Microstructure and Mechanical Properties of PU/PLDL Sponges Intended for Grafting Injured Spinal Cord
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
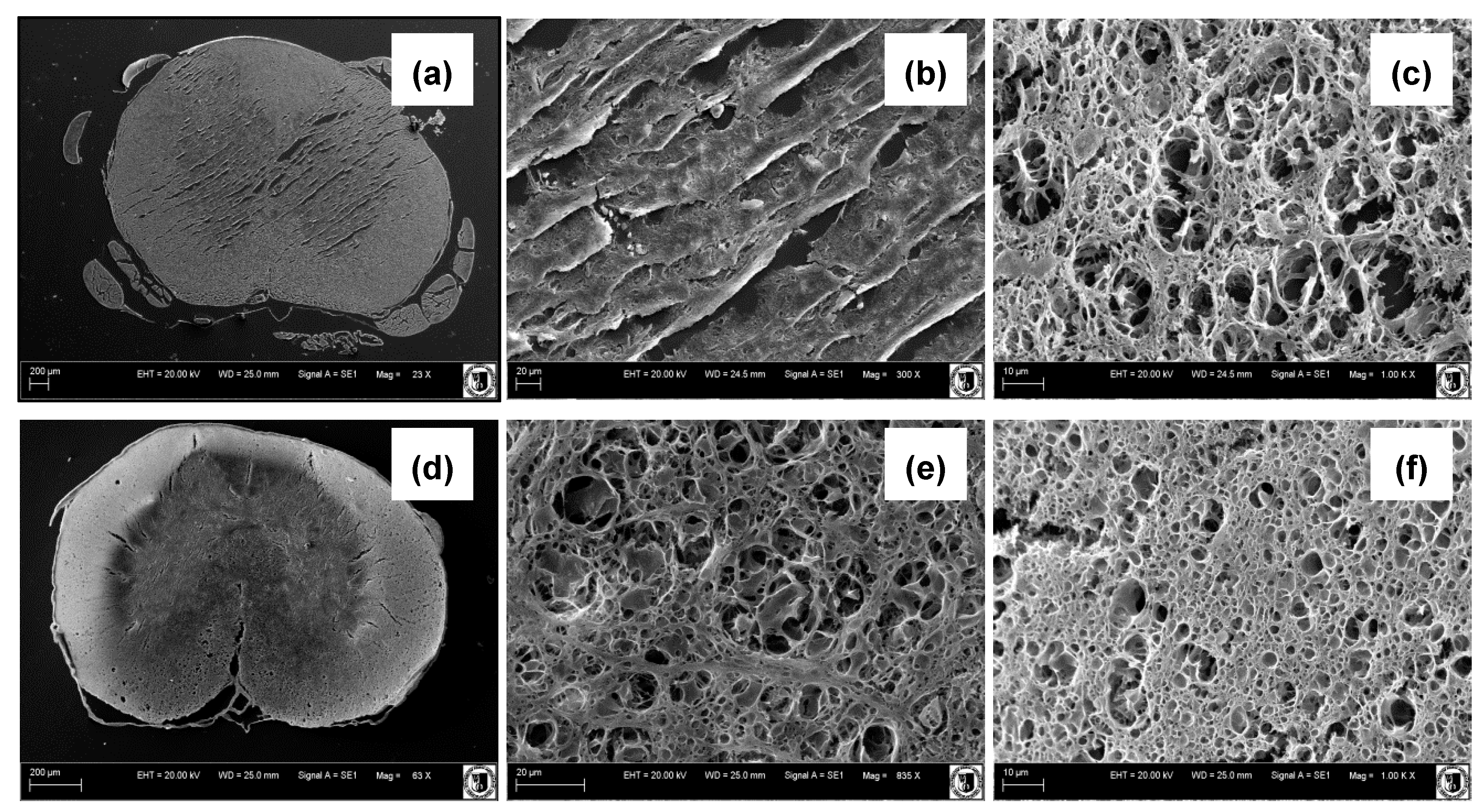
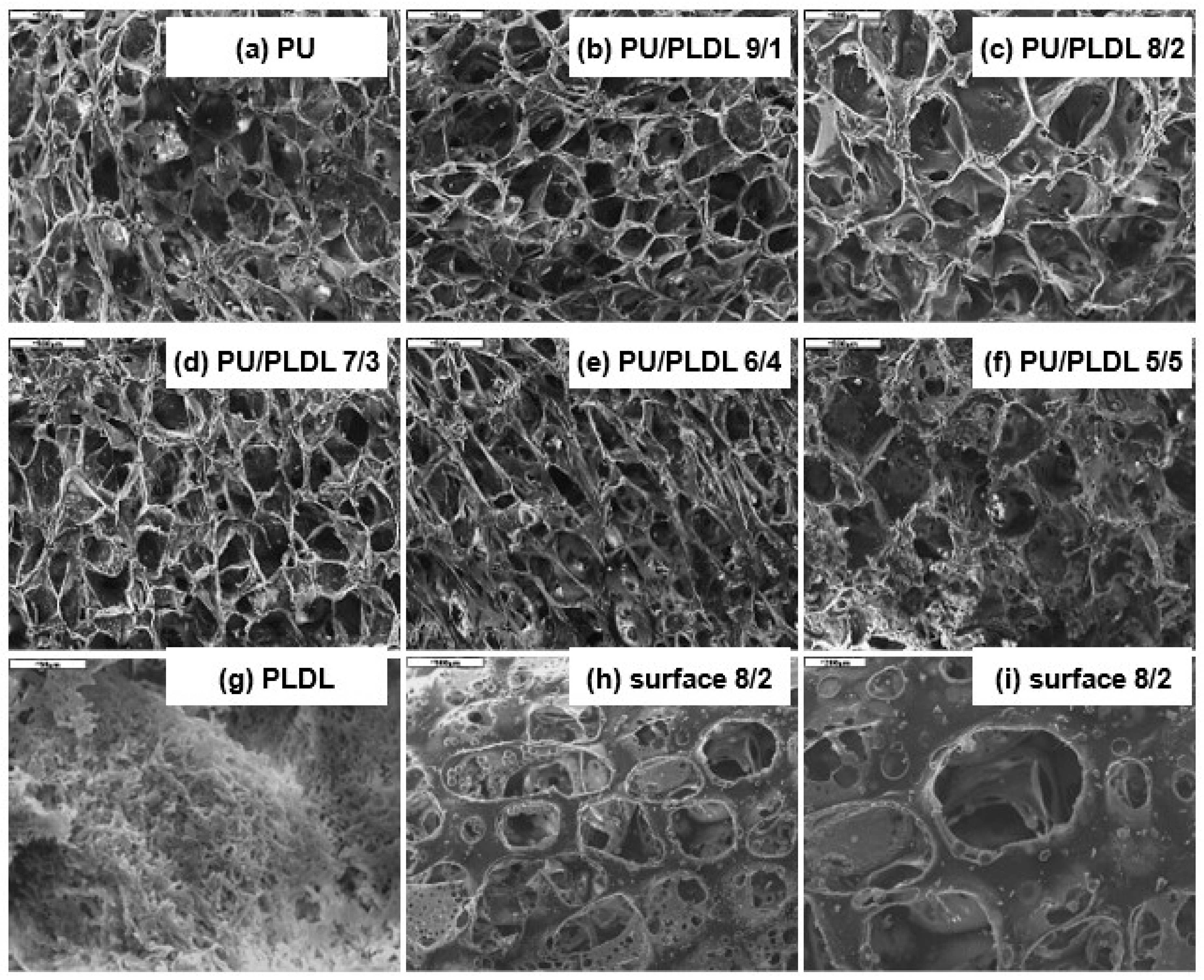
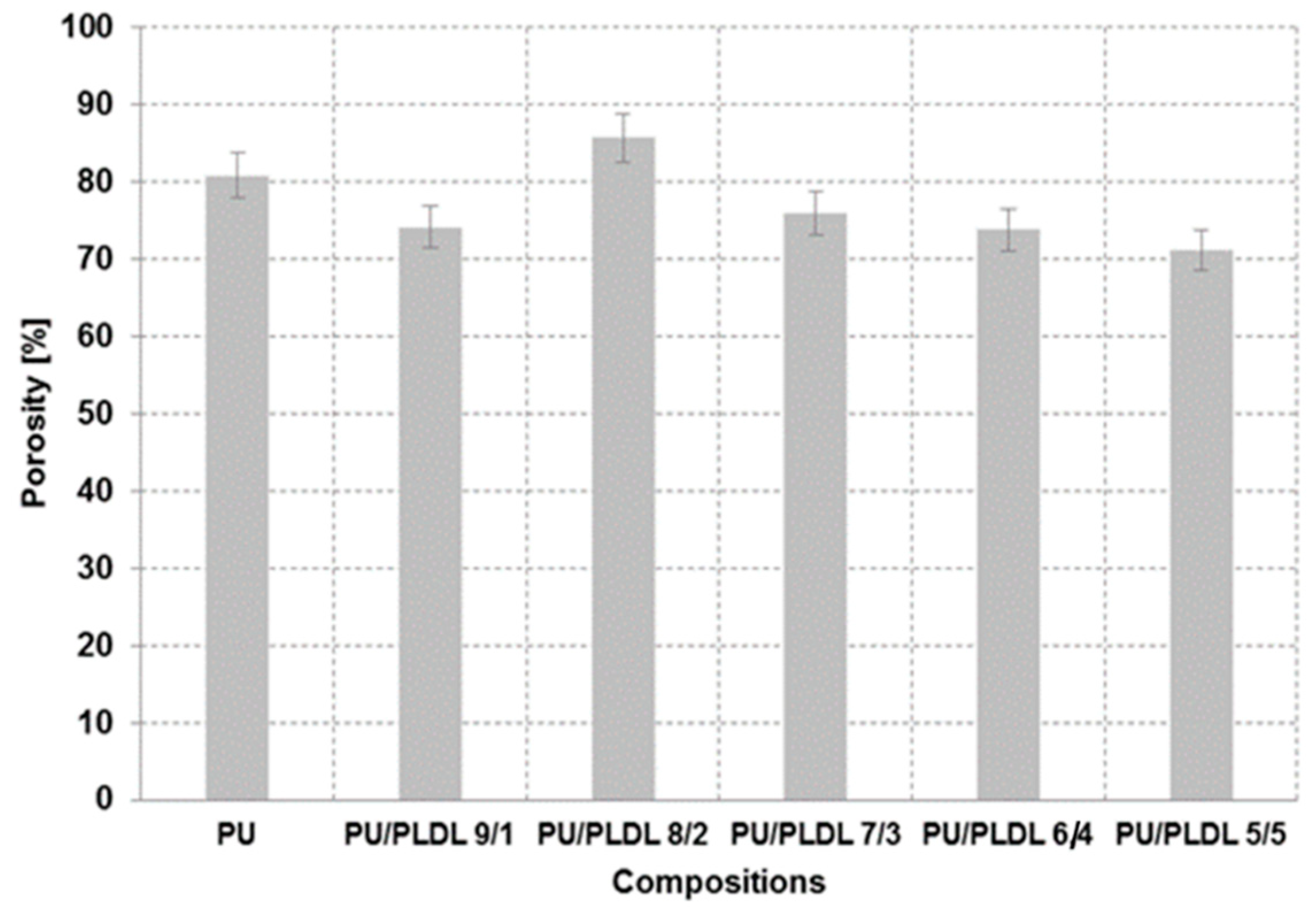
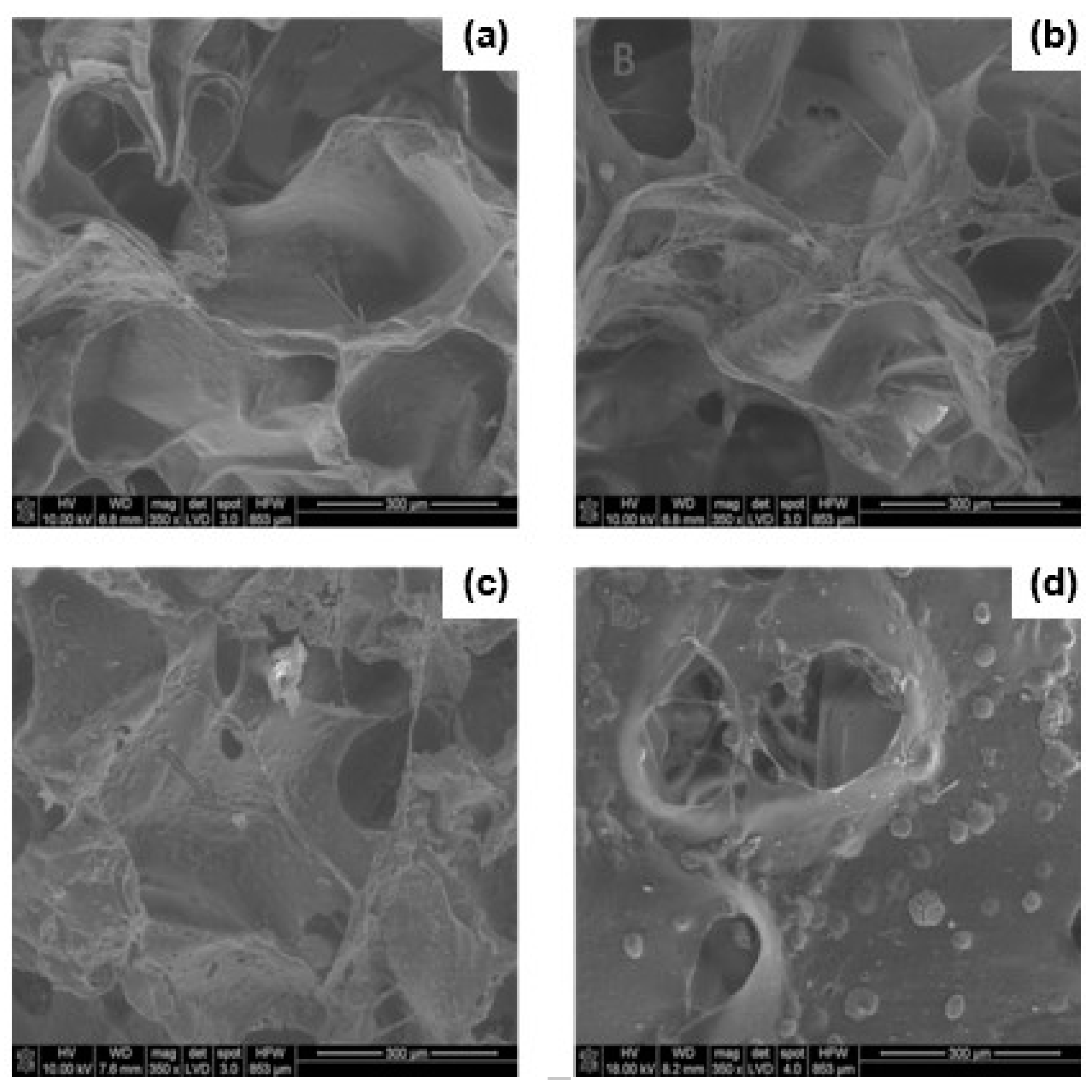
3.1. SEM Studies, Porosity
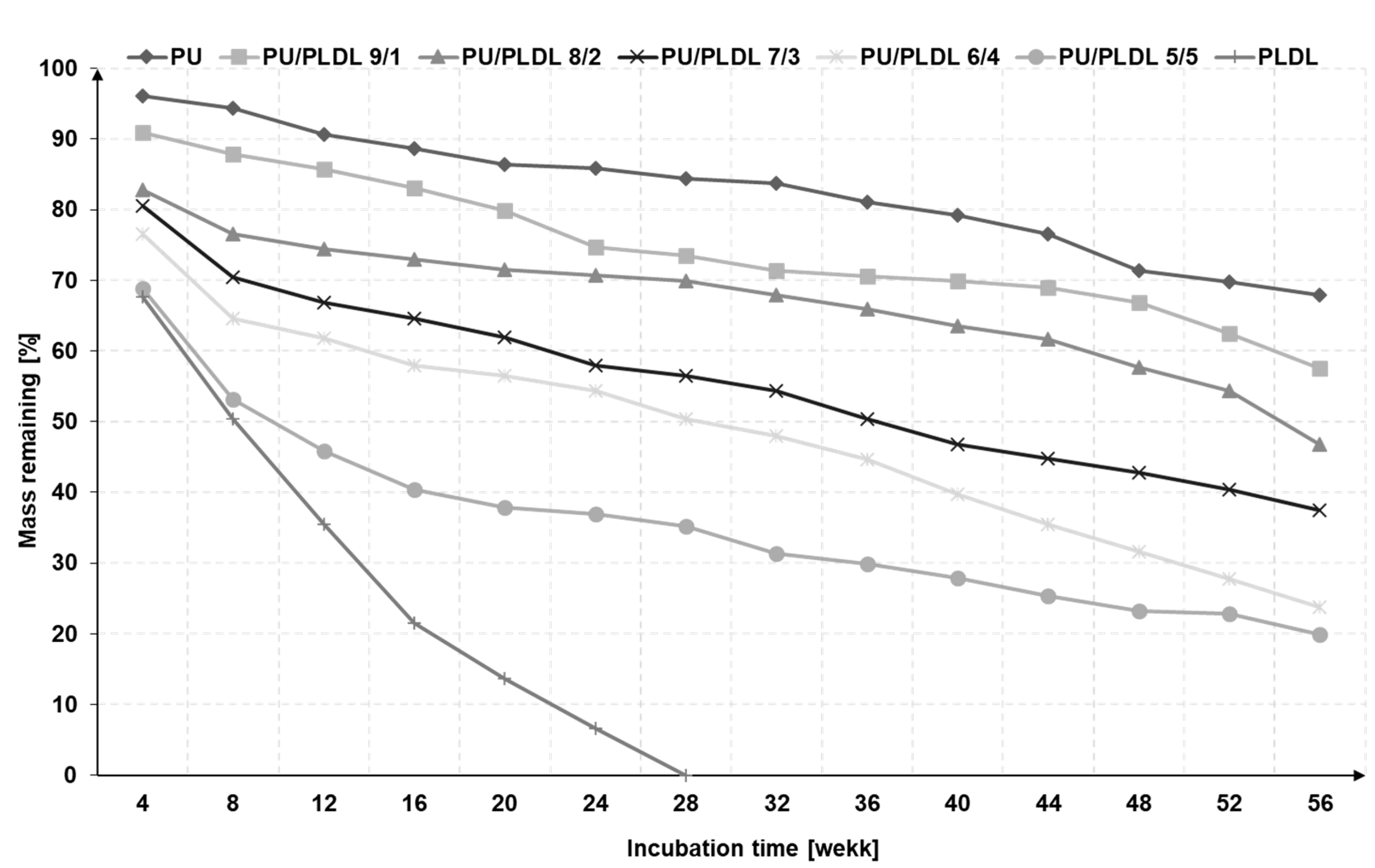
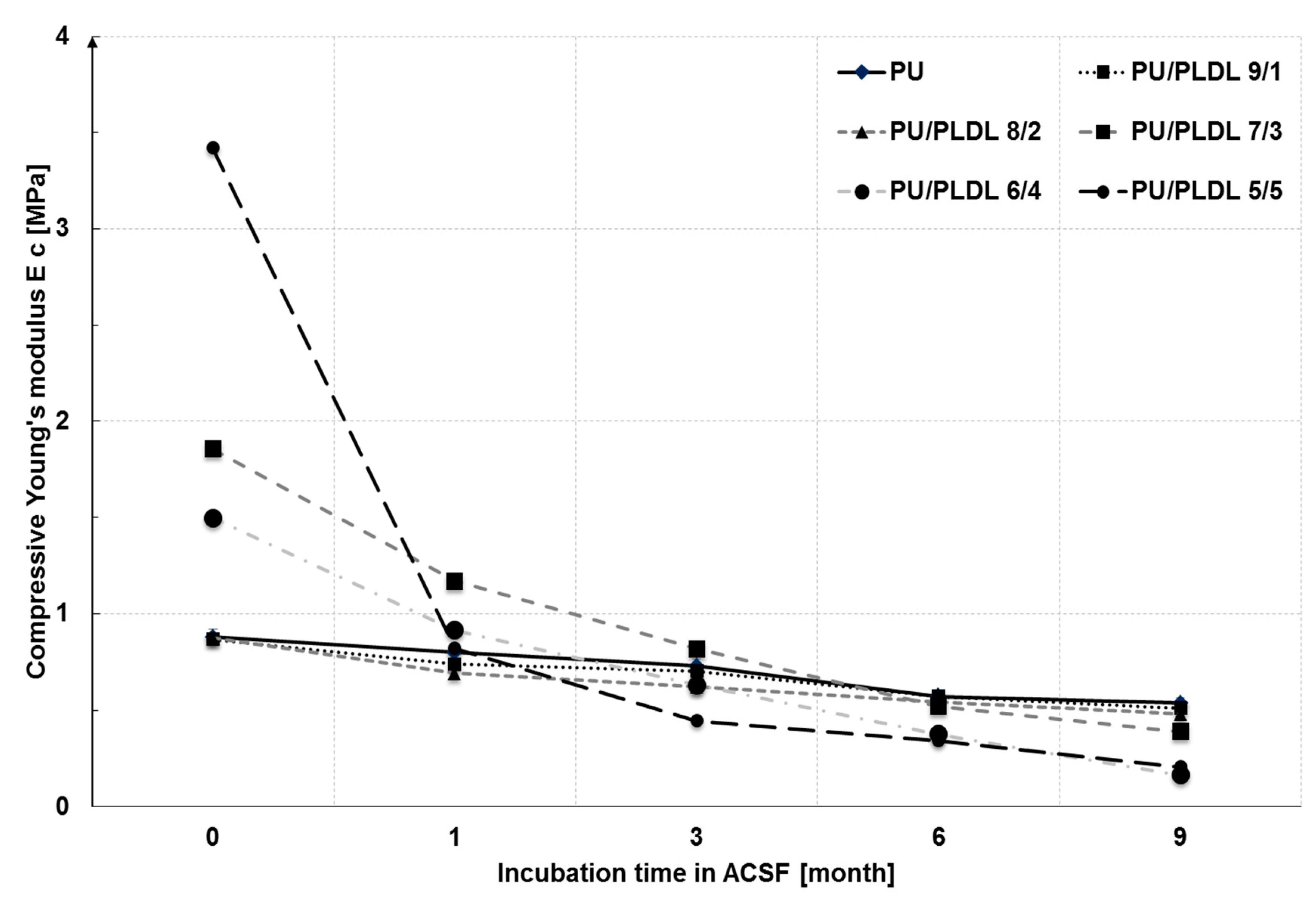
3.2. Degradability of the Sponges in the Artificial Cerebrospinal Fluid
3.3. Mechanical Properties
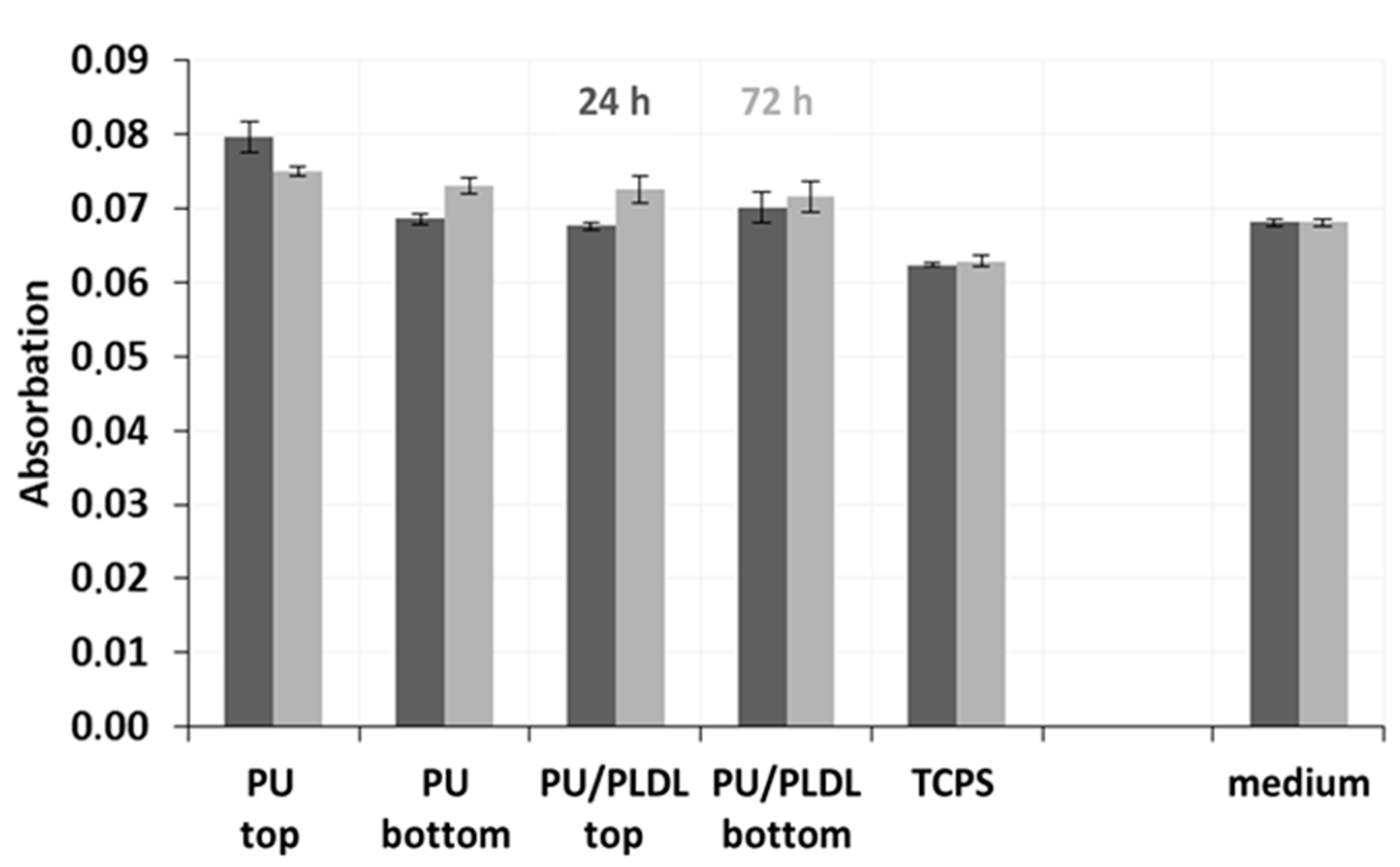
3.4. In Vitro Bioassay
3.5. Summary
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shafiee, A.; Ahmandi, H.; Taheri, B.; Hosseinzadeh, S.; Fatahi, Y.; Soleimani, M.; Atyabi, F.; Dinarvand, R. Appropropriate Scaffold Selection for CNS Tissue Engineering. Avicenna J. Med. Biotechnol. 2020, 12, 203–220. [Google Scholar] [PubMed]
- Amani, H.; Kazerooni, H.; Hassanpoor, H.; Akbarzadeh, A.; Pazoki-Toroudi, H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3524–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snigdha, S.; Thomas, S.; Radhakrishnan, E.K. Polymer based tissue engineering strategies for neural regeneration. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Szarek, D.; Marycz, K.; Lis, A.; Zawada, Z.; Tabakow, P.; Laska, J.; Jarmundowicz, W. Lizard tail spinal cord: A new experimental model of spinal cord injury without limb paralysis. FASEB J. 2016, 30, 1391–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamudzandu, M.; Roach, P.; Fricker, R.A.; Yang, Y. Nanofibrous scaffolds supporting optimal central nervous system regeneration: An evidence-based review. J. Neurorestoratol. 2015, 3, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Assuncao-Silva, R.C.; Gomes, E.D.; Sousa, N.; Silva, N.A.; Salgado, A.J. Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration. Stem Cell Int. 2015, 24, 948040. [Google Scholar] [CrossRef]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M. Regenerative Therapies for Central Nervous System Diseases: A Biomaterials Approach. Neuropsychopharmacol. Rev. 2014, 39, 169–188. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Prabhakaran, M.P.; Ramakrishna, S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen. Biomater. 2015, 2, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Lis, A.; Szarek, D.; Laska, J. Biomaterials Engineering Strategies for Spinal Cord Regeneration: State of the Art. Polim. Med. 2013, 43, 59–80. [Google Scholar]
- Buchet, L.; Lonjon, N.; Perrin, F.-E.; Gilbert, C.; Privat, A.; Fattal, C. Strategies for spinal cord repair after injury: A review of the literature and information. Ann. Phys. Rehabil. Med. 2009, 52, 330–351. [Google Scholar] [CrossRef] [PubMed]
- Koechling, T.; Khalique, H.; Sundstrom, E.; Avila, J.; Lim, F. A culture model for regeneration of human spinal cord neurons. J. Neurosci. Methods 2011, 201, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Priestley, J.V.; Ramer, M.S.; King, V.R.; McMahon, S.B.; Brown, R.A. Stimulating regeneration in the damaged spinal cord. J. Physiol. Paris 2002, 96, 123–133. [Google Scholar] [CrossRef]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Cell therapy for spinal cord regeneration. Adv. Drug Deliv. Rev. 2008, 60, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Madigan, N.N.; McMahon, S.; O’Brien, T.; Yaszemski, M.J.; Windebank, A.J. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir. Physiol. Neurobiol. 2009, 169, 183–199. [Google Scholar] [CrossRef] [Green Version]
- Tabesh, H.; Amoabediny, G.; Salehi Nik, N.; Heydari, M.; Tosefirard, M. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem. Int. 2009, 54, 73–83. [Google Scholar] [CrossRef]
- Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna, W.; Miedzybrodzki, R.; Czyz, M.; Huber, J.; Szarek, D.; Okurowski, S.; Szewczyk, P.; et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transpl. 2013, 22, 1591–1612. [Google Scholar] [CrossRef]
- Keyvan-Foulandi, N.; Raisman, G.; Li, Y. Functional Repair of the Corticospinal Tract by Delayed Transplantation of Olfcatory Ensheathing Cells in Adult Rats. J. Neurosci. 2003, 23, 9428–9434. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Raisman, G.; Li, D.; Li, Y. Transplanted olfactory mucosal cells restore paw reaching function without regeneration of severed corticospinal tract fibers across the lesion. Brain Res. 2009, 1303, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Straley, K.S.; Wong Po Foo, C.h.; Heilshor, S.C. Biomaterial Design Strategies for the Treatment of Spinal Cord Injuries. J. Neurotrauma 2010, 27, 1–19. [Google Scholar] [CrossRef]
- McBane, J.E.; Cai, K.; Labow, R.S.; Santerre, J.P. Co-culturing monocytes with smooth muscle cells improves cell distribution within a degradable polyurethane scaffold and reduces inflammatory cytokines. Acta Biomater. 2012, 8, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.P.; McCandless, S.P.; Lasher RAHitchcock, R.W. The mechanically enhanced phase separation of sprayed polyurethane scaffolds and their effect on the alignment of fibroblasts. Biomaterials 2010, 31, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric Biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, I.-K.; Hoshiba, T.; Jiang, H.-L.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Design of artificial extracellular matrices for tissue engineering. Prog. Polym. Sci. 2011, 36, 238–368. [Google Scholar] [CrossRef]
- Li, B.; Davidson, J.M.; Guelcher, S.A. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials 2009, 30, 3486–3494. [Google Scholar] [CrossRef] [Green Version]
- Sharifpoor, S.; Simmons, C.A.; Labow, R.S.; Santeree, J.P. A study of vascular smooth muscle cell function under cyclic mechanical loading in a polyurethane scaffold with optimized porosity. Acta Biomater. 2010, 6, 4218–4228. [Google Scholar] [CrossRef]
- Spaans, C.J.; Belgraver, V.W.; Rienstra, O.; de Groot, J.H.; Veth, R.P.H.; Pennings, A.J. Solvent-free fabrication of micro-porous polyurethane amide and polyurethane-urea scaffolds for repair and replacement of the knee-joint meniscus. Biomaterials 2000, 21, 2453–2460. [Google Scholar] [CrossRef]
- Guan, J.; Fujimoto, K.L.; Sacks, S.M.; Wagner, W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, A.S.; Lim, S.A.; Martin, D.; Cooper-White, J.J. Polyurethane/poly(lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef]
- Attia, M.; Santerre, J.P.; Kandel, R.A. The response of annulus fibrous cell to fibronectin-coated nanofibrous polyurethane-anionic dihydroxyligomer scaffolds. Biomaterials 2011, 32, 450–460. [Google Scholar] [CrossRef]
- Szarek, D.; Jarmundowicz, W.; Fraczek, A.; Błażewicz, S. Biomaterials in the treatment of peripheral nerve injuries–on overview of methods and materials. Eng. Biomater. 2006, 9, 40–53. [Google Scholar]
- Novikov, L.N.; Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials 2002, 23, 3369–3376. [Google Scholar] [CrossRef]
- Novikova, L.N.; Pettersson, J.; Brohlin, M.; Wiberg, M.; Novikov, L.N. Biodegrdable poly-β-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials 2008, 29, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Moon, L.D.F.; Maquet, V.; Blits, B.; Jerome, R.; Oudega, M. Poly(D,L-lactic acid) macroporous guidance scaffolds seeded with Schwann cells genetically modified to secrete a bi-functional neurotrophin implanted in the completely transected adult rat thoracic spinal cord. Biomaterials 2006, 27, 430–442. [Google Scholar] [CrossRef] [Green Version]
- Maquet, V.; Martin, D.; Scholtes, F.; Franzen, R.; Schoenen, J.; Moonen, G.; Jerome, R. Poly (D, L-lactide) foams modified by poly(ethylene oxide)–block–poly(D, L-lactide) copolymers and a-FGF: In vitro and in vivo evaluation for spinal cord regeneration. Biomaterials 2001, 22, 1137–1146. [Google Scholar] [CrossRef]
- Blacher, S.; Maquet, V.; Schils, F.; Martin, D.; Schoenen, J.; Moonen, G.; Jerome, R.; Pirard, J.-P. Image analysis of the axonal ingrowth into poly(D. L-lactide) porous scaffolds in relation to the 3-D porous structure. Biomaterials 2003, 24, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Patist, C.M.; Borgerhoff Mulder, M.; Gautier, S.E.; Maquet, V.; Jerome, R.; Oudega, M. Freeze-dried poly(D, L–lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials 2004, 25, 1569–1582. [Google Scholar] [CrossRef] [Green Version]
- Oudega, M.; Gautier, S.E.; Chapon, P.; Fragoso, M.; Bates, M.L.; Parel, J.-M.; Bartlett Bunge, M. Axonal regeneration into Schwann cell grafts within resorbable poly(α-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials 2001, 22, 1125–1136. [Google Scholar] [CrossRef]
- Grzesiak, J.; Fryczkowski, R.; Lis, A.; Szarek, D.; Laska, J.; Marycz, K. Characterization of Olfactory Ensheating Glial Cell Cultured on Polyurethane/Polylactide Electrospun Nonwovens. Int. J. Polym. Sci. 2015, 10, 908328. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.T.; Shoichet, M.S. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials 2005, 26, 1507–1514. [Google Scholar] [CrossRef]
- Karchin, A.; Simonovsky, F.I.; Ratner, B.D.; Sanders, J.E. Melt electrospinning of biodegradable polyurethane scaffolds. Acta Biomater. 2011, 7, 3277–3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeganegi, M.; Kandel, R.A.; Santree, J.P. Characterization of a biodegradable electrospun polyurethane nanofiber scaffold: Mechanical properties and cytotoxicity. Acta Biomater. 2010, 6, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Marędziak, M.; Grzesiak, J.; Fryczkowski, R.; Laska, J. Polyurethane/polylactide-based electrospun nonwovens as carriers for human adipose-derived stromal stem cells and chondogenic progenitor cells. Polym. Plast. Technol. Eng. 2016, 55, 1897–1907. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly(lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegrdable polymers–Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Szarek, D.; Laska, J.; Jarmundowicz, W.; Blazewicz, S.; Tabakow, P.; Marycz, K.; Wozniak, Z.; Mierzwa, J. Influence of Alginates on Tubes Nerve Grafts of Different Elasticity–Preliminary in Vivo Study. J. Biomater Nanobiotechnol. 2012, 3, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Szarek, D.; Marycz, K.; Bednarz, P.; Tabakow, P.; Jarmundowicz, W.; Laska, J. Influence of calcium alginate on peripheral nerve regeneration: In Vivo Study. Biotechnol. Appl. Biochem. 2013, 60, 547–556. [Google Scholar] [CrossRef]
- Grzesiak, J.; Marycz, K.; Szarek, D.; Bednarz, P.; Laska, J. Polyurethane/polylactide-based biomaterials combined with rat olfactory bulb-derived glial cells and adipose-derived mesenchymal stromal cells for neural regenerative medicine applications. Mater. Sci. Eng. C 2015, 52, 163–170. [Google Scholar] [CrossRef]
- Marycz, K.; Marędziak, M.; Grzesiak, J.; Szarek, D.; Lis, A.; Laska, J. Polyurethane/polylactide-blend films doped with zinc ions for the growth and expansion of human olfactory ensheathing cells (OECs) and adipose-derived mesenchymal stromal stem cells (ASCs) for regenerative medicine applications. Polymers 2016, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Bednarz, P.; Bernasik, A.; Laska, J. The influence of sterilization on properties of polyurethane/polylactide blend. Sci. Technol. Innov. 2018, 2, 13–18. [Google Scholar] [CrossRef] [Green Version]
- White, A.A.; Panjabi, M.M. Chapter 1: Physical properties and functional biomechanics of the spine. In Clinical Biomechanics of the Spine, 2nd ed.; JB Lippincott: Philadelphia, PA, USA, 1990. [Google Scholar]
- Mazuchowski, E.L.; Thibault, L.E. Biomechanical Properties of the Human Spinal Cord and Pia Matter. In Proceedings of the Summer Bioengineering Conference, Key Biscayne, FL, USA, 25–29 June 2003; Available online: http://www.tulane.edu/~sbc2003/pdfdocs/1205.PDF (accessed on 16 November 2019).
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 28, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cereb. Fluid Res. 2010, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johanson, C.E.; Duncan, J.A., III; Klinge, P.M.; Brinker, T.; Stopa, E.G.; Silverberg, G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and diseases. Cereb. Fluid Res. 2008, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Saunders, N.R.; Habgood, M.D.; Dziegielewska, K.M. Barrier mechanisms in the brain. I. Adult brain. Clin. Exp. Pharmacol. Physiol. 1999, 26, 11–19. [Google Scholar] [CrossRef]
- Wujek, J.R.; Lasek, R.J. Correlation of axonal regeneration and slow component B in two branches of single axon. J. Neurosci. 1983, 3, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Stoll, G.; Muller, H.W. Nerve injury, axonal degeneration and neuronal regeneration: Basic insights. Brain Pathol. 1999, 9, 313–325. [Google Scholar] [CrossRef]
- Ducker, T.B.; Kempe, L.G.; Hayes, G.J. The metabolic background for peripheral nerve surgery. J. Neurosurg. 1969, 30, 270–280. [Google Scholar] [CrossRef]
- Hubbard, J.H. The quality of nerve regeneration: Factors independent of the most skillful repair. Surg. Clin. North Am. 1972, 52, 1099–1108. [Google Scholar] [CrossRef]
- Yiu, G.; Zhigang, H. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]

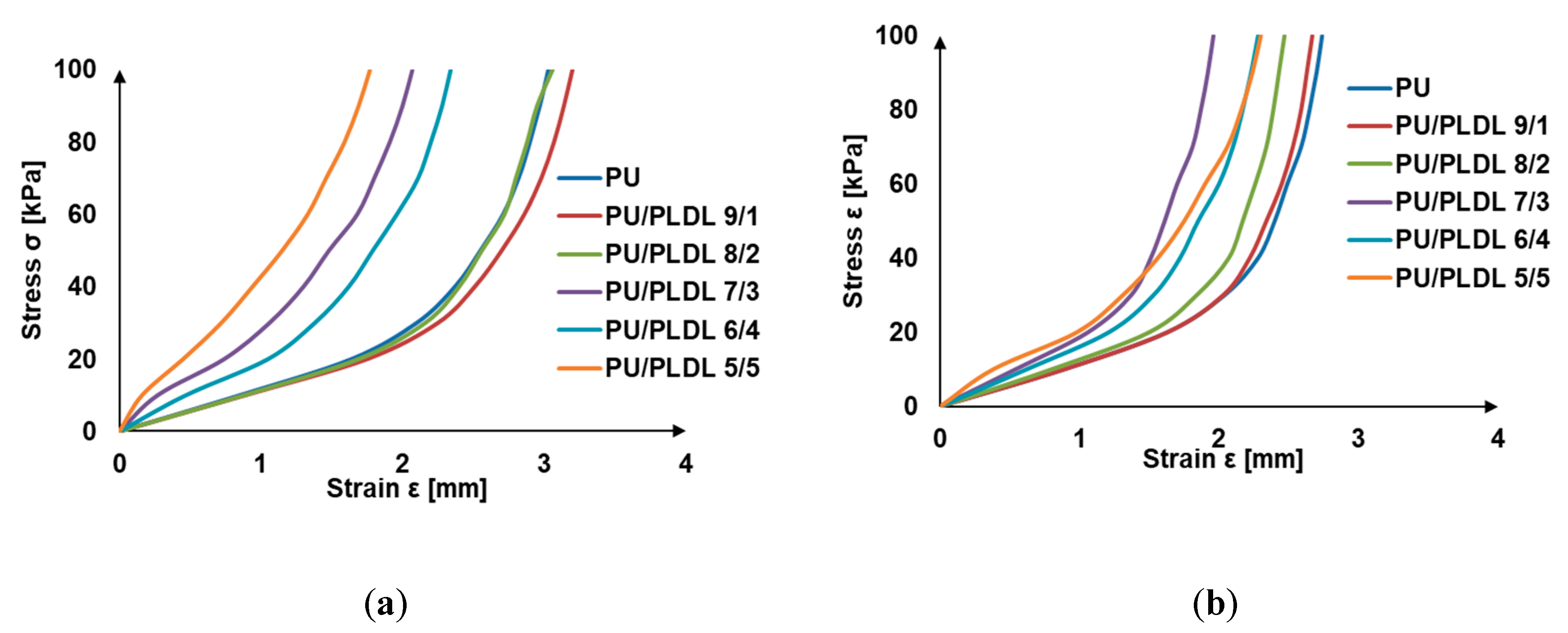


| Sponge Symbol | PU 100% | PU/PLDL 9/1 | PU/PLDL 8/2 | PU/PLDL 7/3 | PU/PLDL 6/4 | PU/PLDL 5/5 |
|---|---|---|---|---|---|---|
| Compressive strength RC0.1 [kPa] | 10.05 ± 0.35 | 10.61 ± 0.81 | 11.84 ± 0.76 | 18.20 ± 1.57 | 17.76 ± 2.21 | 26.93 ± 3.01 |
| Young’s moduli [MPa] | 0.88 ± 0.04 | 0.87 ± 0.05 | 0.85 ± 0.03 | 1.86 ± 0.41 | 1.50 ± 0.13 | 3.42 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis-Bartos, A.; Szarek, D.; Krok-Borkowicz, M.; Marycz, K.; Jarmundowicz, W.; Laska, J. Microstructure and Mechanical Properties of PU/PLDL Sponges Intended for Grafting Injured Spinal Cord. Polymers 2020, 12, 2693. https://doi.org/10.3390/polym12112693
Lis-Bartos A, Szarek D, Krok-Borkowicz M, Marycz K, Jarmundowicz W, Laska J. Microstructure and Mechanical Properties of PU/PLDL Sponges Intended for Grafting Injured Spinal Cord. Polymers. 2020; 12(11):2693. https://doi.org/10.3390/polym12112693
Chicago/Turabian StyleLis-Bartos, Anna, Dariusz Szarek, Małgorzata Krok-Borkowicz, Krzysztof Marycz, Włodzimierz Jarmundowicz, and Jadwiga Laska. 2020. "Microstructure and Mechanical Properties of PU/PLDL Sponges Intended for Grafting Injured Spinal Cord" Polymers 12, no. 11: 2693. https://doi.org/10.3390/polym12112693
APA StyleLis-Bartos, A., Szarek, D., Krok-Borkowicz, M., Marycz, K., Jarmundowicz, W., & Laska, J. (2020). Microstructure and Mechanical Properties of PU/PLDL Sponges Intended for Grafting Injured Spinal Cord. Polymers, 12(11), 2693. https://doi.org/10.3390/polym12112693






