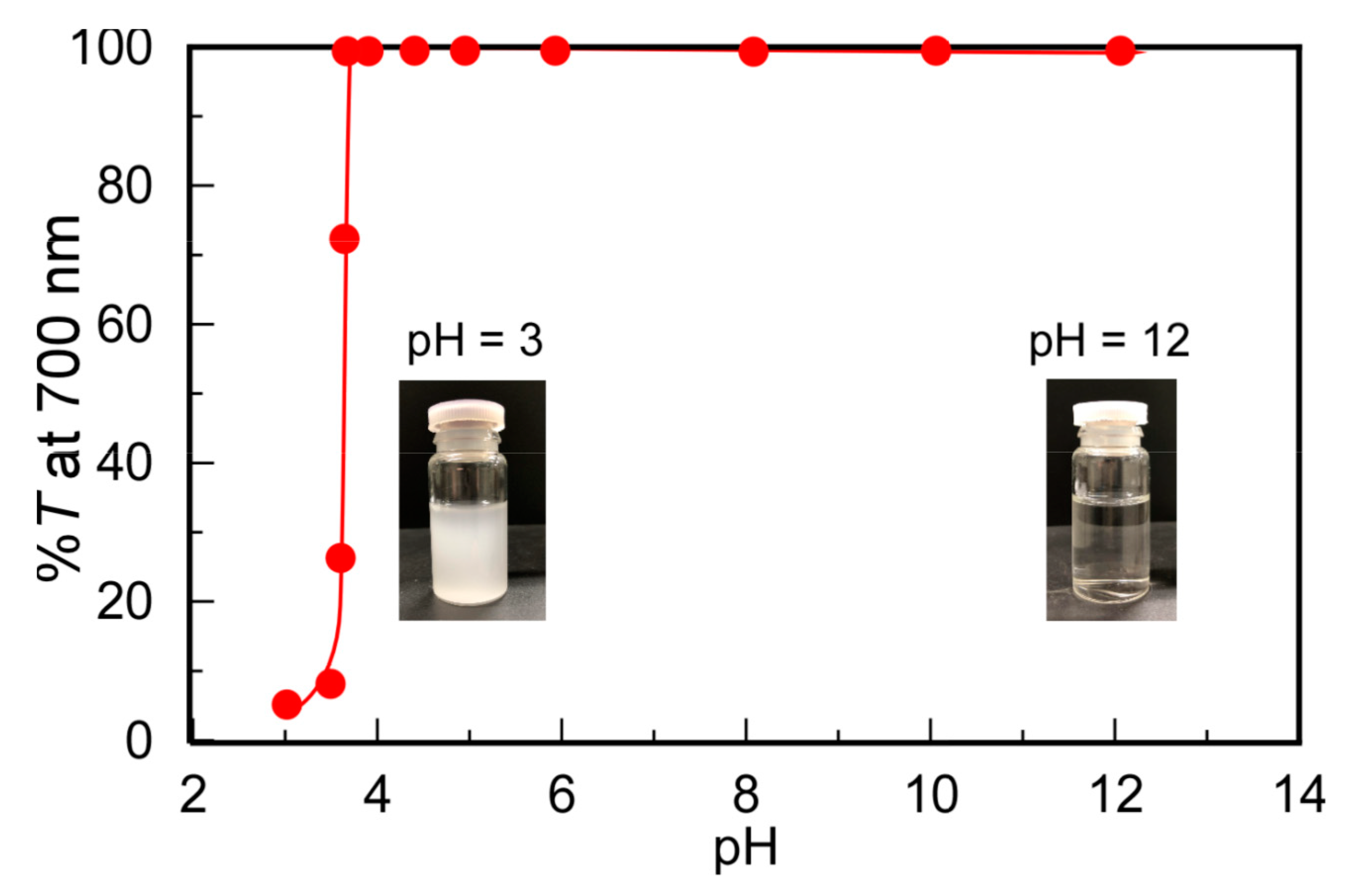
3.3. pH Dependence on Solubility
3.4. pH, Cp, and [NaCl] Dependence on UCST
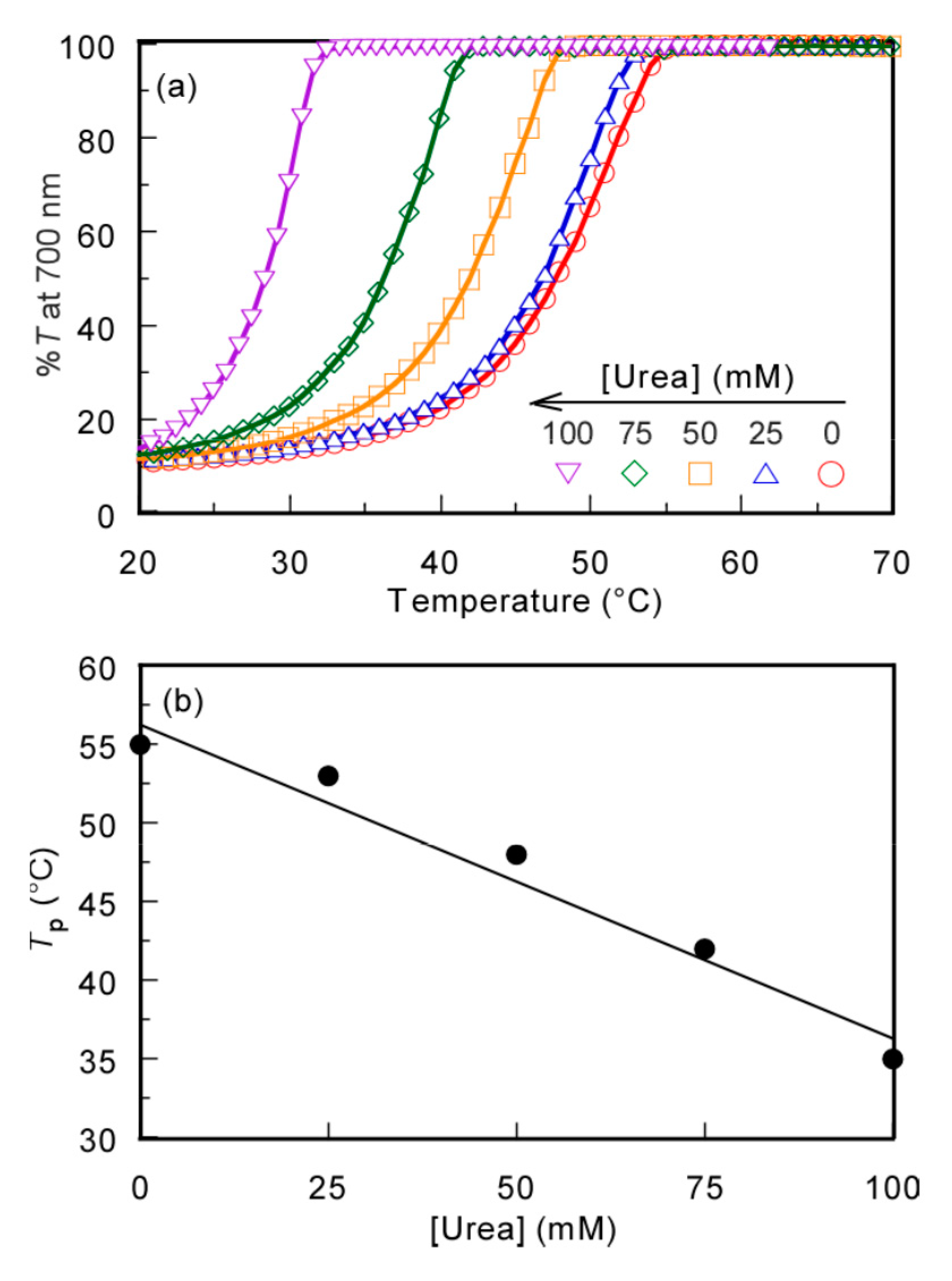
3.5. Effect of Urea on the UCST
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Green, M.D.; Chen, K.; Daengngam, C.; Kotsuchibashi, Y. Stimuli-responsive polymers: Design, synthesis, characterization, and applications. Int. J. Polym. Sci. 2016, 2016, 6480259. [Google Scholar] [CrossRef]
- Khimani, M.; Patel, H.; Patel, V.; Parekh, P.; Vekariya, R.L. Self-assembly of stimuli-responsive block copolymers in aqueous solutions: An overview. Polym. Bull. 2020, 77, 5783–5810. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Allan, S.H.; Patrick, S.S.; Volga, B.; Guohua, C.; Jingping, C.; Chuck, C.; Atsutosh, C.; Zhongli, D.; Liangchang, D.; Robin, F.; et al. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar]
- Eve, R.G.; Jean-Christophe, L. In situ-forming hydrogels―Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar]
- Dirk, S. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar]
- Priest, J.H.; Murray, S.L.; Nelson, R.J.; Hoffman, A.S. Lower critical solution temperatures of aqueous copolymers of N-isopropylacrylamide and other N-substituted acrylamides. ACS Symp. Ser. 1987, 18, 255–264. [Google Scholar]
- Hanneke, M.L.L.; Friso, S.W.; Anja, B.; Lies, B.; Filip, E.D.P.; Ulrich, S.S.; Richard, H. Linear poly(ethylene imine)s by acidic hydrolysis of poly(2-oxazoline)s: Kinetic screening, thermal properties, and temperature-induced solubility transitions. Macromolecules 2010, 43, 927–933. [Google Scholar]
- Doncom, K.E.B.; Willcock, H.; O’reilly, R.K. The direct synthesis of sulfobetaine-containing amphiphilic block copolymers and their self-assembly behavior. Eur. Polym. J. 2017, 87, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, A.; Shimada, N.; Maruyama, A.; Ishihara, K.; Nakai, K.; Yusa, S. Preparation of upper critical solution temperature (UCST) responsive diblock copolymers bearing pendant ureido groups and their micelle formation behavior in water. Soft Matter 2015, 11, 5204–5213. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Contreras, B.A.; Schmalz, H.; Agarwal, S. pH dependent thermoresponsive behavior of acrylamide-acrylonitrile UCST-type copolymers in aqueous media. Polym. Chem. 2016, 7, 1979–1986. [Google Scholar] [CrossRef] [Green Version]
- Seuring, J.; Agarwal, S. First example of a universal and cost-effective approach: Polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. UCST behavior of polyampholytes based on stoichiometric RAFT copolymerization of cationic and anionic monomers. Chem. Commun. 2015, 51, 70–73. [Google Scholar] [CrossRef]
- Abe, K.; Koide, M.; Tsuchida, E. Selective complexation of macromolecules. Macromolecules 1977, 10, 1259–1264. [Google Scholar] [CrossRef]
- Eustace, D.J.; Siano, D.B.; Drake, E.N. Polymer compatibility and interpolymer association in the poly(acrylic acid)-polyacrylamide-water ternary system. J. Appl. Polym. Sci. 1988, 35, 707–716. [Google Scholar] [CrossRef]
- Echeverria, C.; Lopez, D.; Mijangos, C. UCST responsive microgels of poly(acrylamide-acrylic acid) copolymers: Structure and viscoelastic properties. Macromolecules 2009, 42, 9118–9123. [Google Scholar] [CrossRef]
- Li, F.; Xing, Q.; Han, Y.; Li, Y.; Wang, W.; Perera, T.S.H.; Dai, H. Ultrasonically assisted preparation of poly(acrylic acid)/calcium phosphate hybrid nanogels as pH-responsive drug carriers. Mater. Sci. Eng. 2017, 80, 688–697. [Google Scholar] [CrossRef]
- Becerra-Bracamontes, F.; Sanchez-Diaz, J.C.; Gonzalez-Alvarez, A.; Ortega-Gudino, P.; Michel-Valdivia, E.; Martinez-Ruvalcaba, A. Design of a drug delivery system based on poly(acrylamide-co-acrylic acid)/chitosan nanostructured hydrogels. J. Appl. Polym. Sci. 2007, 106, 3939–3944. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci. Technol. Adv. Mater. 2000, 1, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. Part A 2019, 107A, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushita, T.; Chung, U.I.; Nakamura, K.; Kawaguchi, H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef]
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of poly(ethylene glycol) via reversible addition−fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 2009, 42, 376–383. [Google Scholar] [CrossRef]
- Abel, B.A.; Sims, M.B.; McCormick, C.L. Tunable pH- and CO2-responsive sulfonamide-containing polymers by RAFT polymerization. Macromolecules 2015, 48, 5487–5495. [Google Scholar] [CrossRef]
- Geismann, C.; Tomicki, F.; Ulbricht, M. Block copolymer photo-grafted poly(ethylene terephthalate) capillary pore membranes distinctly switchable by two different stimuli. Sep. Sci. Technol. 2009, 44, 3312–3329. [Google Scholar] [CrossRef]
- Paek, K.; Yang, H.; Lee, J.; Park, J.; Kim, B.J. Efficient colorimetric pH sensor based on responsive polymer-quantum dot integrated graphene oxide. ACS Nano 2014, 8, 2848–2856. [Google Scholar] [CrossRef]
- Yan, B.; Han, D.; Boissiere, O.; Ayotte, P.; Zhao, Y. Manipulation of block copolymer vesicles using CO2: Dissociation or “breathing”. Soft Matter 2013, 9, 2011–2016. [Google Scholar] [CrossRef]
- Yin, H.; Feng, Y.; Liu, H.; Mu, M.; Fei, C. Insights into the relationship between CO2 switchability and basicity: Examples of melamine and its derivatives. Langmuir 2014, 30, 9911–9919. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Bodensteiner, M.; Tolstoy, P.M.; Denisov, G.S.; Shenderovich, I.G. P=O moiety as an ambidextrous hydrogen bond acceptor. J. Phys. Chem. C 2018, 122, 1711–1720. [Google Scholar] [CrossRef]
- Pineda-Contreras, B.A.; Liu, F.; Agarwal, S. Importance of compositional homogeneity of macromolecular chains for UCST-type transitions in water: Controlled versus conventional radical polymerization. J. Polym. Sci. A Polym. Chem. 2014, 52, 1878–1884. [Google Scholar] [CrossRef]
- Sagle, L.B.; Zhang, Y.; Litosh, V.A.; Chen, X.; Cho, Y.; Cremer, P.S. Investigating the hydrogen-bonding model of urea denaturation. J. Am. Chem. Soc. 2009, 131, 9304–9310. [Google Scholar] [CrossRef] [PubMed]



| DP(theo) a | Mn(theo) b kDa | DP(NMR) c | Mn(NMR) c kDa | Mn(GPC) d kDa | Mw/Mnd | |
|---|---|---|---|---|---|---|
| PMPC | 99 | 29.5 | 98 | 29.2 | 27.9 | 1.37 |
| PAAc | 95 | 7.07 | 104 | 7.75 | 9.10 | 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukumoto, H.; Ishihara, K.; Yusa, S.-I. Thermo-Responsive Behavior of Mixed Aqueous Solution of Hydrophilic Polymer with Pendant Phosphorylcholine Group and Poly(Acrylic Acid). Polymers 2021, 13, 148. https://doi.org/10.3390/polym13010148
Fukumoto H, Ishihara K, Yusa S-I. Thermo-Responsive Behavior of Mixed Aqueous Solution of Hydrophilic Polymer with Pendant Phosphorylcholine Group and Poly(Acrylic Acid). Polymers. 2021; 13(1):148. https://doi.org/10.3390/polym13010148
Chicago/Turabian StyleFukumoto, Hirokazu, Kazuhiko Ishihara, and Shin-Ichi Yusa. 2021. "Thermo-Responsive Behavior of Mixed Aqueous Solution of Hydrophilic Polymer with Pendant Phosphorylcholine Group and Poly(Acrylic Acid)" Polymers 13, no. 1: 148. https://doi.org/10.3390/polym13010148
APA StyleFukumoto, H., Ishihara, K., & Yusa, S.-I. (2021). Thermo-Responsive Behavior of Mixed Aqueous Solution of Hydrophilic Polymer with Pendant Phosphorylcholine Group and Poly(Acrylic Acid). Polymers, 13(1), 148. https://doi.org/10.3390/polym13010148