Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
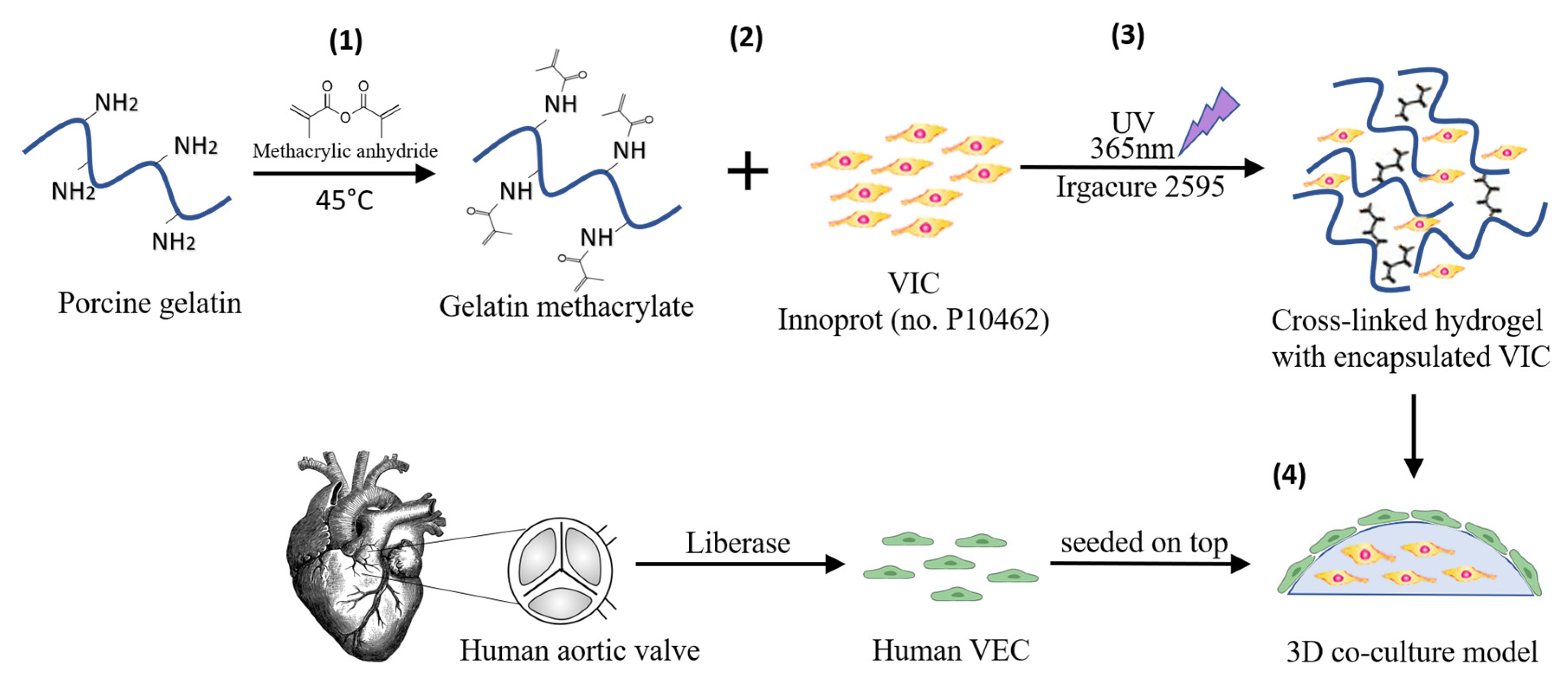
2.2. Three-Dimensional Cell Culture Model
2.3. Gene Expression Analysis by Real-Time Polymerase Chain Reaction (RT-PCR)
2.4. Western Blotting
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Immunofluorescence
3. Results
3.1. High Glucose (HG) Increases MCP-1, TNF-alpha, IL-1β and IL-8 Expression in Valvular Endothelial Cells (VEC) and Valvular Interstitial Cells (VIC) in the 3D Valve Model Based on Gelatin Methacrylate
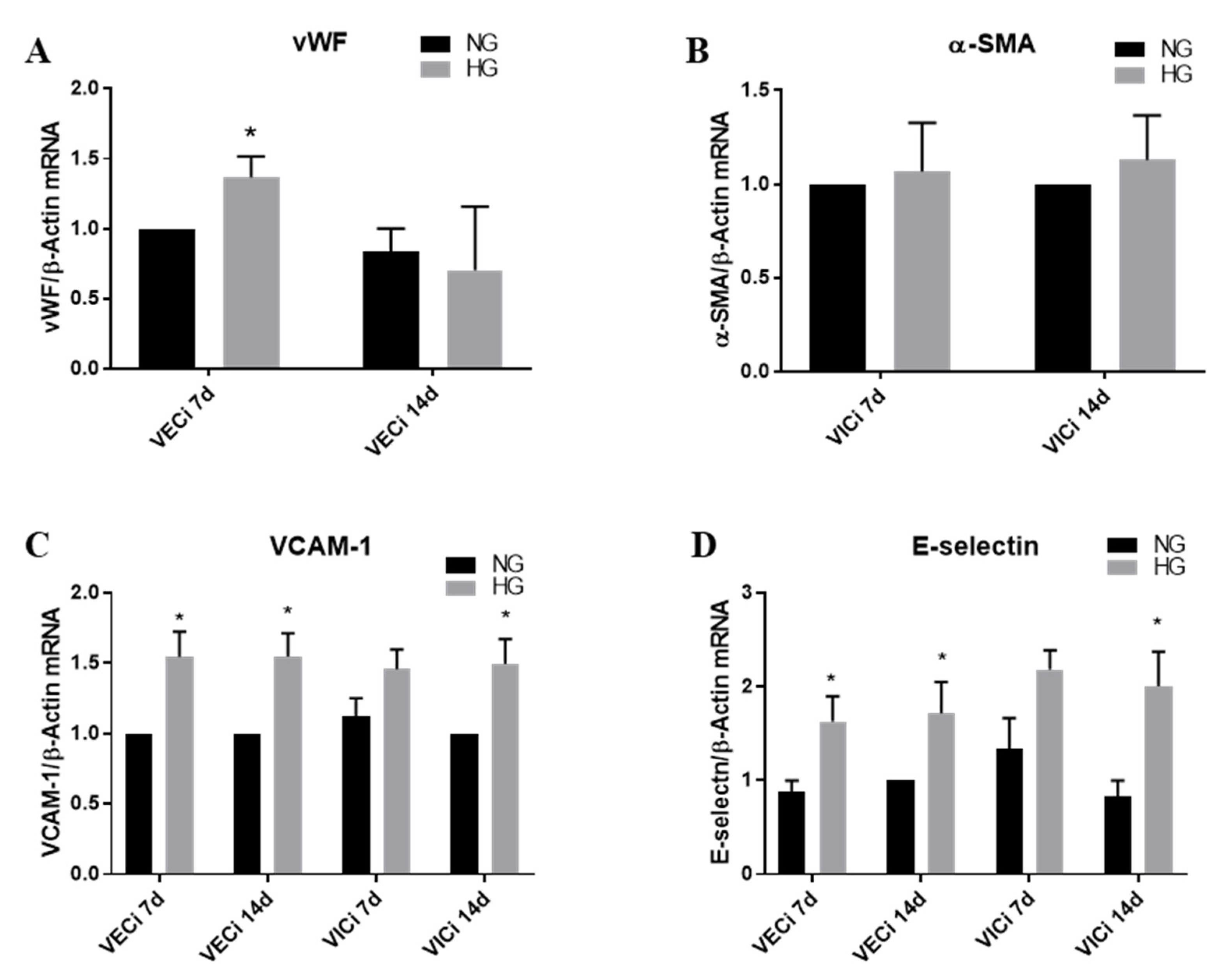
3.2. High Glucose (HG) Increases Von Willebrand Factor (vWF) Expression in VEC and Cell Adhesion Molecules VCAM-1 and E-Selectin in both VEC and VIC
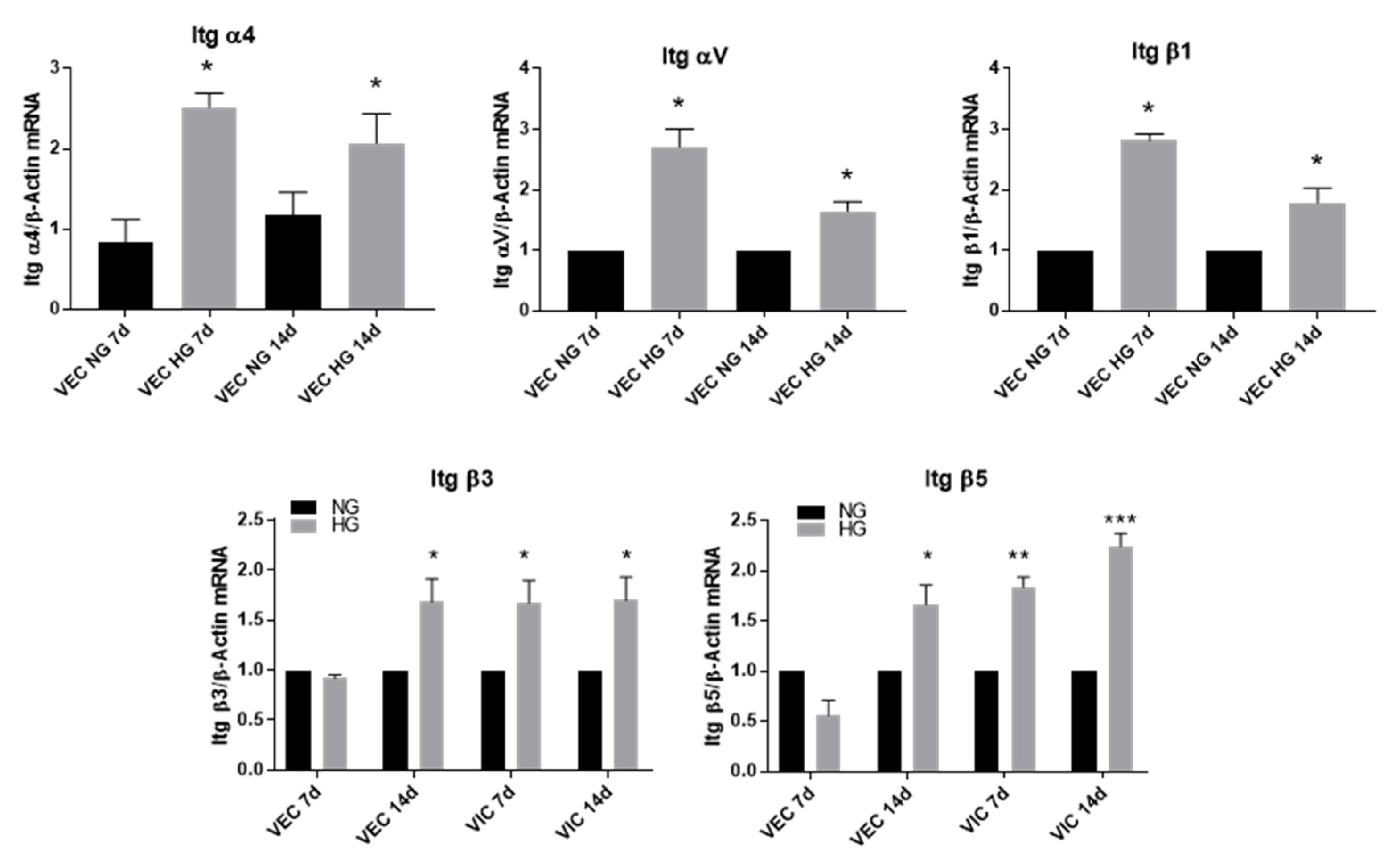
3.3. High Glucose Increases α4, αV and β1 Integrin Chains Gene Expression in VEC and β3 and β5 in VIC
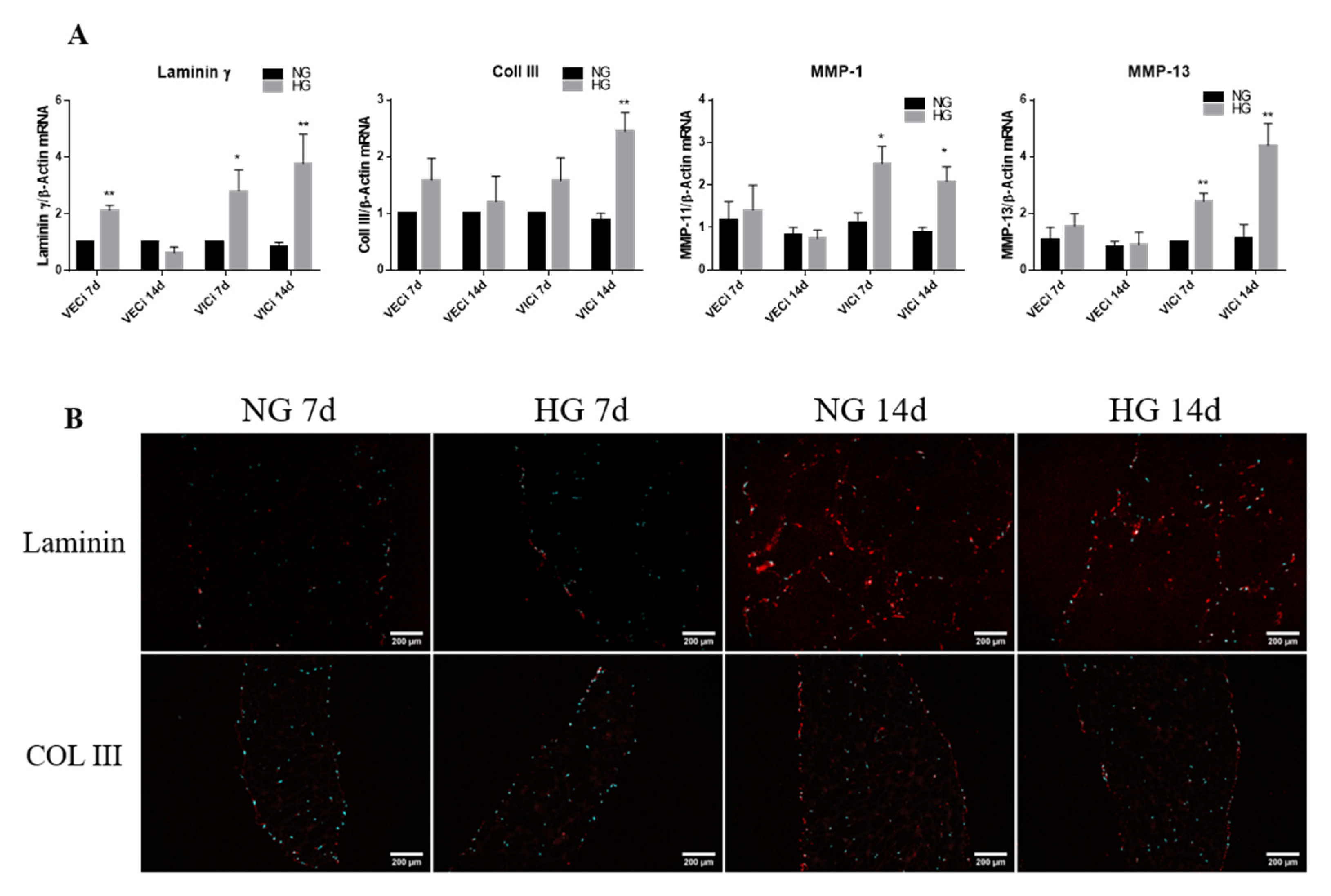
3.4. Exposure of 3D Constructs with Valvular Cells to High Glucose Induces Changes in the Gene Expression of Extracellular Matrix Proteins and Metalloproteases
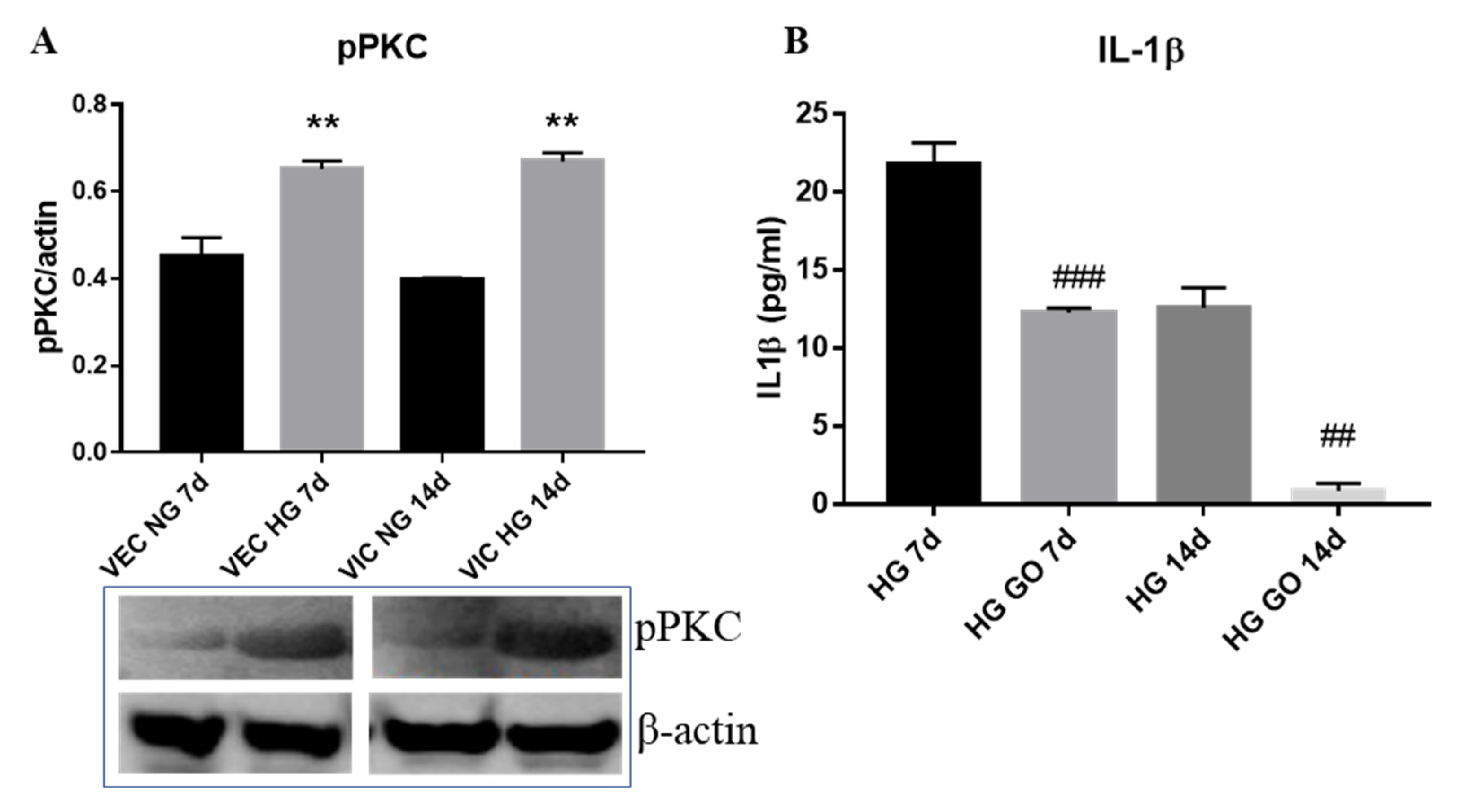
3.5. High Glucose Activates PKC Signaling Pathway in Valvular Cells
4. Discussion
- the gene expression of cytokines (MCP-1, TNF-α, IL-8 and IL-1β), of cell adhesion molecules (VCAM-1 and E-selectin) and of integrins α4, αV, β1, β3 and β5 is significantly increased by HG in VEC after 7 days of culture; moreover, the levels of MCP-1 and IL-1β secreted in the conditioned media by valvular cells from the 3D construct are significantly increased when the constructs are exposed 14 days to HG concentrations, compared to normal glucose conditions;
- the gene expression of cytokines (MCP-1, TNF-α, IL-8 and IL-1β), of cell adhesion molecules (VCAM-1 and E-selectin), of integrins β3 and β5 and of remodeling molecules (collagen III, laminin, MMP-1 and MMP-13) is significantly induced by HG in VIC mainly after 14 days of culture;
- the phosphorylated form of PKC-α is elevated in both, VEC and VIC from 3D constructs exposed to HG and is involved in the production of IL-1β by valvular cells.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natorska, J.; Wypasek, E.; Grudzień, G.; Sobczyk, D.; Marek, G.; Filip, G.; Sadowski, J.; Undas, A. Does Diabetes Accelerate the Progression of Aortic Stenosis through Enhanced Inflammatory Response within Aortic valves? Inflammation 2012, 35, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Banovic, M.; Athithan, L.; McCann, G.P. Aortic stenosis and diabetes mellitus: An ominous combination. Diabetes Vasc. Dis. Res. 2019, 16, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Kamalesh, M.; Ng, C.; El Masry, H.; Eckert, G.; Sawada, S. Does diabetes accelerate progression of calcific aortic stenosis? Eur. J. Echocardiogr. 2009, 10, 723–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The British Diabetic Association. Diabetes in the UK 2013: Key Statistics on Diabetes. Available online: https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-in-the-uk-2013-key-statistics-on-diabetes (accessed on 24 November 2020).
- Larsson, S.C.; Wallin, A.; Håkansson, N.; Stackelberg, O.; Bäck, M.; Wolk, A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int. J. Cardiol. 2018, 262, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific Aortic Stenosis: A Disease of the Valve and the Myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, P.; Bouchareb, R.; Boulanger, M.-C. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J. Immunol. Res. 2015, 2015, 851945. [Google Scholar] [CrossRef]
- Coté, N.; Mahmut, A.; Bosse, Y.; Couture, C.; Pagé, S.; Trahan, S.; Boulanger, M.C.; Fournier, D.; Pibarot, P.; Mathieu, P. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 2013, 36, 573–581. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef] [Green Version]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reid, J.; Northridge, D.B.; Boon, N.A. Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005, 352, 2389–2397. [Google Scholar] [CrossRef]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J. Effect of Lipid Lowering with Rosuvastatin on Progression of Aortic Stenosis. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simionescu, N.; Vasile, E.; Lupu, F.; Popescu, G.; Simionescu, M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am. J. Pathol. 1986, 123, 109–125. [Google Scholar] [PubMed]
- Tucureanu, M.M.; Filippi, A.; Alexandru, N.; Ana Constantinescu, C.; Ciortan, L.; Macarie, R.; Vadana, M.; Voicu, G.; Frunza, S.; Nistor, D.; et al. Diabetes-induced early molecular and functional changes in aortic heart valves in a murine model of atherosclerosis. Diabetes Vasc. Dis. Res. 2019, 16, 562–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selig, J.I.; Ouwens, D.M.; Raschke, S.; Thoresen, G.H.; Fischer, J.W.; Lichtenberg, A.; Akhyari, P.; Barth, M. Impact of hyperinsulinemia and hyperglycemia on valvular interstitial cells—A link between aortic heart valve degeneration and type 2 diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2526–2537. [Google Scholar] [CrossRef]
- Wang, H.; Tibbitt, M.W.; Langer, S.J.; Leinwand, L.A.; Anseth, K.S. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated PI3K/AKT pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 19336–19341. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.A.; Hua, X.; Mishra, K.; Murphy, G.A.; Rosenkranz, A.C.; Horowitz, J.D. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur. J. Pharmacol. 2009, 602, 28–35. [Google Scholar] [CrossRef]
- Richards, J.; El-Hamamsy, I.; Chen, S.; Sarang, Z.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H.; Butcher, J.T. Side-specific endothelial-dependent regulation of aortic valve calcification: Interplay of hemodynamics and nitric oxide signaling. Am. J. Pathol. 2013, 182, 1922–1931. [Google Scholar] [CrossRef] [Green Version]
- Vadana, M.; Cecoltan, S.; Ciortan, L.; Macarie, R.D.; Tucureanu, M.M.; Mihaila, A.C.; Droc, I.; Butoi, E.; Manduteanu, I. Molecular mechanisms involved in high glucose-induced valve calcification in a 3D valve model with human valvular cells. J. Cell. Mol. Med. 2020, 24, 6350–6361. [Google Scholar] [CrossRef]
- Garcia, C.; Feve, B.; Ferré, P.; Halimi, S.; Baizri, H.; Bordier, L.; Guiu, G.; Dupuy, O.; Bauduceau, B.; Mayaudon, H. Diabetes and inflammation: Fundamental aspects and clinical implications. Diabetes Metab. 2010, 36, 327–338. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, B.; Puperi, D.S.; Wu, Y.; West, J.L.; Grande-Allen, K.J. Application of hydrogels in heart valve tissue engineering. J. Long Term Eff. Med. Implants 2015, 25, 105–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voicu, G.; Rebleanu, D.; Constantinescu, C.A.; Fuior, E.V.; Ciortan, L.; Droc, I.; Uritu, C.M.; Pinteala, M.; Manduteanu, I.; Simionescu, M.; et al. Nano-Polyplexes Mediated Transfection of Runx2-shRNA Mitigates the Osteodifferentiation of Human Valvular Interstitial Cells. Pharmaceutics 2020, 12, 507. [Google Scholar] [CrossRef]
- Alushi, B.; Curini, L.; Christopher, M.R.; Grubitzch, H.; Landmesser, U.; Amedei, A.; Lauten, A. Calcific Aortic Valve Disease-Natural History and Future Therapeutic Strategies. Front. Pharmacol. 2020, 11, 685. [Google Scholar] [CrossRef]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef] [Green Version]
- Waheed, H.J.; Khalil, M.; Fawzi, S. Evaluation of Monocyte Chemoattractant Protein-1 (MCP-1) in Type 2 Diabetes Mellitus. Int. J. Sci. Eng. Res. 2015, 6, 791–797. [Google Scholar]
- Kapelouzou, A.; Tsourelis, L.; Kaklamanis, L.; Degiannis, D.; Kogerakis, N.; Cokkinos, D.V. Serum and tissue biomarkers in aortic stenosis. Glob. Cardiol. Sci. Pract. 2015, 2015, 49. [Google Scholar] [CrossRef]
- Manduteanu, I.; Voinea, M.; Serban, G.; Simionescu, M. High glucose induces enhanced monocyte adhesion to valvular endothelial cells via a mechanism involving ICAM-1, VCAM-1 and CD18. Endothelium 1999, 6, 315–324. [Google Scholar] [CrossRef]
- Roth, T.; Podestá, F.; Stepp, M.A.; Boeri, D.; Lorenzi, M. Integrin overexpression induced by high glucose and by human diabetes: Potential pathway to cell dysfunction in diabetic microangiopathy. Proc. Natl. Acad. Sci. USA 1993, 90, 9640–9644. [Google Scholar] [CrossRef] [Green Version]
- Latif, N.; Sarathchandra, P.; Taylor, P.M.; Antoniw, J.; Yacoub, M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005, 14, 218–227. [Google Scholar] [PubMed]
- Butcher, J.T.; Nerem, R.M. Porcine aortic valve interstitial cells in three-dimensional culture: Comparison of phenotype with aortic smooth muscle cells. J. Heart Valve Dis. 2004, 13, 478–486. [Google Scholar] [PubMed]
- Gu, X.; Masters, K.S. Regulation of valvular interstitial cell calcification by adhesive peptide sequences. J. Biomed. Mater. Res. Part A 2010, 93, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, W.S.; Vesey, A.T.; Stirrat, C.; Connell, M.; Lucatelli, C.; Neale, A.; Moles, C.; Vickers, A.; Fletcher, A.; Pawade, T.; et al. Cardiac α(V)β(3) integrin expression following acute myocardial infarction in humans. Heart 2017, 103, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossé, Y.; Miqdad, A.; Fournier, D.; Pépin, A.; Pibarot, P.; Mathieu, P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ. Cardiovasc. Genet. 2009, 2, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, J.S.; Jones, J.A.; Barbour, J.R.; Stroud, R.E.; Clark, L.L.; Kaplan, B.S.; Zeeshan, A.; Bavaria, J.E.; Gorman, J.H., 3rd; Spinale, F.G.; et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J. Thorac. Cardiovasc. Surg. 2007, 133, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, O.; Vieth, M.; Naumann, M. Protein kinase C isozymes regulate matrix metalloproteinase-1 expression and cell invasion in Helicobacter pylori infection. Gut 2013, 62, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zou, Y.; Liu, F. Transforming Growth Factor-Beta1 in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef] [Green Version]


| Gene | GenBank® Accession Number | Sequences of Oligonucleotide Primers | Predicted Size (bp) |
|---|---|---|---|
| vWF | NM_000552 | Fw: 5′-ccttgacctcggacccttatg-3′ Rv: 5′-gatgcccgttcacaccact-3′ | 76 |
| VCAM-1 | NM_080682 | Fw: 5′-gggaagatggtcgtgatcctt-3′ Rv: 5′-ttgggcatagagaccccgtt-3′ | 89 |
| TNF-α | NM_000594 | Fw: 5′-ttcctcagcctcttctccttcc-3′ Rv: 5′-tgatggcagagaggaggttgac-3′ | 427 |
| MCP-1 | NM_002982 | Fw: 5′-cagccagatgcaatcaatgcc-3′ Rv: 5′-tggaatcctgaacccacttct-3′ | 190 |
| α-SMA | NM_001141945.2 | Fw: 5′-actgccttggtgtgtgacaa-3′ Rv: 5′-caccatcaccccctgatgtc-3′ | 120 |
| IL-1β | NM_000576 | Fw: 5′-tggccctaaacagatgaagtgc-3′ Rv: 5′-tcaacacgcaggacaggtacag-3′ | 488 |
| IL-8 | NM_000584 | Fw: 5′-actgagagtgattgagagtggac-3′ Rv: 5′-aaccctctgcacccagttttc-3′ | 112 |
| E-selectin | NM_000450 | Fw: 5′-agagtggagcctggtcttaca-3′ Rv: 5′-cctttgctgacaataagcactgg-3′ | 77 |
| α4 | NM_000885 | Fw: 5′-tacagatgcaggatcggaaaga-3′ Rv: 5′-aggttctccattagggctacc-3′ | 75 |
| αV | NM_002210 | Fw: 5′-gctgtcggagatttcaatggt-3′ Rv: 5′-tctgctcgccagtaaaattgt-3′ | 136 |
| β1 | NM_002211 | Fw: 5′-gtaaccaaccgtagcaaagga-3′ Rv: 5′-tcccctgatcttaatcgcaaaac-3′ | 98 |
| β3 | NM_000212 | Fw: 5′-catgaaggatgatctgtggagc-3′ Rv: 5′-aatccgcaggttactggtgag-3′ | 85 |
| β5 | NM_002213.5 | Fw: 5′-ggaagttcggaaacagagggt-3′ Rv: 5′-ctttcgccagccaatcttctc-3′ | 106 |
| Coll III | NM_000090.3 | Fw: 5′-aggtcctgcgggtaacact-3′ Rv: 5′-actttcacccttgacaccctg-3′ | 226 |
| Laminin | NM_005559.4 | Fw: 5′-gtgatggcaacagcgcaaa-3′ Rv: 5′-gacccagtgatattctctccca-3′ | 116 |
| MMP-1 | NM_002421.3 | Fw:5′-aaaattacacgccagatttgcc-3′ Rv: 5′-ggtgtgacattactccagagttg-3′ | 82 |
| MMP-13 | NM_002427.3 | Fw: 5′-actgagaggctccgagaaatg-3′ Rv: 5′-gaaccccgcatcttggctt-3′ | 103 |
| β-actin | NM_001101.4 | Fw: 5′-catgtacgttgctatccaggc-3′ Rv: 5′-ctccttaatgtcacgcacgat-3′ | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciortan, L.; Macarie, R.D.; Cecoltan, S.; Vadana, M.; Tucureanu, M.M.; Mihaila, A.C.; Droc, I.; Butoi, E.; Manduteanu, I. Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve. Polymers 2020, 12, 2786. https://doi.org/10.3390/polym12122786
Ciortan L, Macarie RD, Cecoltan S, Vadana M, Tucureanu MM, Mihaila AC, Droc I, Butoi E, Manduteanu I. Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve. Polymers. 2020; 12(12):2786. https://doi.org/10.3390/polym12122786
Chicago/Turabian StyleCiortan, Letitia, Razvan Daniel Macarie, Sergiu Cecoltan, Mihaela Vadana, Monica Madalina Tucureanu, Andreea Cristina Mihaila, Ionel Droc, Elena Butoi, and Ileana Manduteanu. 2020. "Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve" Polymers 12, no. 12: 2786. https://doi.org/10.3390/polym12122786
APA StyleCiortan, L., Macarie, R. D., Cecoltan, S., Vadana, M., Tucureanu, M. M., Mihaila, A. C., Droc, I., Butoi, E., & Manduteanu, I. (2020). Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve. Polymers, 12(12), 2786. https://doi.org/10.3390/polym12122786





