A Review of the Use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles
Abstract
1. Introduction
2. Chemistries of Zinc-Based Batteries
3. The Polymer Matrix of the GPEs
- fast segmental motion of polymer chain.
- functional groups promoting the dissolution of salts.
- low glass transition temperature (Tg).
- high molecular weight.
- wide electrochemical window.
- high degradation temperature.
- Good Ionic Conductivity: In GPEs, the ionic conductivity is strongly related to the degree of crystallinity, porosity, and the ability of the polymer to uptake solvent in the matrix [113]. Since ionic conductivity is directly proportional to the concentration of charged species and their electrical mobility, as per σ = ni*qi*µi, it is expected that ionic conductivity to be lower in GPEs as the mobility of ions is higher in liquid electrolytes [114].
- High Cationic Transference Number (t+): Besides the ionic conductivity, the transference number of polymer electrolytes is an important figure of merit when assessing their efficacy. The cationic transference number is the cation mobility relative to the anion in single salts GPEs. In GPEs, working species need to have a transference number close to unity [114].
- Good Chemical and Thermal Stability. For a gel polymer electrolyte to be effective, it needs to maintain high ionic conductivity over a wide temperature range and remain structurally stable during manufacturing, cell assembly, storage, and usage [69]. Introducing inorganic particles such as TiO2, SiO2 or Al2O3 is an strategy to improve the thermal stability of GPEs [32,115,116]. Another strategy is to create a composite of several polymers so that could enhance the thermal stability of GPEs [117].
- Good Mechanical Properties. Since a GPE is “sandwiched” between the cathode and the anode, it should be mechanically stable and not undergo deformation or strain that could jeopardize the stability of the battery itself. At the same time, they must be able to withstand volume change; however, gel polymer electrolytes most of the time show poor mechanical properties due to solvent or plasticizer used trying to increase conductivity. Therefore, there must be a trade-off between ionic conductivity and mechanical endurance.
- Low cost. GPEs with excellent characteristic only would be wearable for commercial application if it can be produced at low cost. It makes necessary an easy fabrication process. We have to keep in mind that the process of developing any new energy storage device must follow the idea underlying in Barnhardt and Benson’s quote for stationary energy storage batteries, “if a battery’s energetic cost is too high, its overall contribution to global warming could negate the environmental benefits of the wind or solar farm it was supposed to support”, but applied to this flexible GPEs as component of wearable electronics gadgets [118].
3.1. Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) (PVdF-HFP)
3.1.1. Zn-MnO2
3.1.2. Zn-Ion
3.2. Polyvinil Alcohol (PVA)
3.2.1. Zn-MnO2
3.2.2. Zn-Air
3.2.3. Zn-Ni
3.2.4. Zn-Ag
3.2.5. Zn-Ion
3.2.6. Zn-Bi2O3
3.3. Polyacrylamide (PAM) and Its Derivatives
3.3.1. Zn-MnO2
3.3.2. Zinc-Air
3.3.3. Zinc-Ion
3.4. Poly-Acrylic Acid (PAA) and Sodium Polyacrylate (PANa)
3.4.1. Zn-MnO2
3.4.2. Zinc-Air
3.4.3. Zn-Ni
3.4.4. Zn-Ag
3.5. Polyethylene Oxide (PEO)
3.5.1. Zn-Air
3.5.2. Zn-Ag
3.6. Biobased GPEs
3.6.1. Starch
3.6.2. Cellulose and Its Derivatives
3.6.3. Gelatine
3.6.4. Xantan Gum
3.6.5. Carrageenan
3.6.6. Chitosan
3.6.7. Guar Gum
3.6.8. Sodium Alginate
3.7. Synthetic Biodegradable Polymers
3.8. Others Polymer Electrolytes
4. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Inamuddin; Rajender, B.; Asiri, A.M. Zinc Batteries: Basics, Development, and Applications; Wiley-Scrivener: Hoboken, NJ, USA, 2020; ISBN 1119662478. [Google Scholar]
- River, M. Newsletter of the International Metals Study Groups.Metals Despatch Newsletter No. 27. Available online: https://www.ilzsg.org/pages/document/p1/list.aspx?ff_aa_document_type=N (accessed on 3 November 2020).
- Anyadike, N. Zinc recycling. International Zinc Association. Lead Zinc 2015. [Google Scholar] [CrossRef]
- Cruz, A.P.S. Lithium statistics and information; U.S. Geological Survey: Reston, VA, USA, 2020; Volume 53, pp. 98–99.
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Garcia-herrero, I.; Margallo, M.; Laso, J.; Onand, R.; Irabien, A. Measuring the Vulnerability of an Energy Intensive Sector to the EU ETS under a Life Cycle Approach: The Case of the Chlor-Alkali Industry. Sustainability 2017, 9, 837. [Google Scholar] [CrossRef]
- Li, Z.; Fuhr, O.; Fichtner, M.; Zhao-Karger, Z. Towards stable and efficient electrolytes for room-temperature rechargeable calcium batteries. Energy Environ. Sci. 2019, 12, 3496–3501. [Google Scholar] [CrossRef]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The aluminum-ion battery: A sustainable and seminal concept? Front. Chem. 2019, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.F.; Ko, J.S.; Rolison, D.R.; Long, J.W. Translating Materials-Level Performance into Device-Relevant Metrics for Zinc-Based Batteries. Joule 2018, 2, 2519–2527. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc–Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Urbina, A.; Abad, J.; López, R.; Toledo, C.; Fernández Romero, A.J. Environmental and economical assessment for a sustainable Zn/air battery. Chemosphere 2020, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, C.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Wang, Z.; Liu, Z.; Tang, Z.; et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018, 11, 941–951. [Google Scholar] [CrossRef]
- Hudak, N. MITRE Technical Report. Water-Based Textile Batteries. Available online: https://www.mitre.org/sites/default/files/publications/pr-19-2959-water-based-textile-batteries.pdf (accessed on 3 November 2020).
- IDC-About-Home. Available online: https://www.idc.com/about (accessed on 15 April 2020).
- The Commission of The European Communities Smart Wearables: Reflection and Orientation Paper. Available online: https://ec.europa.eu/newsroom/document.cfm?doc_id=40542 (accessed on 3 November 2020).
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R. Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Jenax-Flexible Battery. Available online: https://jenaxinc.com/products/batteries/ (accessed on 17 June 2020).
- Stangl, C.; Fuchsbichler, B.; Schmuck, M.; Koller, S. Flexible Batteries. In Flexible Carbon-Based Electronics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 265–287. [Google Scholar]
- Grepow Customized Special Shaped Novel Lipo Battery-Grepow. Available online: https://www.grepow.com/page/shaped-battery.html?gclid=Cj0KCQjw-uH6BRDQARIsAI3I-UdbOx3Y5t8UA63EAO_IEV_d5tjYqNGBrQ3EN8ROT2Xb_QprketisVQaAtNNEALw_wcB (accessed on 9 September 2020).
- He, P.; Chen, Q.; Yan, M.; Xu, X.; Zhou, L.; Mai, L.; Nan, C.-W. Building better zinc-ion batteries: A materials perspective. EnergyChem 2019, 1, 100022. [Google Scholar] [CrossRef]
- Perdu, F. Overview of Existing and Innovative Batteries. Available online: http://science-and-energy.org/wp-content/uploads/2016/03/FPerdu_Houches_final-1.pdf (accessed on 8 August 2020).
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- Hertzberg, B.J.; Huang, A.; Hsieh, A.; Chamoun, M.; Davies, G.; Seo, J.K.; Zhong, Z.; Croft, M.; Erdonmez, C.; Meng, Y.S.; et al. Effect of Multiple Cation Electrolyte Mixtures on Rechargeable Zn-MnO2 Alkaline Battery. Chem. Mater. 2016, 28, 4536–4545. [Google Scholar] [CrossRef]
- Jha, A.R. Next-Generation Batteries and Fuel Cells for Commercial, Military, and Space Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781439850671. [Google Scholar]
- Quinn, J.B.; Waldmann, T.; Richter, K.; Kasper, M.; Wohlfahrt-Mehrens, M. Energy Density of Cylindrical Li-Ion Cells: A Comparison of Commercial 18650 to the 21700 Cells. J. Electrochem. Soc. 2018, 165, A3284–A3291. [Google Scholar] [CrossRef]
- Duracell Zinc-Air Battery-Primary Systems. Available online: https://d2ei442zrkqy2u.cloudfront.net/wp-content/uploads/2016/03/Zinc-Air-Tech-Bulletin.pdf (accessed on 18 August 2020).
- Thin-Film Battery-Molex. Available online: https://www.molex.com/molex/products/family/thinfilm_battery (accessed on 21 June 2020).
- Printed Thin and Flexible SoftBattery|Enfucell Oy. Available online: https://www.enfucell.com/ (accessed on 22 June 2020).
- Imprint Energy. Available online: https://www.imprintenergy.com/ (accessed on 22 June 2020).
- Railanmaa, A.; Kujala, M.; Keskinen, J.; Kololuoma, T.; Lupo, D. Highly flexible and non-toxic natural polymer gel electrolyte for printed supercapacitors for IoT. Appl. Phys. A Mater. Sci. Process. 2019, 125. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Wood, K.N.; Kazyak, E.; Chadwick, A.F.; Chen, K.H.; Zhang, J.G.; Thornton, K.; Dasgupta, N.P. Dendrites and pits: Untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Cent. Sci. 2016. [Google Scholar] [CrossRef]
- He, Y.; Ren, X.; Xu, Y.; Engelhard, M.H.; Li, X.; Xiao, J.; Liu, J.; Zhang, J.G.; Xu, W.; Wang, C. Origin of lithium whisker formation and growth under stress. Nat. Nanotechnol. 2019, 14, 1042–1047. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer (Guildf.) 1973, 14, 589. [Google Scholar] [CrossRef]
- Armand, M.B.; Chabagno, J.M.; Duclot, M.J. Poly-ethers as Solid Electrolytes. In Proceedings of the International Conference on Fast Ion Transport in Solids: Electrodes and Electrolytes, Lake Geneva, WI, USA, 21–25 May 1979; pp. 131–136. [Google Scholar]
- Vincent, C.A. Applications of electroactive polymers. Edited by B. Scrosati. Chapman and Hall, London, 1993. pp. 354. ISBN 0-412-41430-9. Polym. Int. 2005, 33, 343. [Google Scholar] [CrossRef]
- Cameron, G.G. Solid polymer electrolytes: Fundamentals and technological Applications. Fiona M. Gray. VCH Publishers Inc., New York 1991. pp. x + 245, price £44.00. ISBN 0–89573–772–8. Polym. Int. 2007, 32, 436. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Hofmann, D.A. Elektrolyte für Li-Ionen Zellen auf Basis von Polymeren und keramischen Partikeln; Karlsruhe Institute of Technology: Karlsruhe, Germany, 2016; pp. 1–25. [Google Scholar]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Naderi, R. Composite Gel Polymer Electrolyte for Lithium Ion Batteries; South Dakota State University: Brookings, South Dakota, 2016. [Google Scholar]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics (Kiel) 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Di Noto, V.; Lavina, S.; Giffin, G.A.; Negro, E.; Scrosati, B. Polymer electrolytes: Present, past and future. Electrochim. Acta 2011, 57, 4–13. [Google Scholar] [CrossRef]
- Boaretto, N.; Meabe, L.; Martinez-Ibañez, M.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Rechargeable Batteries: From Nanocomposite to Nanohybrid. J. Electrochem. Soc. 2020, 167, 070524. [Google Scholar] [CrossRef]
- Osada, I.; De Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018. [Google Scholar] [CrossRef]
- Matsuura, S.; Shibata, M.; Han, J.; Fujii, K. Polymer Gel Electrolyte Prepared by “Salting-In” Poly(ethylene glycol) into a Phosphonium-Based Ionic Liquid with a Lithium Salt. ACS Appl. Polym. Mater. 2020, 2, 1276–1282. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; InTech: London, UK, 2016; ISBN 9789533077826. [Google Scholar]
- Santos, F.; Tafur, J.P.; Abad, J.; Fernández Romero, A.J. Structural modifications and ionic transport of PVA-KOH hydrogels applied in Zn/Air batteries. J. Electroanal. Chem. 2019, 850, 113380. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional Hydrogels for Next-Generation Batteries and Supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Varshney, P.K.; Gupta, S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics (Kiel) 2011, 17, 479–483. [Google Scholar] [CrossRef]
- Rayung, M.; Aung, M.M.; Azhar, S.C.; Abdullah, L.C.; Su’ait, M.S.; Ahmad, A.; Jamil, S.N.A.M. Bio-based polymer electrolytes for electrochemical devices: Insight into the ionic conductivity performance. Materials 2020, 13, 838. [Google Scholar] [CrossRef]
- Iwaki, Y.O.; Escalona, M.H.; Briones, J.R.; Pawlicka, A. Sodium alginate-based ionic conducting membranes. Mol. Cryst. Liq. Cryst. 2012. [Google Scholar] [CrossRef]
- Selvalakshmi, S.; Mathavan, T.; Selvasekarapandian, S.; Premalatha, M. Study on NH4I composition effect in agar–agar-based biopolymer electrolyte. Ionics (Kiel) 2017. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Krishna Bhat, D. Biopolymer Electrolytes: Fundamentals and Applications in Energy Storage; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128136119. [Google Scholar]
- Spirk, S. Polysaccharides as Battery Components; SpringerBriefs in Molecular Science; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-65968-8. [Google Scholar]
- Mahalakshmi, M.; Selvanayagam, S.; Selvasekarapandian, S.; Moniha, V.; Manjuladevi, R.; Sangeetha, P. Characterization of biopolymer electrolytes based on cellulose acetate with magnesium perchlorate (Mg(ClO4)2) for energy storage devices. J. Sci. Adv. Mater. Devices 2019. [Google Scholar] [CrossRef]
- Di Palma, T.M.; Migliardini, F.; Caputo, D.; Corbo, P. Xanthan and κ-carrageenan based alkaline hydrogels as electrolytes for Al/air batteries. Carbohydr. Polym. 2017, 157, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, J.; Liu, J.; Li, Z.; Jin, S.; Li, Z.; Zhang, S.; Zhou, H. Flexible and stable quasi-solid-state zinc ion battery with conductive guar gum electrolyte. Mater. Today Energy 2019. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Zeng, S.; Zhou, S.; Chen, M.; Di, J.; Li, Q. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Kumar, L.S.; Selvin, P.C.; Selvasekarapandian, S.; Manjuladevi, R.; Monisha, S.; Perumal, P. Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Ionics (Kiel) 2018. [Google Scholar] [CrossRef]
- Di Palma, T.M.; Migliardini, F.; Gaele, M.F.; Corbo, P. Physically cross-linked xanthan hydrogels as solid electrolytes for Al/air batteries. Ionics (Kiel) 2019, 25, 4209–4217. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Xiao, S.Y.; Li, M.X.; Chang, Z.; Wang, F.X.; Gao, J.; Wu, Y.P. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. J. Power Sources 2015. [Google Scholar] [CrossRef]
- Alias, N.S.; Masri, M.N.; Abu Bakar, M.B.; Mazlan, M. Acacia senegal as an alternative material for hydrogel electrolyte implement in battery. Int. J. Adv. Sci. Technol. 2019. [Google Scholar]
- Song, M.-K.; Kim, Y.-T.; Kim, Y.T.; Cho, B.W.; Popov, B.N.; Rhee, H.-W. Thermally Stable Gel Polymer Electrolytes. J. Electrochem. Soc. 2003. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Su, W.N.; Hwang, B.J. In situ analytical techniques for battery interface analysis. Chem. Soc. Rev. 2018, 47, 736–751. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Du, Z.; Duan, H.; Yang, S. Zinc anode with artificial solid electrolyte interface for dendrite-free Ni-Zn secondary battery. J. Colloid Interface Sci. 2019, 555, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, L.; Liu, H.; Amine, A.; Zhao, Q.; Song, Y.; Yang, J.; Wang, K.; Pan, F. Artificial Solid-Electrolyte Interface Facilitating Dendrite-Free Zinc Metal Anodes via Nanowetting Effect. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Song, J.; Gao, Y.; Wang, D.; Liu, S.; Peng, S.; Usher, C.; Goliaszewski, A.; Wang, D. Supremely elastic gel polymer electrolyte enables a reliable electrode structure for silicon-based anodes. Nat. Commun. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, J.; Archer, L. Interphases in Lithium-Sulfur Batteries: Toward Deployable Devices with Competitive Energy Density and Stability. ACS Energy Lett. 2018, 3, 2104–2113. [Google Scholar] [CrossRef]
- Yang, D.; He, L.; Liu, Y.; Yan, W.; Liang, S.; Zhu, Y.; Fu, L.; Chen, Y.; Wu, Y. An acetylene black modified gel polymer electrolyte for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 13679–13686. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal-Air Batteries: Future Electrochemical Energy Storage of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Janek, J.; Schröder, D. Benchmarking Anode Concepts: The Future of Electrically Rechargeable Zinc-Air Batteries. ACS Energy Lett. 2019, 4, 1287–1300. [Google Scholar] [CrossRef]
- Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries. Nano Energy 2020, 70, 104523. [CrossRef]
- Hao, J.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Zhang, S.; Bo, G.; Wang, C.; Guo, Z. Designing Dendrite-Free Zinc Anodes for Advanced Aqueous Zinc Batteries. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, W.; Liu, X.; Lu, X. Challenges and Strategies for Constructing Highly Reversible Zinc Anodes in Aqueous Zinc-Ion Batteries: Recent Progress and Future Perspectives. Adv. Sustain. Syst. 2020. [Google Scholar]
- Zhang, X.G. SECONDARY BATTERIES–ZINC SYSTEMS|Zinc Electrodes: Overview. Encycl. Electrochem. Power Sources 2009, 454–468. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery performance-Dendrite-free cycling. Energy Environ. Sci. 2014. [Google Scholar] [CrossRef]
- Wang, M.; Emre, A.; Tung, S.; Gerber, A.; Wang, D.; Huang, Y.; Cecen, V.; Kotov, N.A. Biomimetic Solid-State Zn2+ Electrolyte for Corrugated Structural Batteries. ACS Nano 2019, 13, 1107–1115. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Han, Q.; Chi, X.; Zhang, S.; Liu, Y.; Zhou, B.; Yang, J.; Liu, Y. Durable, flexible self-standing hydrogel electrolytes enabling high-safety rechargeable solid-state zinc metal batteries. J. Mater. Chem. A 2018, 6, 23046–23054. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, H.; Liang, Q.; Yuan, D.; Xi, S.; Wu, C.; Manalastas, W.; Ma, J.; Fang, W.; Zheng, Y.; et al. High-performance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode. Nano Energy 2019, 62, 94–102. [Google Scholar] [CrossRef]
- Flexible Energy Conversion and Storage Devices; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2018.
- Liu, Y.; Sun, Z.; Tan, K.; Denis, D.K.; Sun, J.; Liang, L.; Hou, L.; Yuan, C. Recent progress in flexible non-lithium based rechargeable batteries. J. Mater. Chem. A 2019, 7, 4353–4382. [Google Scholar] [CrossRef]
- Zeng, L.; Qiu, L.; Cheng, H.M. Towards the practical use of flexible lithium ion batteries. Energy Storage Mater. 2019. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Schaefer, J.L.; Soc, J.E.; Park, B.; Schaefer, J.L. Review—Polymer Electrolytes for Magnesium Batteries: Forging Away from Analogs of Lithium Polymer Electrolytes and Towards the Rechargeable Magnesium Metal Polymer Battery. J. Electrochem. Soc. 2020. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, X.; Wang, Y.; Zhang, H.; Yang, W. Three-dimensional polymer networks for solid-state electrochemical energy storage. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Tilahun, M.; Alloin, F.; Iojoiu, C.; Ashu, R.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for zinc-air batteries: Recent progress, challenges and perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, K.; Tang, D.; Liu, W.; Meng, F.; Huang, Q.; Liu, J. Recent Progress in Electrolytes for Zn–Air Batteries. Front. Chem. 2020, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef]
- COLLINS, D. Handbook of Batteries and Fuel cells By David Linden; McGraw-Hill Book Company GmbH: Hamburg, Germany, 1986; Volume 17, ISBN 0071359788. [Google Scholar]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc-air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X. Flexible Zn-ion batteries based on manganese oxides: Progress and prospect. Carbon Energy 2020, 1–21. [Google Scholar] [CrossRef]
- Armand, M.B. Intercalation electrodes in: Materials for advanced batteries (proceedings of the nato conference). In NATO Conference Series, (Series) 6: Materials Science; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Xu, W.; Wang, Y. Recent Progress on Zinc-Ion Rechargeable Batteries. Nano-Micro Lett. 2019, 11, 1–30. [Google Scholar] [CrossRef]
- Shin, J.; Seo, J.K.; Yaylian, R.; Huang, A.; Meng, Y.S. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. Int. Mater. Rev. 2020, 65, 356–387. [Google Scholar] [CrossRef]
- Liu, X.; Yi, J.; Wu, K.; Jiang, Y.; Liu, Y.; Zhao, B.; Li, W.; Zhang, J. Rechargeable Zn-MnO2 batteries: Advances, challenges and perspectives. Nanotechnology 2020. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry with H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, J.; Cui, J.; Li, J.; Wu, X. A gel polymer electrolyte based on Polyacrylonitrile/organic montmorillonite membrane exhibiting dense structure for lithium ion battery. Solid State Ion. 2018, 315, 102–110. [Google Scholar] [CrossRef]
- Tsao, C.H.; Kuo, P.L. Poly(dimethylsiloxane) hybrid gel polymer electrolytes of a porous structure for lithium ion battery. J. Memb. Sci. 2015, 489, 36–42. [Google Scholar] [CrossRef]
- Jahn, M.; Sedlaikova, M.; Vondrak, J.; Paizek, L. PMMA-Based Electrolytes for Li-Ion Batteries. ECS Trans. 2016, 74, 159–164. [Google Scholar] [CrossRef]
- Rani, M.A.A.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Poly(vinyl chloride) Ionic Liquid Polymer Electrolyte Based on Bis(fluorosulfonyl)Amide for Sodium Secondary Batteries. J. Electrochem. Soc. 2017, 164, H5031–H5035. [Google Scholar] [CrossRef]
- Mittal, V. Polymers for Energy Storage and Conversion. Polym. Energy Storage Convers. 2013. [Google Scholar] [CrossRef]
- Guisao, J.P.T.; Romero, A.J.F. Interaction between Zn2+ cations and n-methyl-2-pyrrolidone in ionic liquid-based Gel Polymer Electrolytes for Zn batteries. Electrochim. Acta 2015, 176, 1447–1453. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Yen, H.J. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Song, D.Y.; Xu, C.; Chen, Y.F.; He, J.R.; Zhao, Y.; Li, P.J.; Lin, W.; Fu, F. Enhanced thermal and electrochemical properties of PVDF-HFP/PMMA polymer electrolyte by TiO2 nanoparticles. Solid State Ion. 2015, 282, 31–36. [Google Scholar] [CrossRef]
- Ramesh, S.; Wen, L.C. Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA-LiCF3SO3-SiO2. Ionics (Kiel) 2010. [Google Scholar] [CrossRef]
- Xiao, Q.; Deng, C.; Wang, Q.; Zhang, Q.; Yue, Y.; Ren, S. In Situ Cross-Linked Gel Polymer Electrolyte Membranes with Excellent Thermal Stability for Lithium Ion Batteries. ACS Omega 2019, 4, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, C.J.; Benson, S.M. On the importance of reducing the energetic and material demands of electrical energy storage. Energy Environ. Sci. 2013, 6, 1083–1092. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Strauss, E.; Peled, E.; Greenbaum, S. Review—On Order and Disorder in Polymer Electrolytes. J. Electrochem. Soc. 2015, 162, A2551–A2566. [Google Scholar] [CrossRef]
- Hu, P.; Chai, J.; Duan, Y.; Liu, Z.; Cui, G.; Chen, L. Progress in nitrile-based polymer electrolytes for high performance lithium batteries. J. Mater. Chem. A 2016, 4, 10070–10083. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, S. Ionic conductivity and physical stability study of gel nework polymer electrolytes. J. Appl. Polym. Sci. 2000, 77, 2957–2962. [Google Scholar] [CrossRef]
- Gohel, K.; Kanchan, D.K.; MacHhi, H.K.; Soni, S.S.; Maheshwaran, C. Gel polymer electrolyte based on PVDF-HFP:PMMA incorporated with propylene carbonate (PC) and diethyl carbonate (DEC) plasticizers: Electrical, morphology, structural and electrochemical properties. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Hirankumar, G.; Mehta, N. Effect of incorporation of different plasticizers on structural and ion transport properties of PVA-LiClO4 based electrolytes. Heliyon 2018, 4. [Google Scholar] [CrossRef]
- Gohel, K.; Kanchan, D.K. Effect of PC:DEC plasticizers on structural and electrical properties of PVDF–HFP:PMMA based gel polymer electrolyte system. J. Mater. Sci. Mater. Electron. 2019, 30, 12260–12268. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Effect of plasticizers on ionic conductivity and dielectric relaxation of PEO-LiClO4 polymer electrolyte. Electrochim. Acta 2015, 171, 59–65. [Google Scholar] [CrossRef]
- Stoeva, Z.; Martin-Litas, I.; Staunton, E.; Andreev, Y.G.; Bruce, P.G. Ionic conductivity in the crystalline polymer electrolytes PEO6:LiXF6, X = P, As, Sb. J. Am. Chem. Soc. 2003. [Google Scholar] [CrossRef]
- Wang, Y.; Sokolov, A.P. Design of superionic polymer electrolytes. Curr. Opin. Chem. Eng. 2015, 7, 113–119. [Google Scholar] [CrossRef]
- Wilson, R.; George, S.C.; Anil Kumar, S.; Thomas, S. Liquid Transport Characteristics in Polymeric Systems: An Introduction; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128098851. [Google Scholar]
- CROW. Available online: http://polymerdatabase.com/index.html (accessed on 16 August 2020).
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and applications of the β phase poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, F.; Foroughi, J.; Zheng, T.; Cheng, Z.; Spinks, G.M. Triaxial braided piezo fiber energy harvesters for self-powered wearable technologies. J. Mater. Chem. A 2019, 7, 8245–8257. [Google Scholar] [CrossRef]
- Mokhtari, F.; Cheng, Z.; Raad, R.; Xi, J.; Foroughi, J. Piezofibers to smart textiles: A review on recent advances and future outlook for wearable technology. J. Mater. Chem. A 2020, 8, 9496–9522. [Google Scholar] [CrossRef]
- Ye, L.; Feng, Z. Polymer electrolytes as solid solvents and their applications. Polym. Electrolytes Fundam. Appl. 2010, 550–582. [Google Scholar] [CrossRef]
- Abbrent, S.; Plestil, J.; Hlavata, D.; Lindgren, J.; Tegenfeldt, J.; Wendsjö, Å. Crystallinity and morphology of PVdF-HFP-based gel electrolytes. Polymer (Guildf.) 2001, 42, 1407–1416. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Polymers; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-1-895198-47-8. [Google Scholar]
- Tsuchida, E.; Ohno, H.; Tsunemi, K. Conduction of lithium ions in polyvinylidene fluoride and its derivatives-I. Electrochim. Acta 1983, 28, 591–595. [Google Scholar] [CrossRef]
- Xu, J.J.; Ye, H.; Huang, J. Novel zinc ion conducting polymer gel electrolytes based on ionic liquids. Electrochem. Commun. 2005, 7, 1309–1317. [Google Scholar] [CrossRef]
- Tafur, J.P.; Santos, F.; Fernández Romero, A.J. Influence of the ionic liquid type on the gel polymer electrolytes properties. Membranes 2015, 5, 752–771. [Google Scholar] [CrossRef] [PubMed]
- Johnsi, M.; Suthanthiraraj, S.A. Preparation, zinc ion transport properties, and battery application based on poly(vinilydene fluoride- co -hexa fluoro propylene) polymer electrolyte system containing titanium dioxide nanofiller. High Perform. Polym. 2015. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, N.; Liu, Z.; Tang, Z.; Zapien, J.A.; Chen, S.; Fan, J.; Zhi, C. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn–MnO2 Battery. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Investigation of the Environmental Stability of Poly(vinyl alcohol)–KOH Polymer Electrolytes for Flexible Zinc–Air Batteries. Front. Chem. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Fan, X.; Liu, X.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. Long-Shelf-Life Polymer Electrolyte Based on Tetraethylammonium Hydroxide for Flexible Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Liu, J.; Zeng, X.; Qu, S.; Han, X.; Deng, Y.; Hu, W.; Lu, J. Atomically Thin Mesoporous Co3O4 Layers Strongly Coupled with N-rGO Nanosheets as High-Performance Bifunctional Catalysts for 1D Knittable Zinc–Air Batteries. Adv. Mater. 2018, 30, 1–9. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Fu, J.; Lee, D.U.; Hassan, F.M.; Bai, Z.; Park, M.G.; Chen, Z. Flexible High-Energy Polymer-Electrolyte-Based Rechargeable Zinc-Air Batteries. Adv. Mater. 2015, 27, 5617–5622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Guo, Z.; Ren, J.; Wang, Y.; Peng, H. Flexible, Stretchable, and Rechargeable Fiber-Shaped Zinc-Air Battery Based on Cross-Stacked Carbon Nanotube Sheets. Angew. Chem. Int. Ed. 2015, 54, 15390–15394. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.M.; Lin, S.J.; Yang, C.C. Alkaline Zn-air and Al-air cells based on novel solid PVA/PAA polymer electrolyte membranes. J. Memb. Sci. 2006, 280, 802–808. [Google Scholar] [CrossRef]
- Kim, H.W.; Lim, J.M.; Lee, H.J.; Eom, S.W.; Hong, Y.T.; Lee, S.Y. Artificially engineered, bicontinuous anion-conducting/-repelling polymeric phases as a selective ion transport channel for rechargeable zinc-air battery separator membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Song, Z.; Ding, J.; Liu, B.; Liu, X.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. A Rechargeable Zn–Air Battery with High Energy Efficiency and Long Life Enabled by a Highly Water-Retentive Gel Electrolyte with Reaction Modifier. Adv. Mater. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Zamarayeva, A.M.; Gaikwad, A.M.; Deckman, I.; Wang, M.; Khau, B.; Steingart, D.A.; Arias, A.C. Fabrication of a High-Performance Flexible Silver–Zinc Wire Battery. Adv. Electron. Mater. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Huang, S.; Wan, F.; Bi, S.; Zhu, J.; Niu, Z.; Chen, J. A Self-Healing Integrated All-in-One Zinc-Ion Battery. Angew. Chem. Int. Ed. 2019, 58, 4313–4317. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Tang, Z.; Liang, G.; Yang, Q.; Li, H.; Ma, L.; Mo, F.; Zhi, C. A mechanically durable and device-level tough Zn-MnO2 battery with high flexibility. Energy Storage Mater. 2019, 23, 636–645. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, H.; Ruan, Z.; Tang, Z.; Liu, Z.; Wang, Z.; Huang, Y.; Pei, Z.; Zapien, J.A.; et al. Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co(iii) rich-electrode. Energy Environ. Sci. 2018, 11, 2521–2530. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Y.; Ji, X.; Zeng, J.; Yang, Q.; Guo, Y.; Huang, Z.; Li, X.; Yu, J.; Zhi, C. A Usage Scenario Independent “Air Chargeable” Flexible Zinc Ion Energy Storage Device. Adv. Energy Mater. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Tan, M.J.; Li, B.; Chee, P.; Ge, X.; Liu, Z.; Zong, Y.; Loh, X.J. Acrylamide-derived freestanding polymer gel electrolyte for flexible metal-air batteries. J. Power Sources 2018, 400, 566–571. [Google Scholar] [CrossRef]
- Miao, H.; Chen, B.; Li, S.; Wu, X.; Wang, Q.; Zhang, C.; Sun, Z.; Li, H. All-solid-state flexible zinc-air battery with polyacrylamide alkaline gel electrolyte. J. Power Sources 2020, 450, 227653. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Liu, Z.; Tang, Z.; Liang, G.; Mo, F.; Yang, Q.; Ma, L.; Zhi, C. A Nanofibrillated Cellulose/Polyacrylamide Electrolyte-Based Flexible and Sewable High-Performance Zn–MnO2 Battery with Superior Shear Resistance. Small 2018, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Liang, G.; Meng, Q.; Liu, Z.; Li, H.; Fan, J.; Zhi, C. A flexible rechargeable aqueous zinc manganese-dioxide battery working at −20 °C. Energy Environ. Sci. 2019, 12, 706–715. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, W.; Wang, K.; Li, C.; Sun, X.; Zhang, X.; Liu, W.; Ma, Y. High-performance solid-state Zn batteries based on a free-standing organic cathode and metal Zn anode with an ordered nano-architecture. Nanoscale Adv. 2020, 2, 296–303. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Liang, G.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.; et al. Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries by a Cross-Linked Polyacrylamide Electrolyte. ACS Nano 2018, 12, 3140–3148. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Clark, M.P.; Chung, H.; Ivey, D.G. Effects of Crosslinker Concentration in Poly(Acrylic Acid)-KOH Gel Electrolyte on Performance of Zinc-Air Batteries. Batter. Supercaps 2020, 3, 409–416. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Whiting, G.L.; Steingart, D.A.; Arias, A.C. Highly flexible, printed alkaline batteries based on mesh-embedded electrodes. Adv. Mater. 2011, 23, 3251–3255. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Pei, Z.; Liu, Z.; Li, H.; Zhu, M.; Fan, J.; Dai, Q.; Zhang, M.; Dai, L.; et al. Solid-State Rechargeable Zn//NiCo and Zn–Air Batteries with Ultralong Lifetime and High Capacity: The Role of a Sodium Polyacrylate Hydrogel Electrolyte. Adv. Energy Mater. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Hu, M.; Wang, J.; Nie, N.; Wang, Y.; Wang, Y.; Zhang, J.; Huang, Y. An intrinsically 400% stretchable and 50% compressible NiCo//Zn battery. Nano Energy 2019, 58, 338–346. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Aasen, D.; Zhalmuratova, D.; Labbe, M.; Chung, H.; Ivey, D.G. Compositional Effects of Gel Polymer Electrolyte and Battery Design for Zinc-Air Batteries. Batter. Supercaps 2020. [Google Scholar] [CrossRef]
- Cao, Z.; Hu, H.; Wu, M.; Tang, K.; Jiang, T. Planar all-solid-state rechargeable Zn-air batteries for compact wearable energy storage. J. Mater. Chem. A 2019, 7, 17581–17593. [Google Scholar] [CrossRef]
- Iwakura, C.; Murakami, H.; Nohara, S.; Furukawa, N.; Inoue, H. Charge-discharge characteristics of nickel/zinc battery with polymer hydrogel electrolyte. J. Power Sources 2005, 152, 291–294. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wang, J.; Hu, M.; Feng, Y.; Wang, P.; Wang, Y.; Nie, N.; Zhang, J.; Chen, H.; et al. Concentrated Hydrogel Electrolyte-Enabled Aqueous Rechargeable NiCo//Zn Battery Working from -20 to 50 °C. ACS Appl. Mater. Interfaces 2019, 11, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Guinot, S.; Salmon, E.; Penneau, J.F.; Fauvarque, J.F. A new class of PEO-based SPEs: Structure, conductivity and application to alkaline secondary batteries. Electrochim. Acta 1998, 43, 1163–1170. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, S.J. Alkaline composite PEO-PVA-glass-fibre-mat polymer electrolyte for Zn-air battery. J. Power Sources 2002, 112, 497–503. [Google Scholar] [CrossRef]
- Rathika, R.; Suthanthiraraj, S.A. Influence of 1-ethyl-3-methylimidazolium bis (trifluoromethyl sulfonyl) imide plasticization on zinc-ion conducting PEO/PVdF blend gel polymer electrolyte. J. Mater. Sci. Mater. Electron. 2018, 29, 19632–19643. [Google Scholar] [CrossRef]
- Sellam; Hashmi, S.A. Enhanced zinc ion transport in gel polymer electrolyte: Effect of nano-sized ZnO dispersion. J. Solid State Electrochem. 2012, 16, 3105–3114. [Google Scholar] [CrossRef]
- Ye, H.; Xu, J.J. Zinc ion conducting polymer electrolytes based on oligomeric polyether/PVDF-HFP blends. J. Power Sources 2007, 165, 500–508. [Google Scholar] [CrossRef]
- Zhao, J.; Sonigara, K.K.; Li, J.; Zhang, J.; Chen, B.; Zhang, J.; Soni, S.S.; Zhou, X.; Cui, G.; Chen, L. A Smart Flexible Zinc Battery with Cooling Recovery Ability. Angew. Chem. Int. Ed. 2017, 56, 7871–7875. [Google Scholar] [CrossRef]
- Zahid, A.R.M.; Masri, M.N.; Hussin, M.H.; Bakar, M.B.A. The preliminary study on cassava (Manihot Esculenta) as gel polymer electrolyte for zinc-air battery. AIP Conf. Proc. 2018, 2030. [Google Scholar] [CrossRef]
- Poosapati, A.; Jang, E.; Madan, D.; Jang, N.; Hu, L.; Lan, Y. Cellulose hydrogel as a flexible gel electrolyte layer. MRS Commun. 2019, 9, 122–128. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Song, X.; Jiang, G.; Zarrin, H.; Xu, P.; Li, K.; Yu, A.; Chen, Z. Laminated Cross-Linked Nanocellulose/Graphene Oxide Electrolyte for Flexible Rechargeable Zinc–Air Batteries. Adv. Energy Mater. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, X.; Lv, R.; Na, B.; Ouyang, J.; Liu, H. Nanostructured Polyaniline-Cellulose Papers for Solid-State Flexible Aqueous Zn-Ion Battery. ACS Sustain. Chem. Eng. 2018, 6, 8697–8703. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Song, X.; Zarrin, H.; Tian, X.; Qiao, J.; Rasen, L.; Li, K.; Chen, Z. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc-air batteries. Energy Environ. Sci. 2016, 9, 663–670. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, F.; Xing, Y.; Qu, W.; Chen, N.; Shang, Y.; Yan, M.; Li, Y.; Li, L.; Chen, R. Flexible Hydrogel Electrolyte with Superior Mechanical Properties Based on Poly(vinyl alcohol) and Bacterial Cellulose for the Solid-State Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 15537–15542. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, Z.; Liu, Z.; Wang, Y.; Tang, Z.; Li, H.; Zhu, M.; Hung, T.F.; Liu, J.; Shi, Z.; et al. A flexible rechargeable zinc-ion wire-shaped battery with shape memory function. J. Mater. Chem. A 2018, 6, 8549–8557. [Google Scholar] [CrossRef]
- Park, J.; Park, M.; Nam, G.; Lee, J.S.; Cho, J. All-solid-state cable-type flexible zinc-air battery. Adv. Mater. 2015, 27, 1396–1401. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Zhang, J.; Jin, S.; Jiang, Y.; Zhang, S.; Li, Z.; Zhi, C.; Du, G.; Zhou, H. Flexible quasi-solid-state zinc ion batteries enabled by highly conductive carrageenan bio-polymer electrolyte. RSC Adv. 2019, 9, 16313–16319. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Majid, S.R.; Arof, A.K. Plasticized chitosan-PVA blend polymer electrolyte based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Xu, N.; Peng, L.; Mao, J.; Gong, Q.; Qiao, J. Alkaline Exchange Polymer Membrane Electrolyte for High Performance of All-Solid-State Electrochemical Devices. ACS Appl. Mater. Interfaces 2018, 10, 29593–29598. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, N.; Fu, J.; Liu, Y.; Qiao, J. High-performance binary cross-linked alkaline anion polymer electrolyte membranes for all-solid-state supercapacitors and flexible rechargeable zinc–air batteries. J. Mater. Chem. A 2019, 7, 11257–11264. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, T.; Xu, N.; Huang, K. A Semisolid Electrolyte for Flexible Zn-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6904–6910. [Google Scholar] [CrossRef]
- Sownthari, K.; Suthanthiraraj, S.A. Synthesis and characterization of an electrolyte system based on a biodegradable polymer. Express Polym. Lett. 2013, 7, 495–504. [Google Scholar] [CrossRef]
- Kumar, G.G.; Sampath, S. Electrochemical Characterization of a Zinc-Based Gel-Polymer Electrolyte and Its Application in Rechargeable Batteries. J. Electrochem. Soc. 2003, 150, A608. [Google Scholar] [CrossRef]
- Lu, C.; Hoang, T.K.A.; Doan, T.N.L.; Acton, M.; Zhao, H.; Guan, W.; Chen, P. Influence of different silica gelling agents on the performance of aqueous gel electrolytes. J. Ind. Eng. Chem. 2016, 42, 101–106. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Song, M.; Liang, P.; Zhang, X.; Tiep, N.H.; Zhao, H.; Wang, J.; Wang, R.; Zhang, H.; et al. A High-Rate and Stable Quasi-Solid-State Zinc-Ion Battery with Novel 2D Layered Zinc Orthovanadate Array. Adv. Mater. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Tafur, J.P.; Fernández Romero, A.J. Electrical and spectroscopic characterization of PVdF-HFP and TFSI-ionic liquids-based gel polymer electrolyte membranes. Influence of ZnTf2 salt. J. Memb. Sci. 2014, 469, 499–506. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Muchakayala, R.; Song, S. Fabrication and characterization of Zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn(Tf)2 complex system. J. Mater. Sci. Mater. Electron. 2020, 31, 6160–6173. [Google Scholar] [CrossRef]
- Johnsi, M.; Austin Suthanthiraraj, S. Electrochemical and structural properties of a polymer electrolyte system based on the effect of CeO2 nanofiller with PVDF-co-HFP for energy storage devices. Ionics (Kiel) 2016. [Google Scholar] [CrossRef]
- Ho, C.C.; Evans, J.W.; Wright, P.K. Direct write dispenser printing of a zinc microbattery with an ionic liquid gel electrolyte. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Wang, Z.; Winslow, R.; Madan, D.; Wright, P.K.; Evans, J.W.; Keif, M.; Rong, X. Development of MnO2 cathode inks for flexographically printed rechargeable zinc-based battery. J. Power Sources 2014, 268, 246–254. [Google Scholar] [CrossRef]
- Tafur, J.P.; Abad, J.; Román, E.; Fernández Romero, A.J. Charge storage mechanism of MnO2 cathodes in Zn/MnO2 batteries using ionic liquid-based gel polymer electrolytes. Electrochem. Commun. 2015, 60, 190–194. [Google Scholar] [CrossRef]
- Kumar, G.G.; Sampath, S. Electrochemical characterization of poly(vinylidenefluoride)-zinc triflate gel polymer electrolyte and its application in solid-state zinc batteries. Solid State Ion. 2003, 160, 289–300. [Google Scholar] [CrossRef]
- Liu, Z.; Zein El Abedin, S.; Endres, F. Electrodeposition and stripping of zinc from an ionic liquid polymer gel electrolyte for rechargeable zinc-based batteries. J. Solid State Electrochem. 2014, 18, 2683–2691. [Google Scholar] [CrossRef]
- Kim, B.; Winslow, R.; Lin, I.; Gururangan, K.; Evans, J.; Wright, P. Layer-by-layer fully printed Zn-MnO2 batteries with improved internal resistance and cycle life. J. Phys. Conf. Ser. 2015, 660. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Saini, I.; Sharma, A.; Dhiman, R.; Aggarwal, S.; Ram, S.; Sharma, P.K. Grafted SiC nanocrystals: For enhanced optical, electrical and mechanical properties of polyvinyl alcohol. J. Alloys Compd. 2017, 714, 172–180. [Google Scholar] [CrossRef]
- Dattola, E.; Parrotta, E.I.; Scalise, S.; Perozziello, G.; Limongi, T.; Candeloro, P.; Coluccio, M.L.; Maletta, C.; Bruno, L.; De Angelis, M.T.; et al. Development of 3D PVA scaffolds for cardiac tissue engineering and cell screening applications. RSC Adv. 2019, 9, 4246–4257. [Google Scholar] [CrossRef]
- Yang, C.C. Chemical composition and XRD analyses for alkaline composite PVA polymer electrolyte. Mater. Lett. 2004, 58, 33–38. [Google Scholar] [CrossRef]
- Lewandowski, A.; Skorupska, K.; Malinska, J. Novel poly(vinyl alcohol)-KOH-H2O alkaline polymer electrolyte. Solid State Ion. 2000, 133, 265–271. [Google Scholar] [CrossRef]
- Yuan, A.; Zhao, J. Composite alkaline polymer electrolytes and its application to nickel-metal hydride batteries. Electrochim. Acta 2006, 51, 2454–2462. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Yuan, J.; Zhu, Y.; Li, L. Alkaline poly(vinyl alcohol)/poly(acrylic acid) polymer electrolyte membrane for Ni-MH battery application. Ionics (Kiel) 2014, 21, 141–148. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Sang, S. Preparation of alkaline solid polymer electrolyte based on PVA-TiO 2-KOH-H2O and its performance in Zn-Ni battery. J. Phys. Chem. Solids 2008, 69, 2691–2695. [Google Scholar] [CrossRef]
- Yang, C.C. Polymer Ni-MH battery based on PEO-PVA-KOH polymer electrolyte. J. Power Sources 2002, 109, 22–31. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, X.J.; Hou, Y.Y.; Gao, X.W.; Liu, L.L.; Wu, Y.P.; Shimizu, M. A new single-ion polymer electrolyte based on polyvinyl alcohol for lithium ion batteries. Electrochim. Acta 2013, 87, 113–118. [Google Scholar] [CrossRef]
- Caldeira, I.; Lüdtke, A.; Tavares, F.; Cholant, C.; Balboni, R.; Flores, W.H.; Galio, A.; Pawlicka, A.; Avellaneda, C.O. Ecologically friendly xanthan gum-PVA matrix for solid polymeric electrolytes. Ionics (Kiel) 2018, 24, 413–420. [Google Scholar] [CrossRef]
- Martinez-Laserna, E.; Gandiaga, I.; Sarasketa-Zabala, E.; Badeda, J.; Stroe, D.I.; Swierczynski, M.; Goikoetxea, A. Battery second life: Hype, hope or reality? A critical review of the state of the art. Renew. Sustain. Energy Rev. 2018, 93, 701–718. [Google Scholar] [CrossRef]
- Yang, J.M.; Chiang, C.Y.; Wang, H.Z.; Yang, C.C. Two step modification of poly(vinyl alcohol) by UV radiation with 2-hydroxy ethyl methacrylate and sol-gel process for the application of polymer electrolyte membrane. J. Memb. Sci. 2009, 341, 186–194. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Han, J.; Zhang, X.; Sun, X.; Li, C.; Liu, W.; Li, Q.; Ma, Y. High-Performance Cable-Type Flexible Rechargeable Zn Battery Based on MnO2@CNT Fiber Microelectrode. ACS Appl. Mater. Interfaces 2018, 10, 24573–24582. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, Q.; Li, L.; Sun, J.; Man, P.; Zhou, Z.; Li, Q.; Guo, J.; Xie, L.; Li, C.; et al. High-performance flexible all-solid-state aqueous rechargeable Zn-MnO2 microbatteries integrated with wearable pressure sensors. J. Mater. Chem. A 2018, 6, 14594–14601. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Qi, K.; Xin, J.H. Functionalization of cotton with carbon nanotubes. J. Mater. Chem. 2008, 18, 3454–3460. [Google Scholar] [CrossRef]
- Shi, Q.; Sun, J.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Advanced Functional Fiber and Smart Textile. Adv. Fiber Mater. 2019, 1, 3–31. [Google Scholar] [CrossRef]
- Casey, G. Future Soldier 2030 Initiative. Available online: https://www.wired.com/images_blogs/dangerroom/2009/05/dplus2009_11641-1.pdf (accessed on 3 November 2020).
- Qu, S.; Liu, B.; Fan, X.; Liu, X.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. 3D Foam Anode and Hydrogel Electrolyte for High-Performance and Stable Flexible Zinc–Air Battery. ChemistrySelect 2020, 5, 8305–8310. [Google Scholar] [CrossRef]
- Lin, C.; Shinde, S.S.; Li, X.; Kim, D.H.; Li, N.; Sun, Y.; Song, X.; Zhang, H.; Lee, C.H.; Lee, S.U.; et al. Solid-State Rechargeable Zinc–Air Battery with Long Shelf Life Based on Nanoengineered Polymer Electrolyte. ChemSusChem 2018. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.; Cai, W.; He, W.; Liu, M.; Shao, Z.; Ni, M. Co3O4 Nanosheets as Active Material for Hybrid Zn Batteries. Small 2018. [Google Scholar] [CrossRef]
- Guan, C.; Sumboja, A.; Wu, H.; Ren, W.; Liu, X.; Zhang, H.; Liu, Z.; Cheng, C.; Pennycook, S.J.; Wang, J. Hollow Co3O4 Nanosphere Embedded in Carbon Arrays for Stable and Flexible Solid-State Zinc–Air Batteries. Adv. Mater. 2017. [Google Scholar] [CrossRef]
- Guan, C.; Sumboja, A.; Zang, W.; Qian, Y.; Zhang, H.; Liu, X.; Liu, Z.; Zhao, D.; Pennycook, S.J.; Wang, J. Decorating Co/CoNx nanoparticles in nitrogen-doped carbon nanoarrays for flexible and rechargeable zinc-air batteries. Energy Storage Mater. 2019, 16, 243–250. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Hou, C.; Wang, Z.; Liang, C.; Zhao, C.; Tong, Y.; Lu, X.; Yang, S. Nitrogen-Doped Co3O4 Mesoporous Nanowire Arrays as an Additive-Free Air-Cathode for Flexible Solid-State Zinc–Air Batteries. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Zhong, C.; Liu, Z.; Liu, J.; Ma, L.; Deng, Y.; Han, X.; Wu, T.; Hu, W.; et al. Ultrathin Co3O4 Layers with Large Contact Area on Carbon Fibers as High-Performance Electrode for Flexible Zinc–Air Battery Integrated with Flexible Display. Adv. Energy Mater. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, H.; Bao, D.; Yan, J.; Zhang, X. In Situ Coupling of Strung Co4N and Intertwined N-C Fibers toward Free-Standing Bifunctional Cathode for Robust, Efficient, and Flexible Zn-Air Batteries. J. Am. Chem. Soc. 2016, 138, 10226–10231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Abbas, S.C.; Huang, Y.; Lv, J.; Wu, M.; Wang, Y. Robust and Highly Active FeNi@NCNT Nanowire Arrays as Integrated Air Electrode for Flexible Solid-State Rechargeable Zn-Air Batteries. Adv. Mater. Interfaces 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Domenech, B.; Bastos-Arrieta, J.; Alonso, A.; Macanas, J.; Munoz, M.; Muraviev, D.N. Bifunctional Polymer-Metal Nanocomposite Ion Exchange Materials. Ion Exch. Technol. 2012. [Google Scholar] [CrossRef]
- Gan, W.; Zhou, D.; Zhou, L.; Zhang, Z.; Zhao, J. Zinc electrode with anion conducting polyvinyl alcohol/poly(diallyldimethylammonium chloride) film coated ZnO for secondary zinc air batteries. Electrochim. Acta 2015, 182, 430–436. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Walther, F.; Sann, J.; Janek, J.; Schröder, D. Homogeneous Coating with an Anion-Exchange Ionomer Improves the Cycling Stability of Secondary Batteries with Zinc Anodes. ACS Appl. Mater. Interfaces 2018, 10, 8640–8648. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Dai, L.; Yao, J. Scalable Fabrication of Nanoporous Carbon Fiber Films as Bifunctional Catalytic Electrodes for Flexible Zn-Air Batteries. Adv. Mater. 2016, 28, 3000–3006. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhang, S.Y.; Wang, B.; Xia, Z.J.; Tang, C.; Zhang, Q. A porphyrin covalent organic framework cathode for flexible Zn-air batteries. Energy Environ. Sci. 2018, 11, 1723–1729. [Google Scholar] [CrossRef]
- Lai, S.B.; Jamesh, M.I.; Wu, X.C.; Dong, Y.L.; Wang, J.H.; Gao, M.; Liu, J.F.; Sun, X.M. A promising energy storage system: Rechargeable Ni–Zn battery. Rare Met. 2017, 36, 381–396. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science (80-) 2017, 356, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Q.; Sun, J.; Li, T.; Songfeng, S.; Zhu, Z.; He, B.; Zhou, Z.; Li, Q.; Yao, Y. High-performance quasi-solid-state flexible aqueous rechargeable ag-zn battery based on metal-organic framework-derived ag nanowires. ACS Energy Lett. 2018, 3, 2761–2768. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, C.; Kim, J.H.; de Andrade, M.J.; Baughman, R.H.; Kim, S.J. Biscrolled Carbon Nanotube Yarn Structured Silver-Zinc Battery. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ip, W.S.; Lau, Y.Y.; Sun, J.; Zeng, J.; Yeung, N.S.S.; Ng, W.S.; Li, H.; Pei, Z.; Xue, Q.; et al. Weavable, Conductive Yarn-Based NiCo//Zn Textile Battery with High Energy Density and Rate Capability. ACS Nano 2017, 11, 8953–8961. [Google Scholar] [CrossRef]
- Zeng, Y.; Meng, Y.; Lai, Z.; Zhang, X.; Yu, M.; Fang, P.; Wu, M.; Tong, Y.; Lu, X. An Ultrastable and High-Performance Flexible Fiber-Shaped Ni–Zn Battery based on a Ni–NiO Heterostructured Nanosheet Cathode. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Guan, C.; Zhou, C.; Fan, Z.; Ke, Q.; Zhang, G.; Liu, C.; Wang, J. A Flexible Quasi-Solid-State Nickel–Zinc Battery with High Energy and Power Densities Based on 3D Electrode Design. Adv. Mater. 2016, 28, 8732–8739. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Z.; Wang, Y.; Li, H.; Zhang, J. In-situ growth of ZnO nanoplates on graphene for the application of high rate flexible quasi-solid-state Ni-Zn secondary battery. J. Power Sources 2018, 407, 137–146. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Xiao, D. Three-dimensional nanotube-array anode enables a flexible Ni/Zn fibrous battery to ultrafast charge and discharge in seconds. Energy Storage Mater. 2018, 12, 232–240. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Songfeng, E.; Li, T.; Zhu, Z.; He, B.; Zhou, Z.; Man, P.; Li, Q.; Yao, Y. An ultra-high endurance and high-performance quasi-solid-state fiber-shaped Zn-Ag 2 O battery to harvest wind energy. J. Mater. Chem. A 2019, 7, 2034–2040. [Google Scholar] [CrossRef]
- Lee, J.A.; Spinks, G.; Wallace, G. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 2013. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1–27. [Google Scholar] [CrossRef]
- Cao, H.; Wan, F.; Zhang, L.; Dai, X.; Huang, S.; Liu, L.; Niu, Z. Highly compressible zinc-ion batteries with stable performance. J. Mater. Chem. A 2019, 7, 11734–11741. [Google Scholar] [CrossRef]
- Qiu, W.; Li, Y.; You, A.; Zhang, Z.; Li, G.; Lu, X.; Tong, Y. High-performance flexible quasi-solid-state Zn-MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem. A 2017, 5, 14838–14846. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian Blue Analogs as Battery Materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef]
- Lu, K.; Song, B.; Zhang, Y.; Ma, H.; Zhang, J. Encapsulation of zinc hexacyanoferrate nanocubes with manganese oxide nanosheets for high-performance rechargeable zinc ion batteries. J. Mater. Chem. A 2017, 5, 23628–23633. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries. Adv. Mater. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Deng, S.; Wu, W.; Zeng, Y.; Xia, X.; Pan, G.; Tong, Y.; Lu, X. 3D CNTs Networks Enable MnO2 Cathodes with High Capacity and Superior Rate Capability for Flexible Rechargeable Zn–MnO2 Batteries. Small Methods 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, F.; Huang, S.; Wang, S.; Niu, Z.; Chen, J. A chemically self-charging aqueous zinc-ion battery. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Zuo, W.; Zhu, W.; Zhao, D.; Sun, Y.; Li, Y.; Liu, J.; Lou, X.W.D. Bismuth oxide: A versatile high-capacity electrode material for rechargeable aqueous metal-ion batteries. Energy Environ. Sci. 2016, 9, 2881–2891. [Google Scholar] [CrossRef]
- Lun, Y.H.V.; Tung, S.L.D. Sustainable energy. Green Energy Technol. 2020, 17–33. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1–30. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Rabiee, A.; Ershad-Langroudi, A.; Jamshidi, H. Polyacrylamide-based polyampholytes and their applications. Rev. Chem. Eng. 2014, 30, 501–519. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Wang, D.; Liang, G.; Zhu, Y.; Mo, F.; Huang, Z.; Li, X.; Ma, L.; Tang, T.; et al. A Flexible Solid-State Aqueous Zinc Hybrid Battery with Flat and High-Voltage Discharge Plateau. Adv. Energy Mater. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Cericola, D.; Novák, P.; Wokaun, A.; Kötz, R. Hybridization of electrochemical capacitors and rechargeable batteries: An experimental analysis of the different possible approaches utilizing activated carbon, Li4Ti5O12 and LiMn2O 4. J. Power Sources 2011, 196, 10305–10313. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Pei, Z.; Li, H.; Wang, Z.; Liu, Z.; Tang, Z.; Zapien, J.A.; Zhi, C. Flexible waterproof rechargeable hybrid zinc batteries initiated by multifunctional oxygen vacancies-rich cobalt oxide. ACS Nano 2018, 12, 8597–8605. [Google Scholar] [CrossRef]
- Watts, J. How the Race for Cobalt Risks Turning it from Miracle Metal to Deadly Chemical. Available online: https://www.theguardian.com/global-development/2019/dec/18/how-the-race-for-cobalt-risks-turning-it-from-miracle-metal-to-deadly-chemical (accessed on 19 June 2020).
- Sun, G.; Xiao, Y.; Lu, B.; Jin, X.; Yang, H.; Dai, C.; Zhang, X.; Zhao, Y.; Qu, L. Hybrid Energy Storage Device: Combination of Zinc-Ion Supercapacitor and Zinc-Air Battery in Mild Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 7239–7248. [Google Scholar] [CrossRef]
- Quan, Y.; Chen, M.; Zhou, W.; Tian, Q.; Chen, J. High-Performance Anti-freezing Flexible Zn-MnO2 Battery Based on Polyacrylamide/Graphene Oxide/Ethylene Glycol Gel Electrolyte. Front. Chem. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Mo, F.; Li, H.; Pei, Z.; Liang, G.; Ma, L.; Yang, Q.; Wang, D.; Huang, Y.; Zhi, C. A smart safe rechargeable zinc ion battery based on sol-gel transition electrolytes. Sci. Bull. 2018, 63, 1077–1086. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, L.; Zhu, Y.; Qin, P.; Li, H.; Mo, F.; Wang, D.; Liang, G.; Yang, Q.; Liu, W.; et al. Inhibiting Grain Pulverization and Sulfur Dissolution of Bismuth Sulfide by Ionic Liquid Enhanced Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) for High-Performance Zinc-Ion Batteries. ACS Nano 2019, 13, 7270–7280. [Google Scholar] [CrossRef] [PubMed]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. (Eng. Ed.) 2008, 17, 451–477. [Google Scholar]
- Tran, T.N.T.; Chung, H.J.; Ivey, D.G. A study of alkaline gel polymer electrolytes for rechargeable zinc–air batteries. Electrochim. Acta 2019, 327, 135021. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super-Stretchable Zinc–Air Batteries Based on an Alkaline-Tolerant Dual-Network Hydrogel Electrolyte. Adv. Energy Mater. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Wang, J.; Hu, M.; Mo, F.; Liang, G.; Zhi, C. An Intrinsically Self-Healing NiCo||Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2018, 57, 9810–9813. [Google Scholar] [CrossRef]
- Gong, Z.; Niu, F.; Zhang, G.; Li, J.; Li, G.; Huang, W.; Deng, H.; Sun, R.; Wong, C. Effects of composition on the properties of dual physically cross-linked hydrogel composed of polyvinyl alcohol and poly (acrylamide-co-acrylic acid). J. Polym. Res. 2017, 24, 1–7. [Google Scholar] [CrossRef]
- Fukao, K.; Tanaka, K.; Kiyama, R.; Nonoyama, T.; Nonoyama, T.; Gong, J.P.; Gong, J.P. Hydrogels toughened by biominerals providing energy-dissipative sacrificial bonds. J. Mater. Chem. B 2020, 8, 5184–5188. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly(vinyl alcohol)/poly(acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Uddin, M.M.; Roy, C.K.; Hossen, M.J.; Sujan, M.I.; Azam, M.S. Mechanically tough and highly stretchable poly(acrylic acid) hydrogel cross-linked by 2D graphene oxide. RSC Adv. 2020. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, H.; Cao, Y.; Ai, X. Preparation and electrochemical characterization of the alkaline polymer gel electrolyte polymerized from acrylic acid and KOH solution. Electrochim. Acta 2004, 49, 2533–2539. [Google Scholar] [CrossRef]
- Lao-atiman, W.; Julaphatachote, T.; Boonmongkolras, P.; Kheawhom, S. Printed Transparent Thin Film Zn-MnO2 Battery. J. Electrochem. Soc. 2017, 164, A859–A863. [Google Scholar] [CrossRef]
- Kwon, O.; Hwang, H.J.; Ji, Y.; Jeon, O.S.; Kim, J.P.; Lee, C.; Shul, Y.G. Transparent Bendable Secondary Zinc-Air Batteries by Controlled Void Ionic Separators. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chaduang, S.; Lao-atiman, W.; Kheawhom, S. Improving Performance and Cyclability of Printed Flexible Zinc-Air Batteries Using Carbopol Gel Electrolyte. ECS Trans. 2017, 77, 55–62. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, X.; Wu, Z.; Mitra, S. Development of flexible zinc–air battery with nanocomposite electrodes and a novel separator. J. Energy Chem. 2017, 26, 129–138. [Google Scholar] [CrossRef]
- Mohamad, A.A. Zn/gelled 6 M KOH/O2 zinc-air battery. J. Power Sources 2006. [Google Scholar] [CrossRef]
- Braam, K.; Subramanian, V. A stencil printed, high energy density silver oxide battery using a novel photopolymerizable poly(acrylic acid) separator. Adv. Mater. 2015, 27, 689–694. [Google Scholar] [CrossRef]
- Berchmans, S.; Bandodkar, A.J.; Jia, W.; Ramírez, J.; Meng, Y.S.; Wang, J. An epidermal alkaline rechargeable Ag-Zn printable tattoo battery for wearable electronics. J. Mater. Chem. A 2014, 2, 15788–15795. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Antony, R.; Alwin, S.; Balakumar, S.; Sahaya Shajan, X. A review on PEO based solid polymer electrolytes (SPEs) complexed with LiX (X=Tf, BOB) for rechargeable lithium ion batteries. Mater. Sci. Forum 2015, 807, 41–63. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Insights into the use of polyethylene oxide in energy storage/conversion devices: A critical review. J. Phys. D. Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Hu, M.; Zeng, J.; Mu, Y.; Guo, Y.; Yu, J.; Ma, X.; Qiu, Y.; Huang, Y. A flexible, electrochromic, rechargeable Zn//PPy battery with a short circuit chromatic warning function. J. Mater. Chem. A 2018, 6, 11113–11118. [Google Scholar] [CrossRef]
- Mitha, A.; Mi, H.; Dong, W.; Cho, I.S.; Ly, J.; Yoo, S.; Bang, S.; Hoang, T.K.A.; Chen, P. Thixotropic gel electrolyte containing poly(ethylene glycol) with high zinc ion concentration for the secondary aqueous Zn/LiMn 2 O 4 battery. J. Electroanal. Chem. 2019, 836, 1–6. [Google Scholar] [CrossRef]
- Lyu, L.; Gao, Y.; Wang, Y.; Xiao, L.; Lu, J.; Zhuang, L. Improving the cycling performance of silver-zinc battery by introducing PEG-200 as electrolyte additive. Chem. Phys. Lett. 2019, 723, 102–110. [Google Scholar] [CrossRef]
- Banik, S.J.; Akolkar, R. Suppressing Dendrite Growth during Zinc Electrodeposition by PEG-200 Additive. J. Electrochem. Soc. 2013. [Google Scholar] [CrossRef]
- Plancha, M.J.C.; Rangel, C.M.; Sequeira, C.A.C. Pseudo-equilibrium phase diagrams for PEO-Zn salts-based electrolytes. Solid State Ion. 1999, 116, 293–300. [Google Scholar] [CrossRef]
- Fauvarque, J.F.; Guinot, S.; Bouzir, N.; Salmon, E.; Penneau, J.F. Alkaline poly(ethylene oxide) solid polymer electrolytes. Application to nickel secondary batteries. Electrochim. Acta 1995, 40, 2449–2453. [Google Scholar] [CrossRef]
- Vassal, N.; Salmon, E.; Fauvarque, J.F. Electrochemical properties of an alkaline solid polymer electrolyte based on P(ECH-co-EO). Electrochim. Acta 2000, 45, 1527–1532. [Google Scholar] [CrossRef]
- Braam, K.T.; Volkman, S.K.; Subramanian, V. Characterization and optimization of a printed, primary silver-zinc battery. J. Power Sources 2012, 199, 367–372. [Google Scholar] [CrossRef]
- Armelin, E.; Pérez-Madrigal, M.M.; Alemán, C.; Díaz, D.D. Current status and challenges of biohydrogels for applications as supercapacitors and secondary batteries. J. Mater. Chem. A 2016, 4, 8952–8968. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Ruso, J.; Messina, P. Biopolymers in Regenerative Medicine: Overview, Current Advances and Future Trends. In Biopolymers for Medical Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–37. ISBN 9781315368863. [Google Scholar]
- Nomura, K.; Terwilliger, P. A glimpse of biodegradable polymers and biomedical applications. e-Polymers 2019, 385–410. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Masri, M.N.; Nazeri, M.F.M.; Ng, C.Y.; Mohamad, A.A. Tapioca binder for porous zinc anodes electrode in zinc–air batteries. J. King Saud Univ. Eng. Sci. 2015, 27, 217–224. [Google Scholar] [CrossRef]
- Masri, M.N.; Nazeri, M.F.M.; Mohamad, A.A. Sago Gel Polymer Electrolyte for Zinc-Air Battery. Adv. Sci. Technol. 2010, 72, 305–308. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Biopolymer Electrolytes for Solar Cells and Electrochemical Cells. In Biopolymer Electrolytes: Fundamentals and Applications in Energy Storage; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–149. ISBN 9780128134474. [Google Scholar]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Garcia, D.M.E.; Pereira, A.S.T.M.; Almeida, A.C.; Roma, U.S.; Soler, A.B.A.; Lacharmoise, P.D.; Das Mercês Ferreira, I.M.; Simaõ, C.C.D. Large-Area Paper Batteries with Ag and Zn/Ag Screen-Printed Electrodes. ACS Omega 2019, 4, 16781–16788. [Google Scholar] [CrossRef]
- Fu, J.; Hassan, F.M.; Li, J.; Lee, D.U.; Ghannoum, A.R.; Lui, G.; Hoque, M.A.; Chen, Z. Flexible Rechargeable Zinc-Air Batteries through Morphological Emulation of Human Hair Array. Adv. Mater. 2016, 28, 6421–6428. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Cai, X.; Yao, J.; Li, M.; Zhang, X.; Liu, Q. High alkaline tolerant electrolyte membrane with improved conductivity and mechanical strength via lithium chloride/dimethylacetamide dissolved microcrystalline cellulose for Zn-Air batteries. Electrochim. Acta 2016, 220, 635–642. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.H.; Yu, J.Y.; Kim, D.H.; Lee, S.U.; Lee, J.H. Hierarchically Designed 3D Holey C2N Aerogels as Bifunctional Oxygen Electrodes for Flexible and Rechargeable Zn-Air Batteries. ACS Nano 2018. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Lee, C.H.; Jung, J.Y.; Wagh, N.K.; Kim, S.H.; Kim, D.H.; Lin, C.; Lee, S.U.; Lee, J.H. Unveiling dual-linkage 3D hexaiminobenzene metal-organic frameworks towards long-lasting advanced reversible Zn-air batteries. Energy Environ. Sci. 2019, 12, 727–738. [Google Scholar] [CrossRef]
- Hasan, A.M.A.; Abdel-Raouf, M.E.-S. Cellulose-Based Superabsorbent Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 245–267. ISBN 9783319778297. [Google Scholar]
- Zhang, Q.; Li, C.; Li, Q.; Pan, Z.; Sun, J.; Zhou, Z.; He, B.; Man, P.; Xie, L.; Kang, L.; et al. Flexible and High-Voltage Coaxial-Fiber Aqueous Rechargeable Zinc-Ion Battery. Nano Lett. 2019, 19, 4035–4042. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Z.; Li, H.; Xiong, Y.; Liu, Z.; Tang, Z.; Huang, Y.; Rogach, A.L.; Zhi, C. Light-permeable, photoluminescent microbatteries embedded in the color filter of a screen. Energy Environ. Sci. 2018, 11, 2414–2422. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, Z.; Ng, W.S.; Li, H.; Tang, Z.; Liu, Z.; Wang, Y.; Hu, H.; Zhi, C. Integrating a Triboelectric Nanogenerator and a Zinc-Ion Battery on a Designed Flexible 3D Spacer Fabric. Small Methods 2018, 2, 1800150. [Google Scholar] [CrossRef]
- Liu, J.; Gu, L.; Cui, N.; Xu, Q.; Qin, Y.; Yang, R. Fabric-Based Triboelectric Nanogenerators. Research 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, G.; Zhou, F.; Zhang, S.; Deng, C. Toward Tailorable Zn-Ion Textile Batteries with High Energy Density and Ultrafast Capability: Building High-Performance Textile Electrode in 3D Hierarchical Branched Design. Small 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Mirza, A.; Ahmad, R. Novel recyclable (Xanthan gum/montmorillonite) bionanocomposite for the removal of Pb (II) from synthetic and industrial wastewater. Environ. Technol. Innov. 2018, 11, 241–252. [Google Scholar] [CrossRef]
- Wan, C.T.C.; López Barreiro, D.; Forner-Cuenca, A.; Barotta, J.W.; Hawker, M.J.; Han, G.; Loh, H.C.; Masic, A.; Kaplan, D.L.; Chiang, Y.M.; et al. Exploration of Biomass-Derived Activated Carbons for Use in Vanadium Redox Flow Batteries. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Biswal, A.; Minakshi, M.; Tripathy, B.C. Probing the electrochemical properties of biopolymer modified EMD nanoflakes through electrodeposition for high performance alkaline batteries. Dalt. Trans. 2016, 45, 5557–5567. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, D.; Yuan, Z.; Xie, W.; Wu, Y.; Li, R.; Zhao, Y.; Luo, D.; Cen, L.; Chen, B.; et al. A Fully Biodegradable Battery for Self-Powered Transient Implants. Small 2018, 1800994, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Pan, S.; Linsley, C.; Li, X. Manufacturing and characterization of Zn-WC as potential biodegradable material. Procedia Manuf. 2019. [Google Scholar] [CrossRef]
- Huang, X. Materials and applications of bioresorbable electronics. J. Semicond. 2018, 39, 1–10. [Google Scholar] [CrossRef]
- Majdecka, D.; Dramiska, S.; Stolarczyk, K.; Kizling, M.; Krysiski, P.; Golimowski, J.; Bilewicz, R. Sandwich Biobattery with Enzymatic Cathode and Zinc Anode for Powering Sensors. ECS Trans. 2014, 61, 1–7. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. An introduction of Biopolymer Electrolytes. In Biopolymer Electrolytes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–34. ISBN 9780128134474. [Google Scholar]
- Bharti, V.; Kumar, P.; Paul, J. Materials Today: Proceedings Development of polymer electrolyte membranes based on biodegradable polymer. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Tsang, M.; Armutlulu, A.; Martinez, A.W.; Allen, S.A.B.; Allen, M.G. Biodegradable magnesium/iron batteries with polycaprolactone encapsulation: A microfabricated power source for transient implantable devices. Microsyst. Nanoeng. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Tan, L.; Deng, Y.; Cao, Q.; Jing, B.; Wang, X.; Liu, Y. Gel electrolytes based on polyacrylonitrile/thermoplastic polyurethane/polystyrene for lithium-ion batteries. Ionics (Kiel) 2019, 25, 3673–3682. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Polyacrylonitrile-nanofiber-based gel polymer electrolyte for novel aqueous sodium-ion battery based on a Na4Mn9O18 cathode and Zn metal anode. Polymers 2018, 10, 853. [Google Scholar] [CrossRef]
- Patel, A.R.; Mankoč, B.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Fumed silica-based organogels and “aqueous-organic” bigels. RSC Adv. 2015, 5, 9703–9708. [Google Scholar] [CrossRef]
- Candhadai Murali, S.P.; Samuel, A.S. Zinc ion conducting blended polymer electrolytes based on room temperature ionic liquid and ceramic filler. J. Appl. Polym. Sci. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Yang, C.C.; Yang, J.M.; Wu, C.Y. Poly(vinyl alcohol)/poly(vinyl chloride) composite polymer membranes for secondary zinc electrodes. J. Power Sources 2009, 191, 669–677. [Google Scholar] [CrossRef]
- Mahmood, Z.; Yameen, M.; Jahangeer, M.; Riaz, M.; Ghaffar, A.; Javid, I. Lignin as Natural Antioxidant Capacity. Lignin Trends Appl. 2018. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-based hydrogels: Synthesis and applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef]
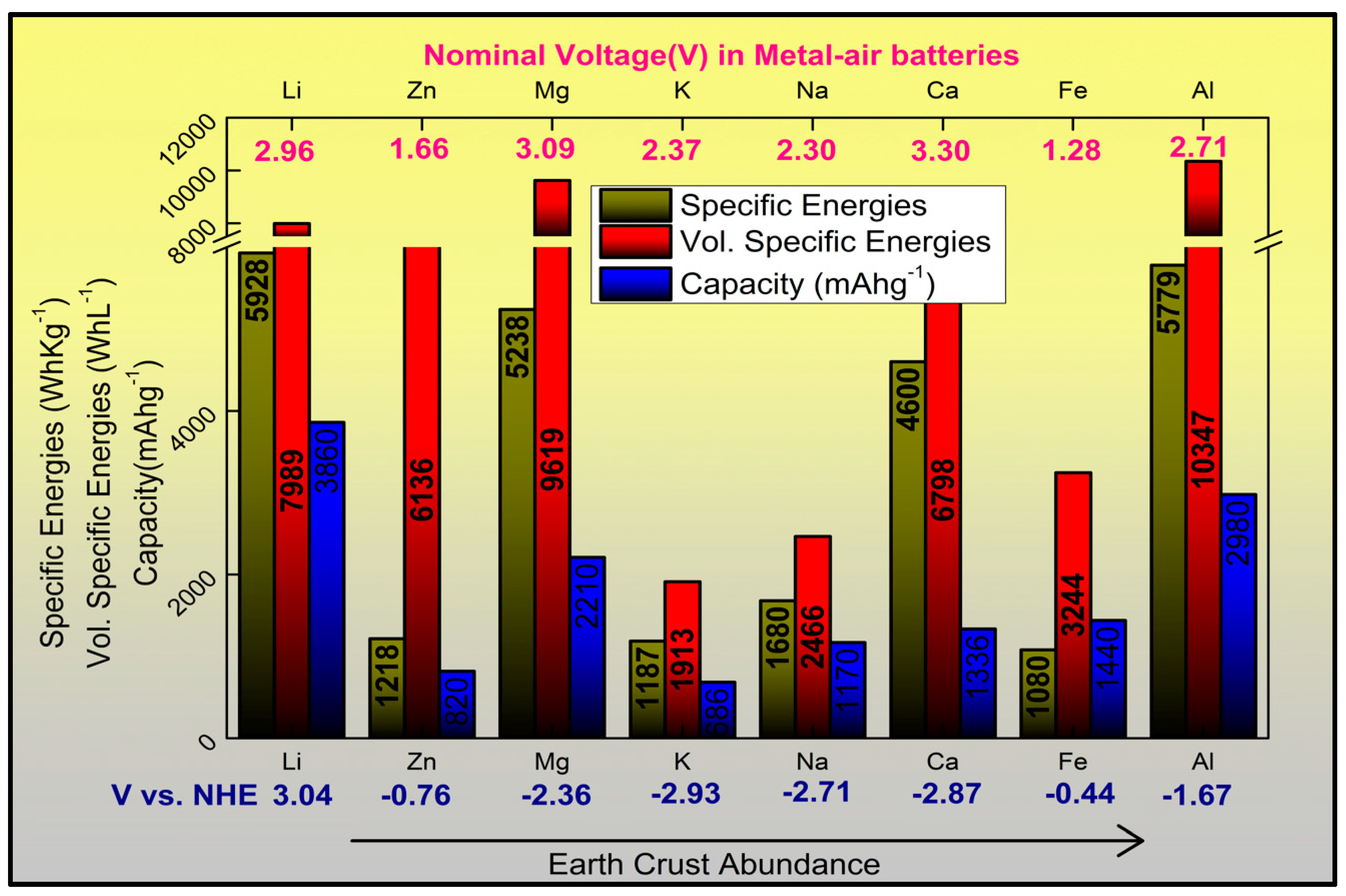
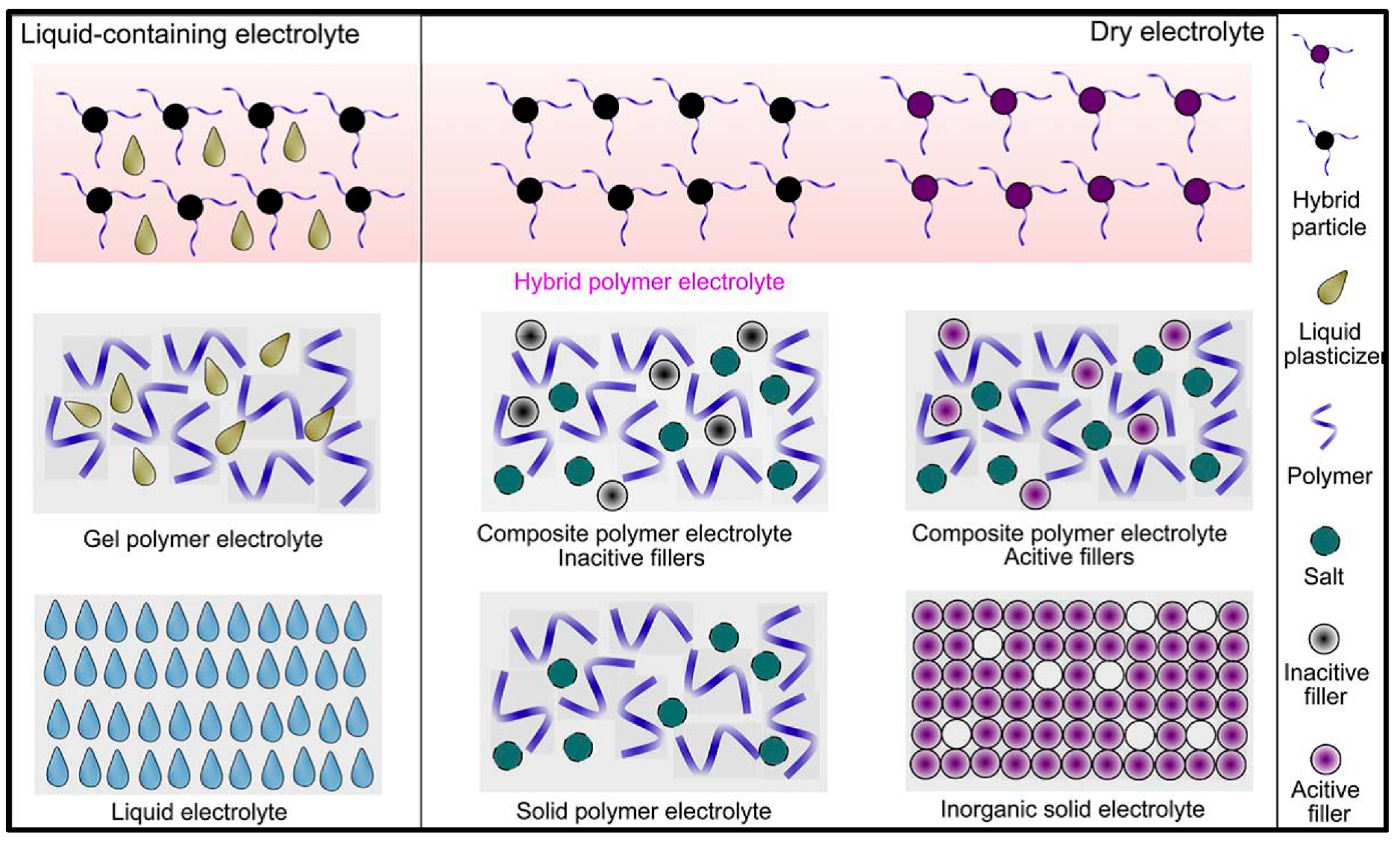

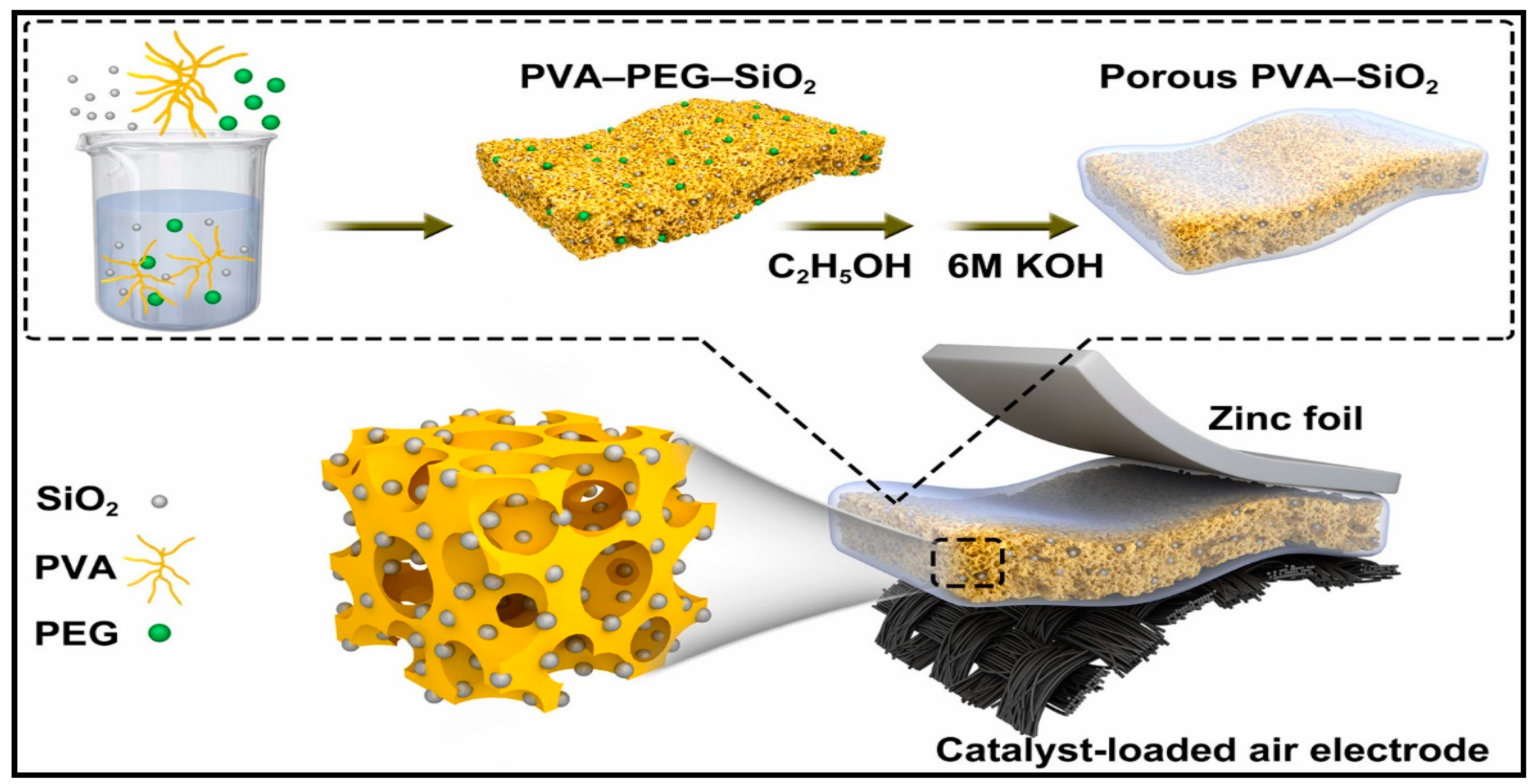
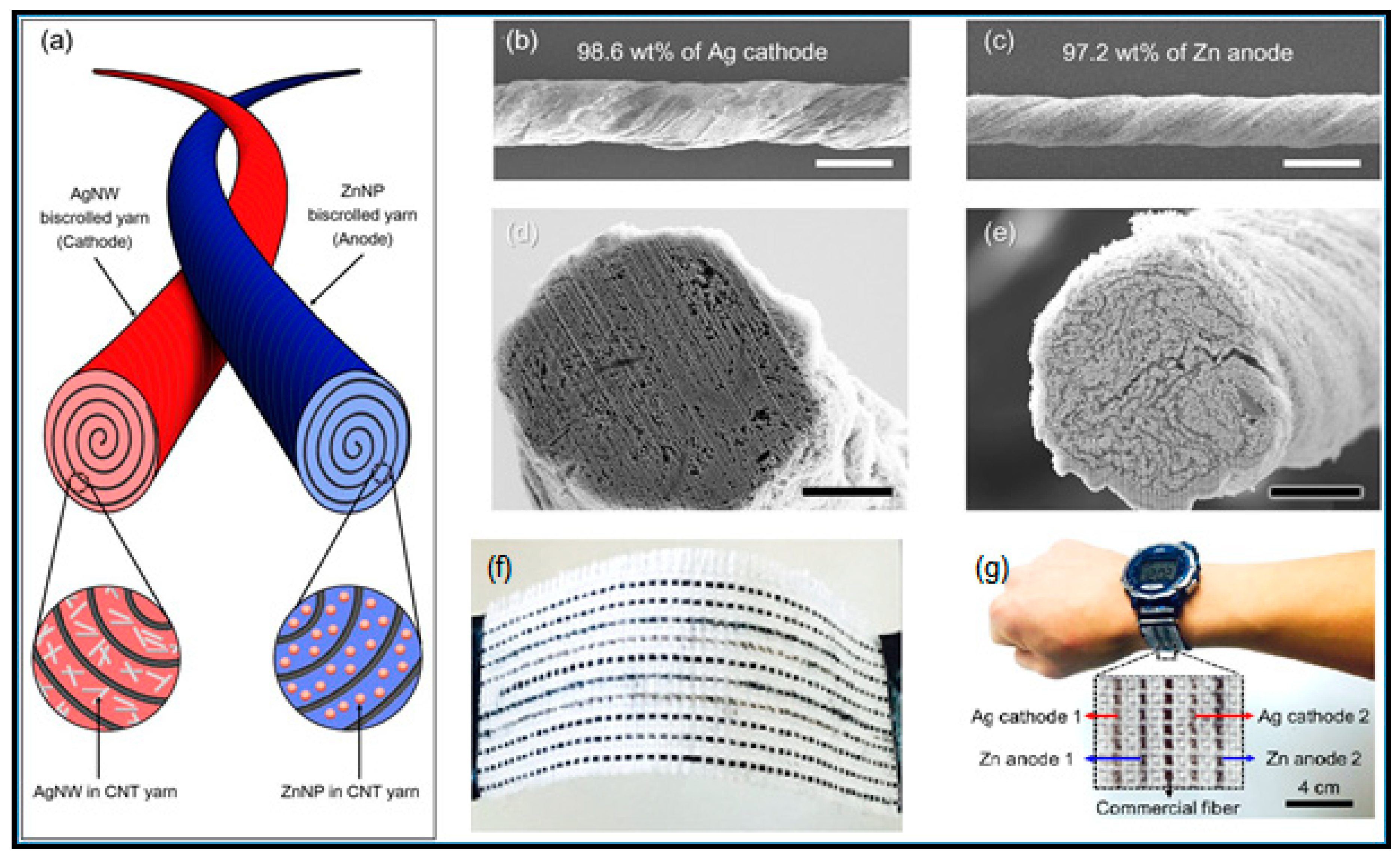

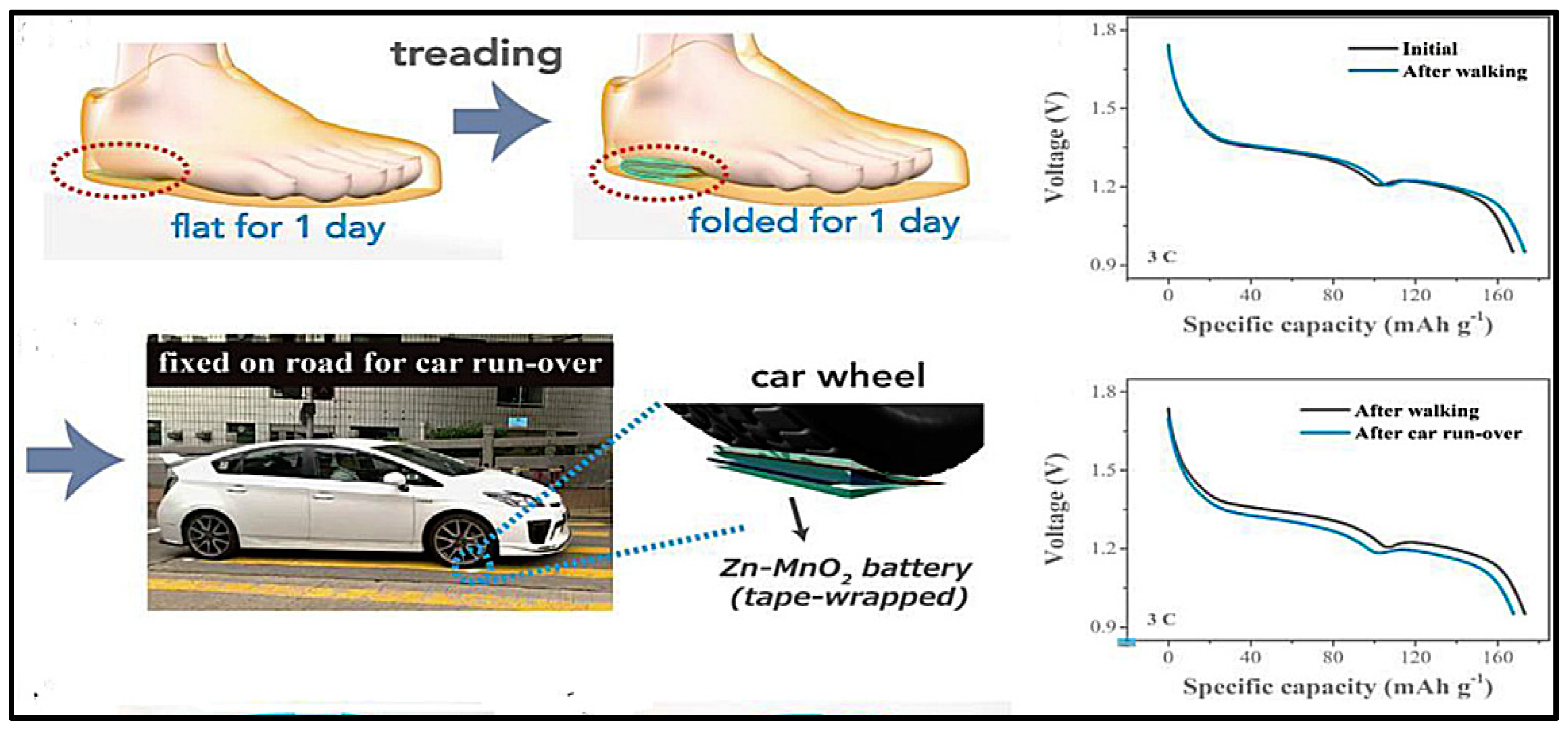

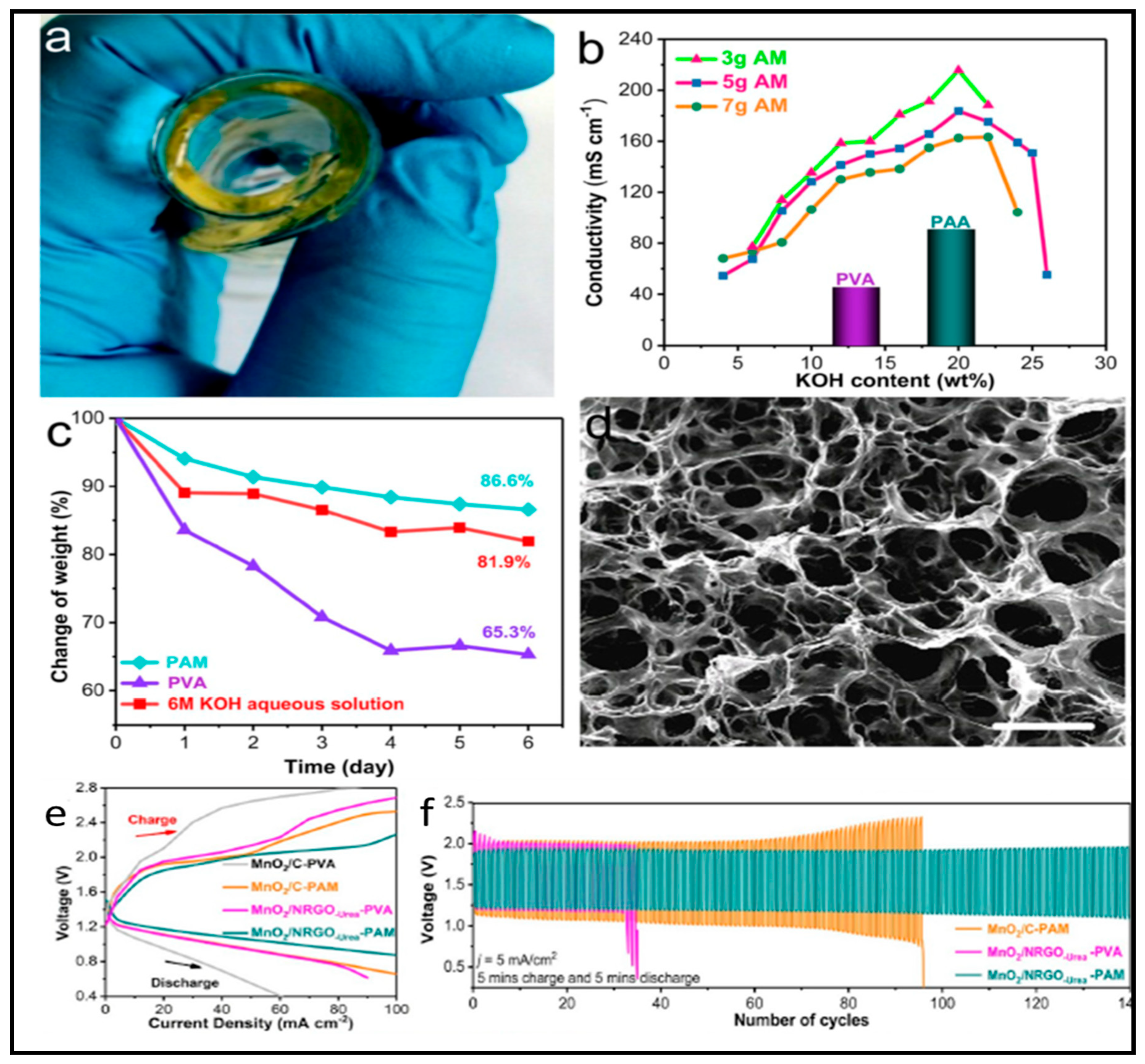
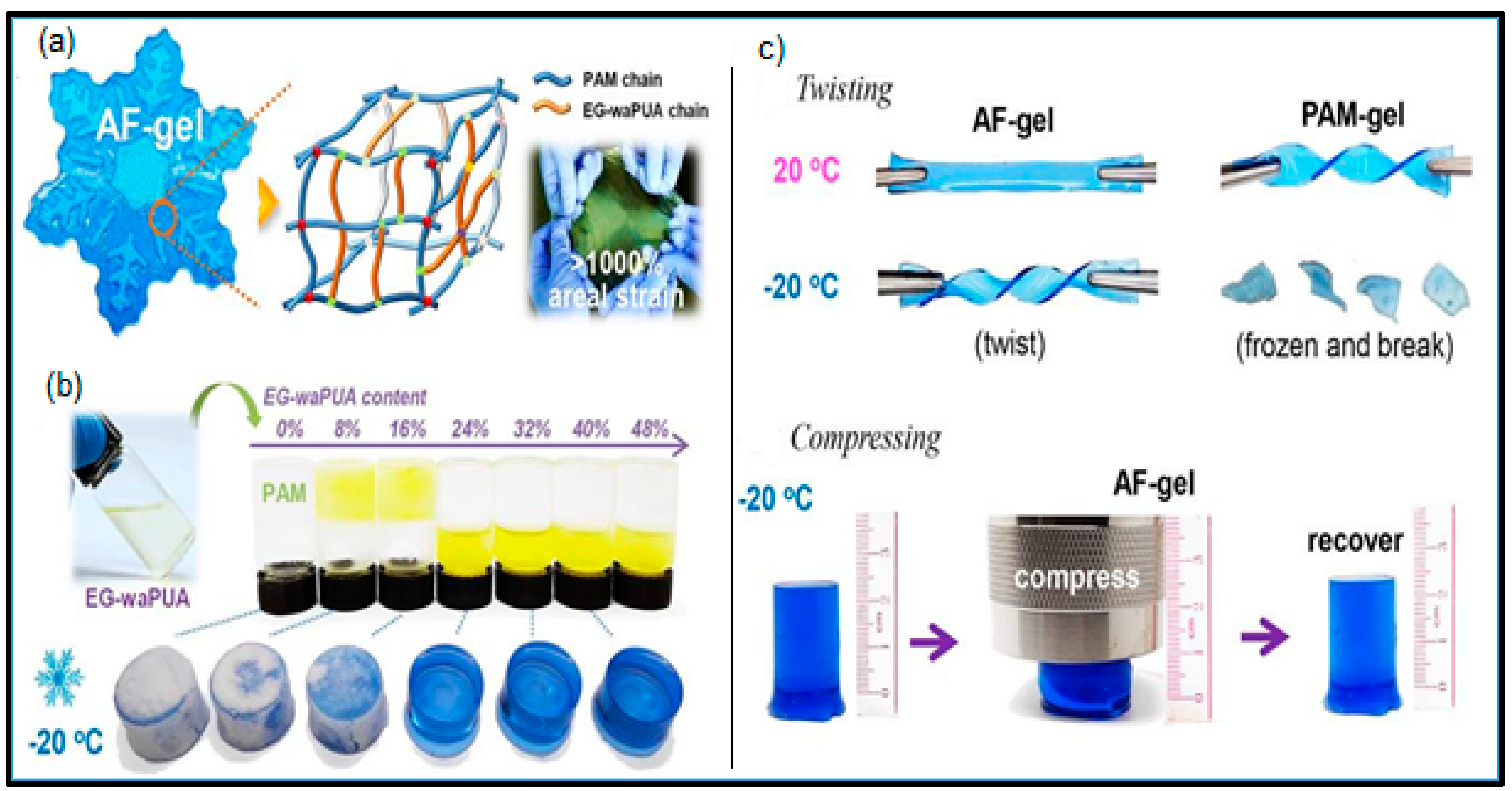

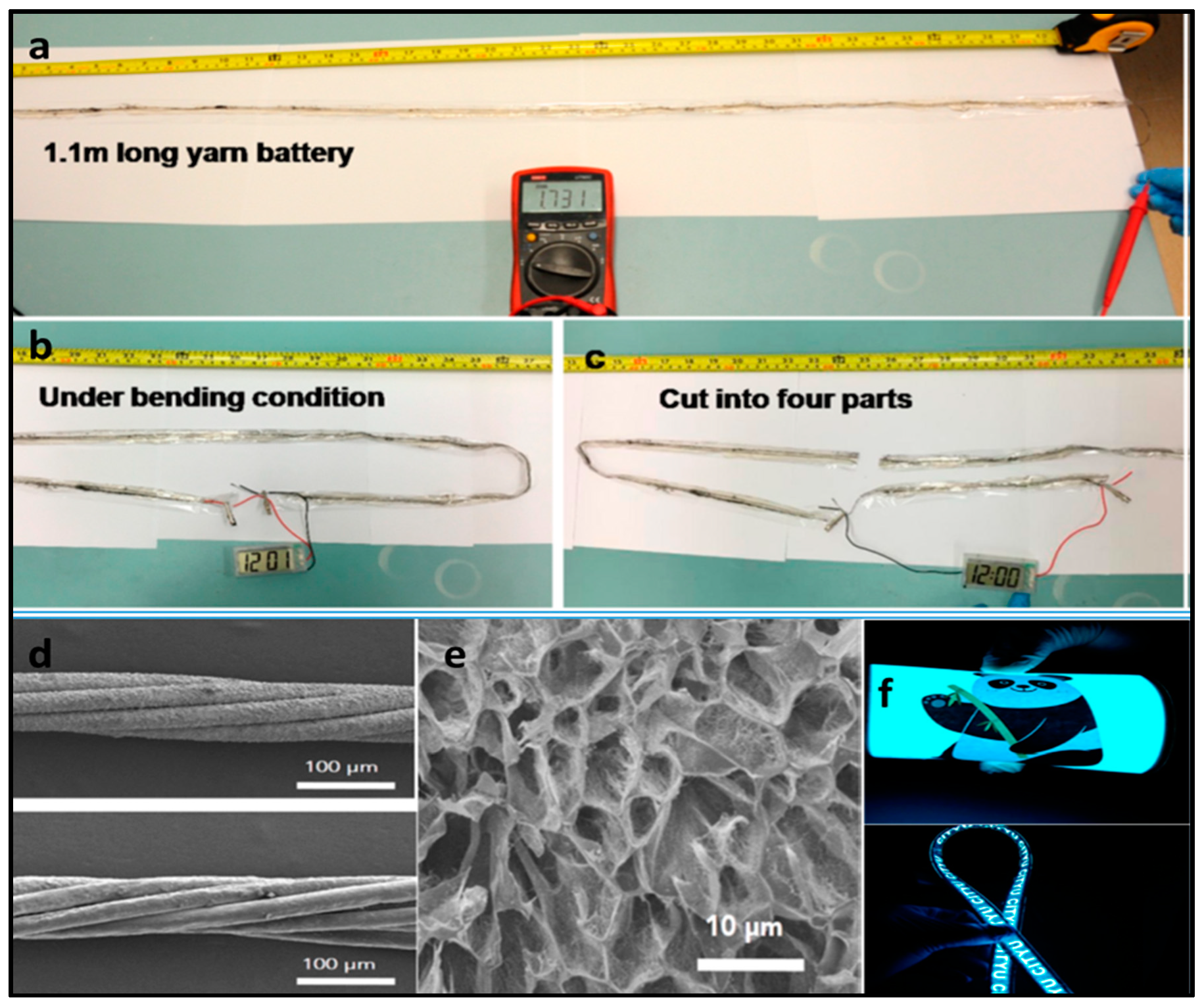

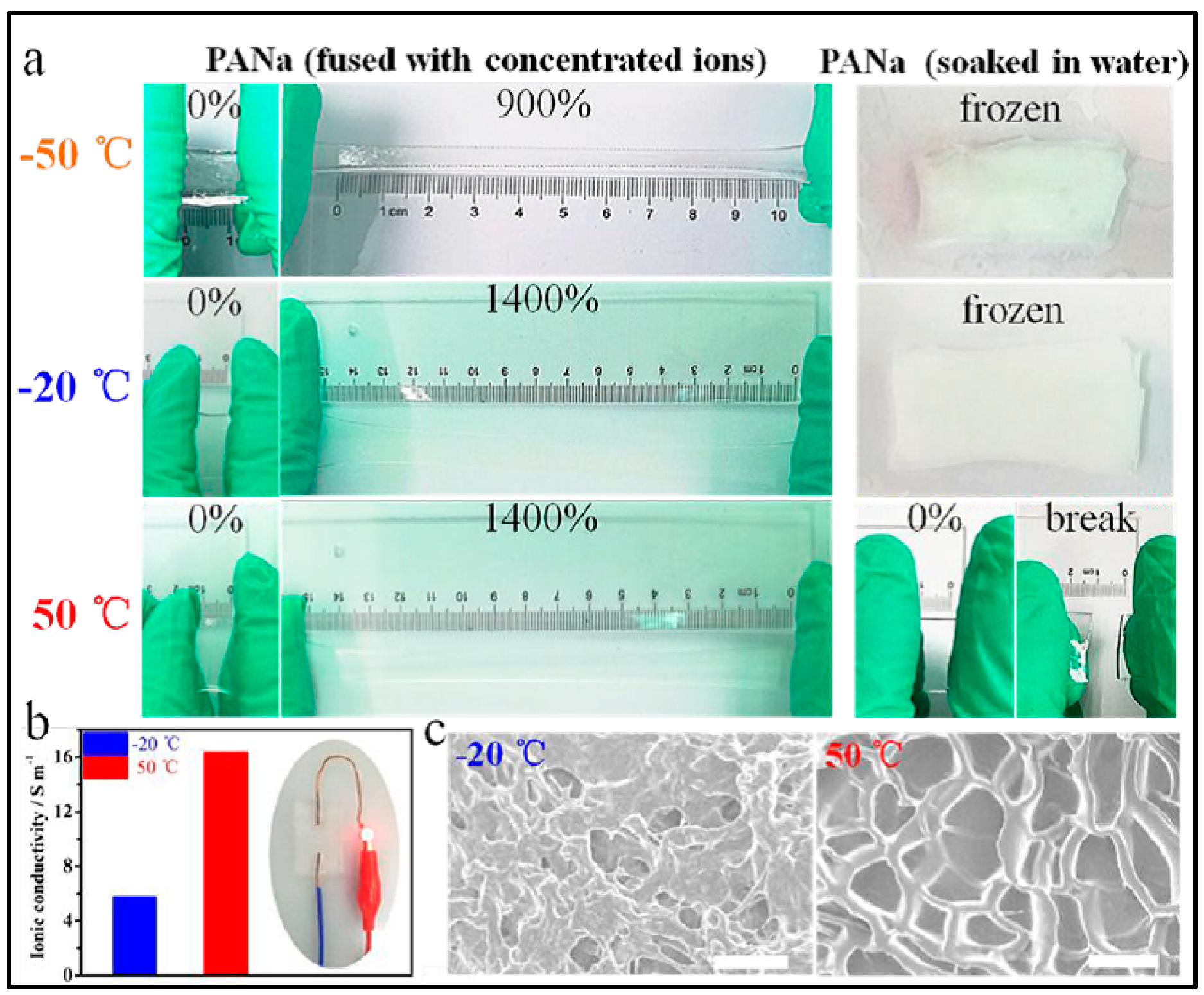
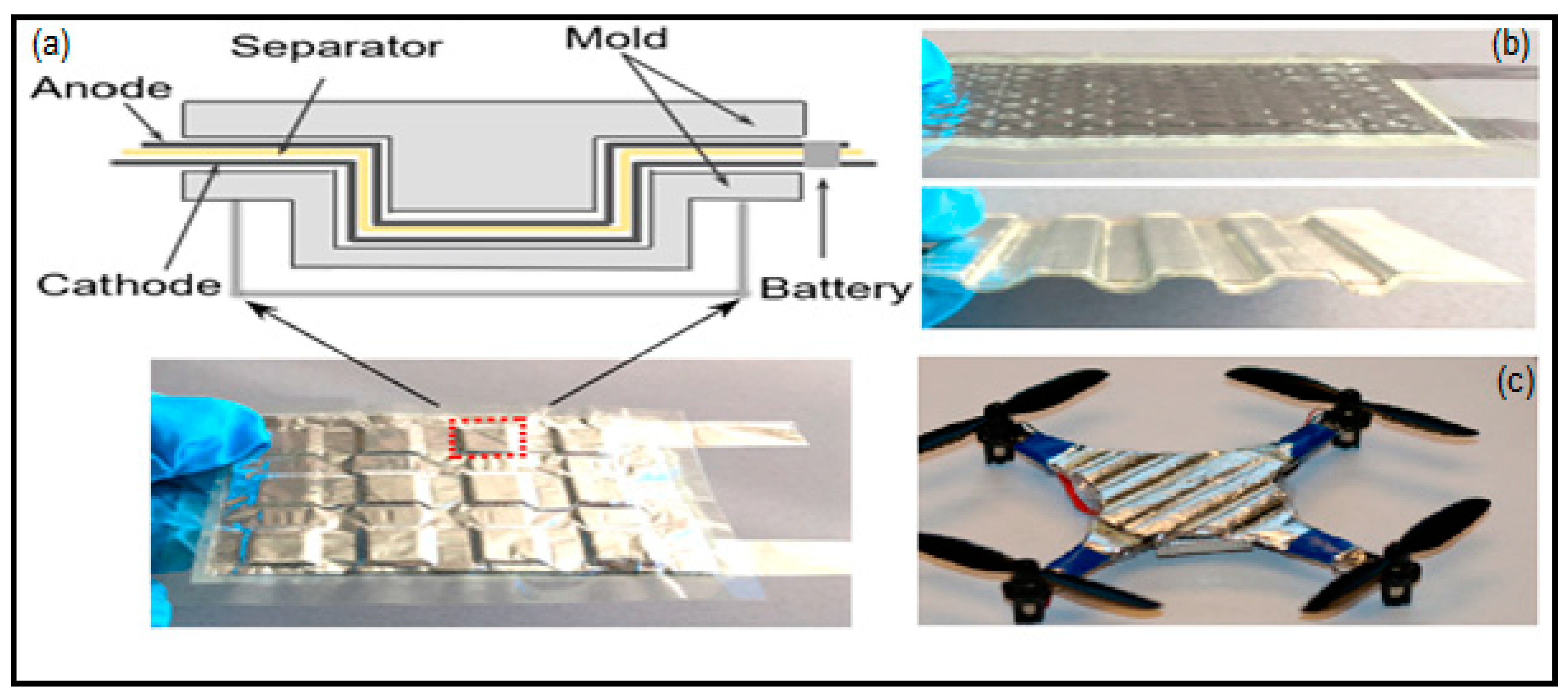
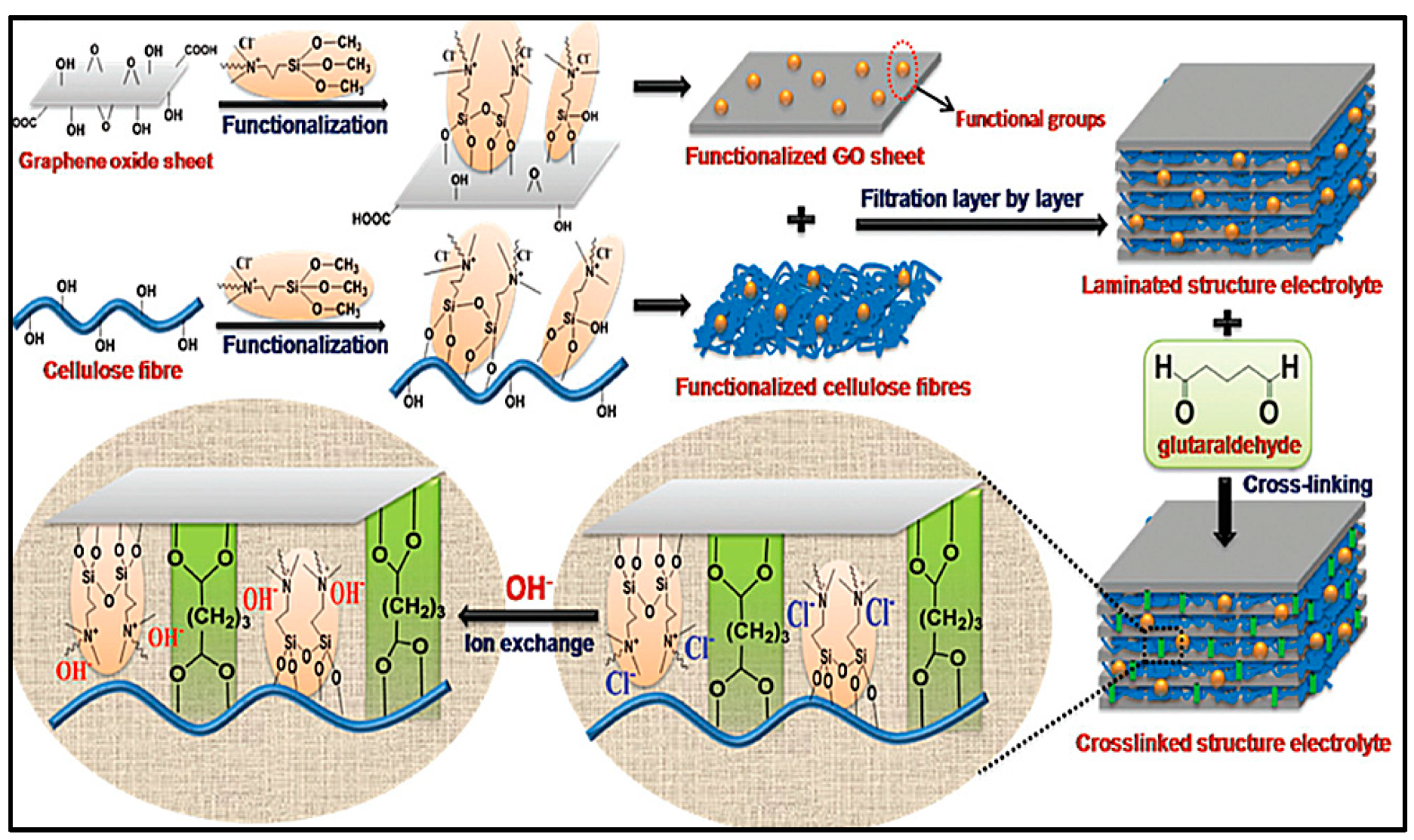
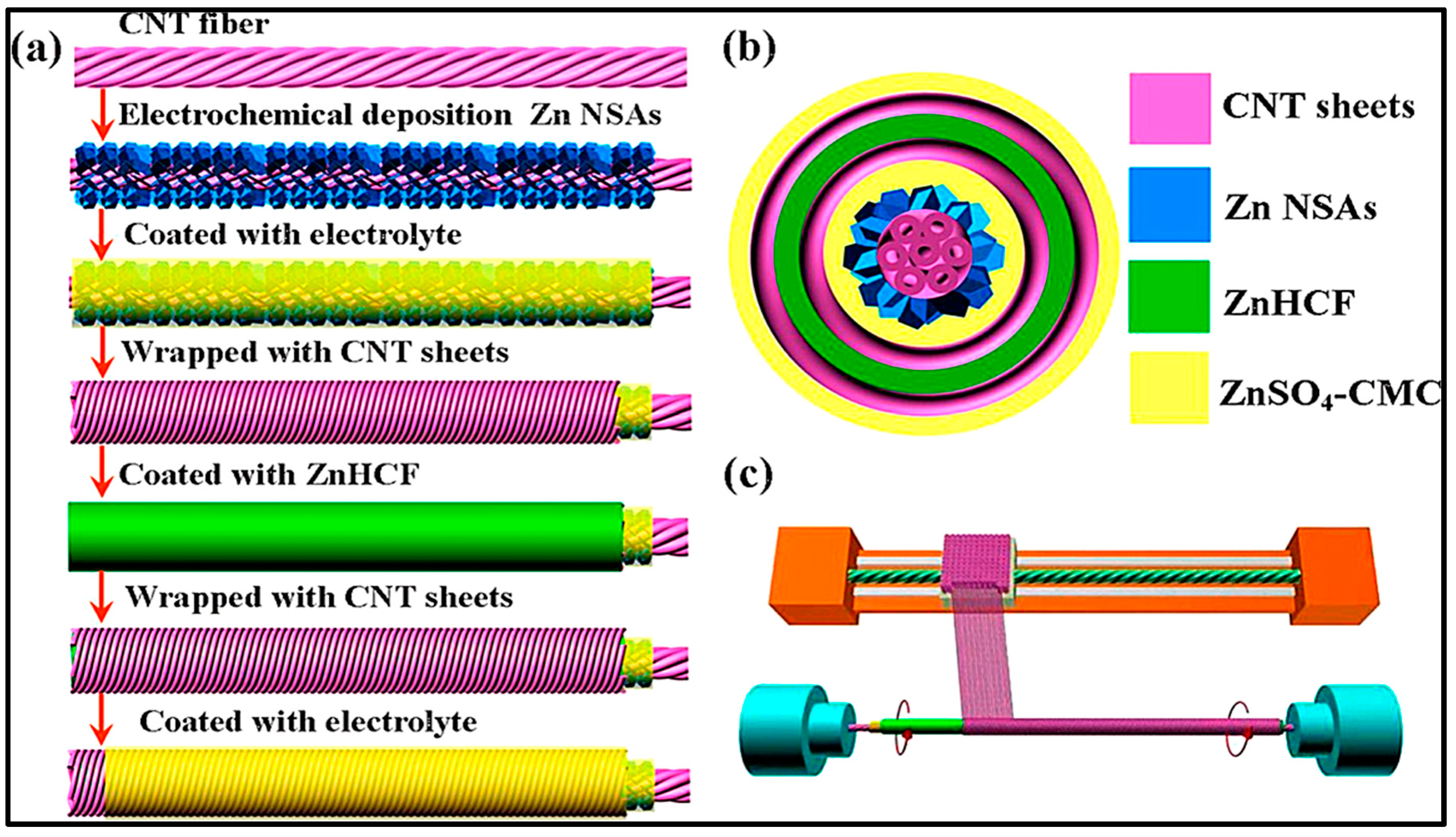
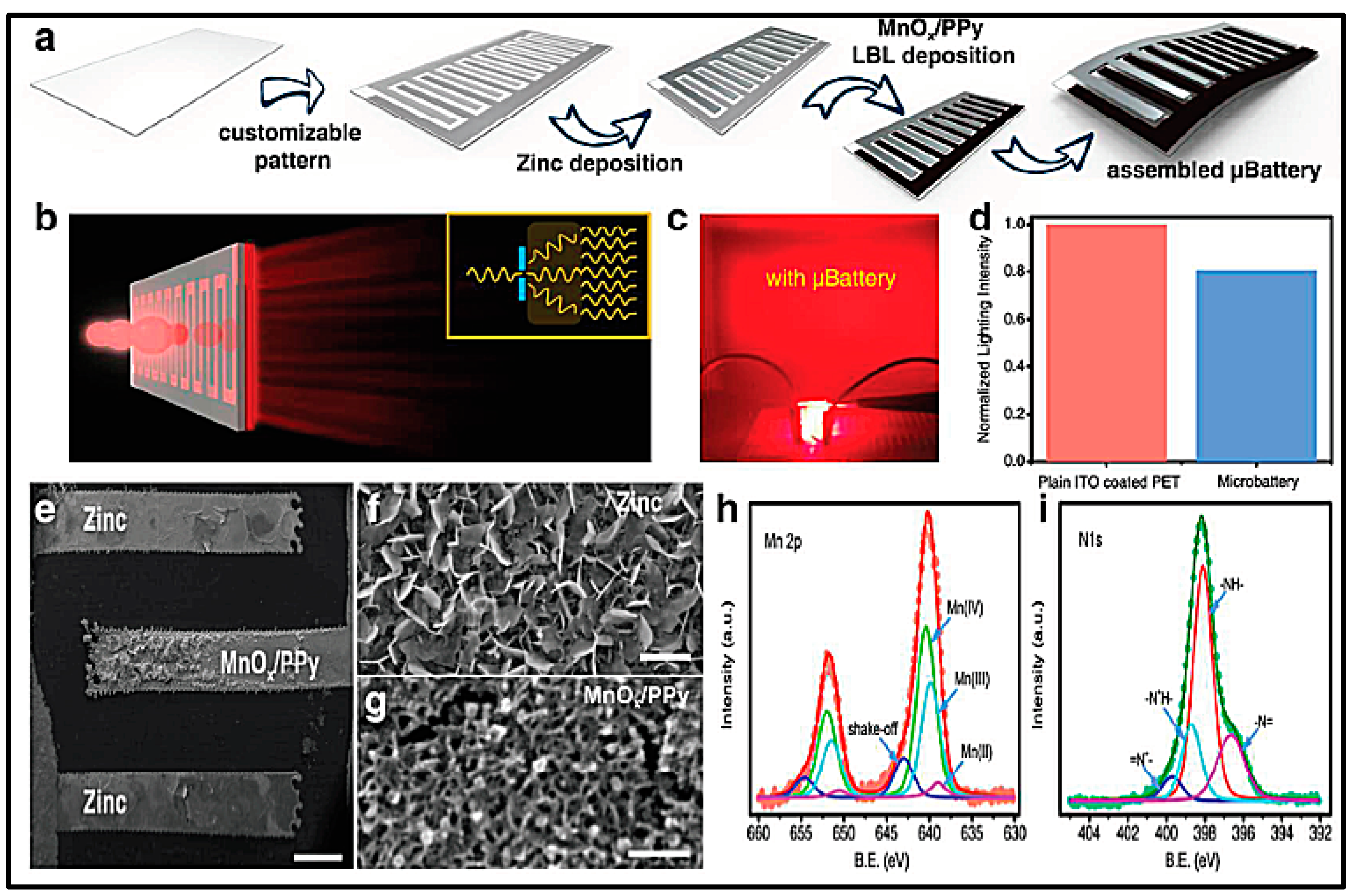
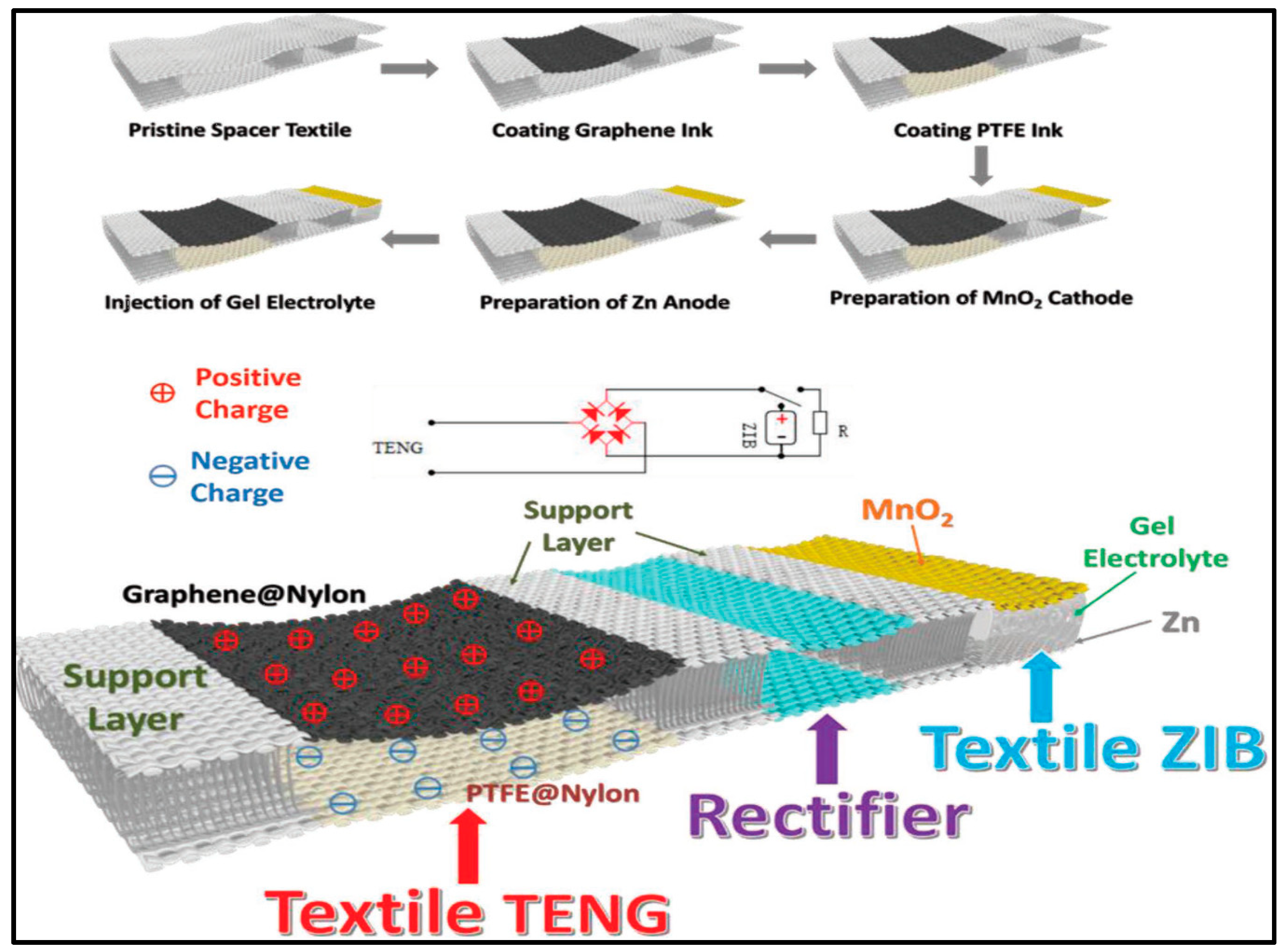
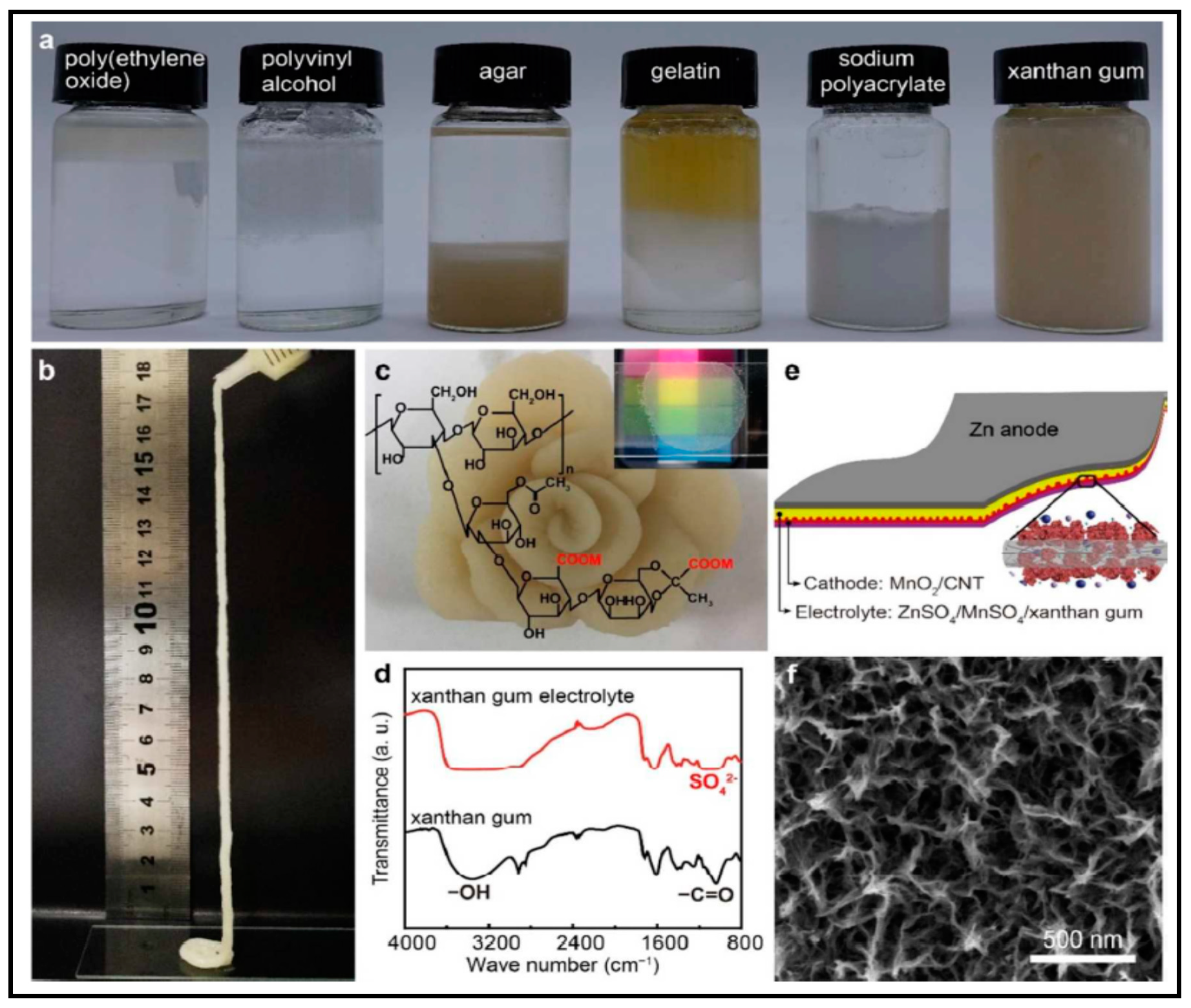

| Batteries | Capacity (Whkg−1) | Capacity (Whl−1) | Life Cycles | Voltage (V) | Commercially Available Batteries [25,26,27] | |
|---|---|---|---|---|---|---|
| Capacity (Whkg−1) | Capacity (Whl−1) | |||||
| Ni-Cd | 45–80 | 50–150 | 300–2000 | 1.2 | 48 | 122 |
| Ni-MH | 60–120 | 140–300 | 180–200 | 1.25 | 52 | 175 |
| Lead-acid | 33–42 | 60–110 | 200–300 | 2.1 | 25 | 85 |
| Li-ion | 160–300 | 250–693 | 400–1200 | 3.2–3.8 | 114–240 | 314–680 |
| Zn-ion | 80–120 | >500 | >1000 | 0.6–1.75 | - | - |
| Zn-Ag | 81–276 | 4–970 | <100 | 1.6 | 163 | 308 |
| Zn-MnO2 | 145 | 400 | >500 | 1.5 | 163 | 398 |
| Zn-Air | 200–250 | 270 | 100–200 | 1.0–1.2 | 442 | 1673 |
| Zn-Ni | 70–100 | 280 | 500 | 1.7 | 75 | 170 |
| Polymer | Monomer | Glass Trans. Tª (°C) [129] | Crosslinking Methods |
|---|---|---|---|
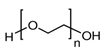 Polyethylene oxide (PEO) | –[CH2–CH2–O]n– | −64 | Physical crosslinking: UV radiation. |
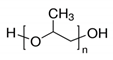 Polypropylene oxide (PPO) | –[CH2–CH(–CH3)O]n– | −60 | Chemical crosslinking: Polysiloxane. |
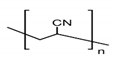 Polyacrylonitrile (PAN) | –[CH2–CH(–CN)]n– | 109 | Physical crosslinking: γ-rays radiation. Chemical crosslinking: Hydrazine |
 Polyvinylidenefluoride (PVdF) | –[CH2–CF2]n– | −34 | Physical crosslinking: UV radiation. Chemical crosslinking: Diamines, dithioles, etc. |
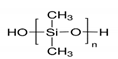 Poly(dimethylsiloxane) (PDMS) | –[(CH3)2–SiO)]n– | −127 | Chemical crosslinking: pentaerythritol-derived. |
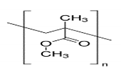 Poly(methylmethacrylate) | –[CH2C(–CH3)(–OOCH3)]n– | 105 | Chemical crosslinking: Ethyleneglycol dimethacrylate, 1,6-diaminohexane, etc. |
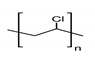 Poly(vinyl chloride) (PVC) | –(CH2CHCl)n– | 83 | Physical crosslinking: radiation. Chemical crosslinking: silanes, peroxides. |
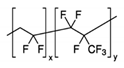 Poly(vinylidene fluoride-co- hexafluoropropylene) (PVdF-HFP) | –(–CH2CF2–)x–co– [–CF2CF(CF3)–]y– | −65 | Physical crosslinking: electrospinning., radiation Chemical crosslinking: diamines, carbamates.peroxides, etc. |
 Poly(vinyl alcohol) (PVA) | –[CH2CH(OH)]n– | 80 | Physical crosslinking: freezing/thawing, H-bonding. Chemical crosslinking: GA, borax. |
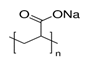 Sodium polyacrylate (PANa) | –(CH2CHCONH2)n– | - | Physical crosslinking: UV radiation. Chemical crosslinking: SiO2, FeCl3. |
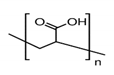 Polyacrilic acid (PAA) | –(C3H4O2)n– | 101 | Physical crosslinking: H-bonding. Chemical crosslinking: FeCl3, vinyl hybrid silica nanoparticles. |
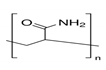 Polyacrylamide (PAM) | CH2=CHC(O)NH2– | 165 | Chemical crosslinking: (bisacrylamide, typically: N,N’-methylenebisacrylamide (MBA). |
 Poly(ε-caprolactone) (PCL) | –(C6H10O2)n– | −66 | Physical crosslinking: radiation. Chemical crosslinking: peroxides. |
| Polymer Matrix | Salts | Salts Conc. sk. (Soaked) | Ionic Conductivity (S/cm) | Ta (°C) | Mechanical Strength (MPa) | Ref. No. | Battery Type | Remarks |
|---|---|---|---|---|---|---|---|---|
| PVDF-HFP | Zn(TFSI)2/ ZnTf2 + IL EMIMTFSI | 0.2 M | 1.31∙10−3 | 25 | - | [138] | - | There is no visual difference between the membrane containing Zn salt and the membrane without Zn salt, which suggests homogeneous blending. Electrochemical stability window with anodic stability limit at 2.8 V vs. Zn2+/Zn and single phase behavior from −50 °C to 100 °C |
| PVDF-HFP | ZnTf2/ EMIMTf | 30% | 1.96∙10−3 | 30 | - | [139] | Zn-MnO2 | Inclusion of ILs inside the membranes is necessary to obtain GPEs with good electrical characteristics; poor results are obtained for IL-free GPEs. NMP residual is fundamental to rise the cation transport number |
| PVDF-HFP | ZnTf2/ TiO2 | 25%/ 5% | 3.4∙10−4 | 25 | - | [140] | Zn-ion | Cation transport due to the migration of Zn2+ ions has been enhanced, in the case of TiO2 up to 5 wt%, owing to the formation of a space charge region, which would provide an enhanced mobility of Zn2+ |
| PVDF-HFP/PEO | Zn(BF4)2/ [EMIM]BF4 | 25% | 16.9∙10−3 | 25 | 9.12 | [141] | Zn-ion | Reversibility and stability of metallic Zn in ILZE cell under fixed galvanostatic condition shows a dense and dendrite-free morphology, which contains no zinc oxides or Hydroxides(>3000 cycles). The solid hydrogen free Zn/cobalt ferricyanide battery shows over 30,000 cycles with 90% capacity retained at a ≈100% columbic efficiency and can work at wide temperature range of −20 to 70 °C. |
| PVA | LiCl/ZnCl2/ MnSO4 | 3 M LiCl/ 2 M ZnCl2/ 0.4 M MnSO4 | 0.897∙10−2 | / | / | [142] | Zn-MnO2 | The protective layer of PEDOT as well as the mild neutral electrolyte suppress the structural pulverization and dissolution of MnO2 and help the rechargeable Zn–MnO2 battery to exhibits a favorable capacity retention |
| PVA | KOH | 3 g | 30.3∙10−3 | 25 | - | [143] | Zn-air | Water plays a significant role in the transport of ions for the PVA–KOH GPE, which consists of a PVA polymer matrix and KOH aqueous electrolyte for mitigating ohmic polarization and promoting the resulting reaction kinetics |
| PVA | KOH | 12 M(sk.) | 0.34 | 20 | - | [51] | Zn-air | The Grotthuss mechanism significantly contributes to or is the central mechanism for hydroxyl anion transport through PVA-KOH channels. Confinement of Zn2+ close to Zn electrode by GPE makes necessary the OH– transport through the membrane as the only ionic species causing conductivity |
| PVA/TEAOH | KOH | 18 M | 30∙10−3 | - | - | [144] | Zn-air | Tetraethylammonium hydroxide (TEAOH) replaces KOH as the ionic conductor in the TEAOH−PVA electrolyte. A greatly improved shelf life of the TEAOH−PVA electrolyte is achieved compared to normal PVA-KOH. The special NR4+ structure of TEAOH is relatively stable because the positive charge of the TEA+ remains the same regardless of the pH of the environment. TEAOH is hygroscopic which binds the water in the polymer electrolyte more tightly. |
| PVA | KOH | 0.15 M | 3.33∙10–4 | - | - | [145] | Zn-air | Fiber-shaped zinc–air batteries are fabricated via a continuous method with a atomically thin mesoporous Co3O4 layers in situ coupled with N-rGO nanosheets as the bifunctional catalyst synthetized by high-yield method. The potential gap ∆E between Ej = 10 and E1/2 is generally used to assess the bifunctional activity of catalysts. The lower value of ∆E means better bifunctional activity. |
| PVA | KOH/ SiO2 | 6 M/5% | 57.3∙10−3 | 25 | - | [146] | Zn-air | Porous PVA-based nanocomposite GPE containing 5 wt% SiO2 exhibits good ionic conductivity and electrolyte retention capability as well as good thermal and mechanical properties. SiO2 contribute to the electrolyte retention property due to the presence of high levels of bound water in the GPE formed through SiO2. |
| PVA/GA | KOH | 2% | 15∙10−3 | - | - | [147] | Zn-air | Battery components can be prefabricated as sheets of customized shape and size to fit space and energy needs for a variety of applications. |
| PVA/PEO | KOH | 8.3% | 0.3 | 25 | - | [148] | Zn-air | PEO was added to improve the mechanical properties of the electrolyte (0.83%). Aligned carbon nanotube (CNT) as sheets are materials that show conductivities of 102–103 S∙cm−1 and high tensile strengths in the order of 102–103 MPa. The number of CNT layer and the way CNT sheet electrode in rolled around hydrogel determines the performance of the battery. |
| PVA/PAA | KOH | 32% (sk.) | 0.301 | RT | - | [149] | Zn-air | Ionic conductivity of the alkaline PVA/PAA polymer electrolyte membrane increased as the PAA content increased. |
| PVA/PAA/ NAFION | KOH | 6 M (sk.) | 6.6∙10−3 | RT | 39.4 | [150] | Zn-air | Nafion, can be suggested as a potential single cation conductor suppressing Zn(OH)42− crossover without deteriorating OH- conduction. Bicontinuous phases provide synergistic effects of blends. Into porous regions of the intermolecular condensed PVA/PAA nanofibres mat, Nafion is impregnated as an anion-repelling phase. |
| PVA/PAA/GO (PVAA–GO) | KOH/ KI | 4 M KOH/ 2 M KI (sk.) | 0.155 | - | 67 | [151] | Zn-air | I− anions were employed as a soluble reaction modifier additive to reduce the charging potential alleviating degradation of the carbon-supported catalyst electrode. Hydrogen bonds of PVA have been partially replaced by hydrogen bonds among PVA, PAA, and GO. |
| PVA | KOH | - | 10∙10−3 | - | - | [152] | Zn/Ag | Cathode architecture with silver nanoparticle ink embedded into the conductive thread. Void spaces in the dendritic Zn deposit make it more flexible than compact Zn. batteries with 10 wt% KOH demonstrate stable capacity with lower silver migration. |
| PVA | Zn(CF3SO3)2 | 2 M | 12∙10−3 | - | - | [153] | Zn-ion | Freezing/thawing of PVA forms more crystalline microdomains, which serve as cross-links to achieve a porous network structure with pore size of 50–500 nm. When Zn(CF3SO3)2 is incorporated, gel fraction of free-moving PVA chain segments attract each other re-establishing hydrogen bonds and healing the fracture. |
| PAAM/ Zn-Alginate | ZnSO4/ MnSO4 | 2 M ZnSO4 + 0.1 M MnSO4 | 43∙10−3 | - | - | [154] | Zn-MnO2 | Dual-crosslinked energy-dissipative hydrogel electrolyte endows the battery with outstanding flexibility and exceptional stability. Under heavy stress the covalently crosslinked PAAm network is deformed but maintain shape and strength of the hydrogel, whereas alginate network breaks, leading to effective energy dissipation. |
| PAAM | ZnSO4/ CoSO4 | 2 M ZnSO4/ 2 M CoSO4 | 0.12 | - | - | [155] | Zn/rich-Co3O4 | Highly reversible conversion reaction in Zn/Co(III) rich-Co3O4 system in mild aqueous electrolyte. The freeze-dried PAM hydrogel possesses interconnected macro-pores which allows ions to transfer freely in the electrolyte allow. Hence fast kinetic in the process of charge-discharge. High content of Co(III) in the Co(III) rich-Co3O4 leads to highly effective redox reaction. |
| PAAM PANa | ZnSO4/ KOH-Zn(Ac)2 | 2 M ZnSO4/ 6 M KOH+ 0.2 M Zn(Ac)2 | 0.12/ ≈0.15 | - | - | [156] | Zn-ion capacitor/Zn-air | U-shaped electrode is dual-functional with one part as a capacitor electrode and another part as an air electrode with two different electrolytes. The zinc-ion capacity maintains constant even overall 20,000 cycles. The high energy density of the zinc-air part could supply sufficient energy for zinc-ion capacitors. |
| PAM | KOH | 6 M(sk.) | 0.33 | - | - | [157] | Zn-air | The highly crosslinked PAM film was also found compatible with aqueous saline neutral pH GPE for flexible aluminum-air (Al-air) batteries. The optimal crosslinker (MBA) concentration was 0.2 mol% Catalyst powder was just simply spread onto PAM to form a catalyst layer in the side air-cathode. |
| PAM | KOH | 20% | 0.215 | - | - | [158] | Zn-air | The oxygen catalytic activity of MnO2/NRGO-Urea is much higher than that of MnO2/C and NRGO, suggesting the synergistic effect of MnO2 and NRGO |
| NFC/PAM | ZnSO4/ MnSO4 | 2 M ZnSO4/ 0.2 M MnSO4 (sk.) | 22.8∙10−3 | RT | - | [159] | Zn-MnO2 | With the addition of cellulose (NFC), a much larger and stable porous structure is formed. Sewing, enhance the shear tolerance of the battery. |
| EG-WAPUA/ PAM | ZnSO4/ MnSO4 | 2 M ZnSO4/ 0.1 M MnSO4 | 14.6∙10−3 | −20 | [160] | Zn-MnO2 | The alcohols molecules must be anchored onto the polymer chains through covalent bonds to form a stable unified matrix to get anti-freezing hydrogel electrolytes. Covalent cross-linking bonding and physical hydrogen-bonding endows the synthesized hydrogel with excellent flexibility. Water molecules connect hydroxyl groups of the EG- waPUA and carbonyl groups of PAM chains firmly locking water molecules disrupting the formation of water crystal lattices. | |
| PAAM | ZnSO4 | 1 M ZnSO4 | 5.56∙10−3 | - | - | [161] | Zn/ PANI | Vertically conducting PANI nanowires deposited on the CNT film with high crystallinity degree and uniform orientation, provides high electrical conductivity and substantial mechanical strength |
| PAM | ZnSO4/MnSO4 | 2 M ZnSO4/ 0.1 M MnSO4 | 1.73∙10−3 | RT | 0.27 | [162] | Zn-MnO2 | MnSO4 suppresses the dissolution of Mn2+ from MnO2 into electrolyte stabilizing the MnO2 cathode. Eco-flex silicone endows a superior waterproof performance to the flexible battery. Double-helix carbon nanotube (CNT) yarns are used as substrates. Roll-dip-coating and roll-electrodeposition continuous processes were used to produce the MnO2 yarn cathode and zinc yarn anode continuously. |
| PAA | KOH | 7.5 M | 0.36 | 65 | - | [163] | Zn-air | Solvodynamic radii of Zn(OH)42– calculated from the Stokes–Einstein equation had a range of values from 0.35 to 0.41 nm. For hydroxide ions, the thermochemical radius is 0.152 nm. Water retention is similar for PAA–KOH with 0.3, 0.5 and 0.7 mol% MBA. Higher crosslinking creates a denser network. When polymer structure then collapses, the network voids available for retaining water are minimized and water retention is decreased. Shape change on the Zn surface is also reduced with increasing MBA but ZAB cyclability is reduced. |
| PAA | KOH/ZnO | 8 M KOH/ Saturated | 55∙10−3 | - | - | [164] | Zn-MnO2 | Silver composite ink as current collector and nylon mesh embedded with electroactive inks show good flexibility avoiding cracks and delamination. The shear thinning behavior of the GPE can be used advantageously to allow printing with a lower pressure head |
| PANa | Zn(Ac)2/ KOH | 0.2 M Zn(Ac)2/ 6 M KOH | 0.17 | - | ≈0.15 | [165] | Zn/NiCo Zn–air | Electrostatic interactions between the acrylate ions along the PANa backbone and Zn2+ facilitated the formation of quasi-SEI eliminating zinc dendrites confirmed by SEM and TEM images of the Zn anode. PANa exhibited a tunable electrochemical performance depending on the concentration of water, OH− and/or Zn2+. The flexible quasi-SEI accommodated interface fluctuation during repeated charging/discharging without breakdown. |
| PANa | Zn(Ac)2/ KOH | 0.2 M Zn(Ac)2/ 6 M KOH(sk.) | 0.2 | - | - | [166] | Zn/NiCo | Resistance of CNT papers coated with Au foil drops from 31.1 to 0.8 Ω. Facilitating uniform electrodeposition of nickel cobalt hydroxide and zinc. PANa hydrogel was first pre-stretched to over 400% strain |
| PAA | Zn(Ac)2/ ZnO | 0.1–0.5 M Zn(Ac)2/ZnO | 0.28 | - | 0.047 | [167] | Zn-air | In aqueous electrolyte 6 M KOH, ZnO was found to be saturated at 0.5 M. The addition of 0.25 M ZnO reduces ionic conductivity of PAA–KOH. All the 0.25 M ZnO appears to be Zn(OH)42– in a highly alkaline solution (~6.5 M KOH). ZnO to reduce water activity and Zn corrosion. |
| P-(AM-co-AA) | Zn(Ac)2/ KOH | 0.2 M Zn(Ac)2/ 6 M KOH (sk.) | 0.148 | - | 0.052 | [168] | Zn-air | (Fe3C@N-doped carbons) as bifunctional non-noble-metal electro- catalyst at the air electrode with highly ordered graphitized structure. Abundant carboxylic acid groups (PAA) hinder packing of polymer chains, endowing PAA with amorphous properties. Silicone encapsulation slows down losses of water locked by GPE. |
| PAAK | KOH | 7.3 MKOH | 0.6 | 25 | - | [169] | Zn/Ni | The high solubility of Zn(OH)42− in alkaline solution results in the shape change of the zinc electrode, and the poor charge–discharge characteristics. |
| PANa | Zn(Ac)2/ KOH | 0.2 M Zn(Ac)2/ 6 M KOH (sk.) | 0.12 | 24 | - | [170] | Zn/Ni | PANa hydrogel soaked by concentrated ions can be easily stretched to 1400% in both cold (−20 °C) and hot (50 °C) environments. Concentrated ions reduce the freezing point of the hydrogel. Different ionic conductivity at different temperatures is substantiated by their microstructure, at −20 °C fewer micropores are observed and the micropores are smaller |
| PEO(H2O) | KOH | 30% | 5–10∙10−4 | RT | - | [171] | Zn/Ni, Zn/Cd | Conductivity log(s) decreases almost linearly with 1000/T temperature, an Arrhenius-type behavior, which is often observed for semi-crystalline polymer. The presence, at high O/K ratios, of diffraction peaks not observed in the PEO nor in the KOH, suggests the existence of a different crystalline entity |
| PEO/ PVA(H2O) | KOH | - | 4–5∙10−2 | RT | - | [172] | Zn-air | The surface morphology of film has a micro-porous structure consisting of many small pores with a dimension of about 0.1–0.2 µm. The cell with the solid polymer electrolyte has higher capacity and higher use of zinc on account of the much smaller pore size compared to PE/PP and cellulose separator. |
| PEO/PVdF | Zn(Ac)2/EMIMTFSI | 15% Zn(Ac)2/7% EMIMTFSI | 1.63∙10−4 | RT | - | [173] | - | PEO (90 wt%)/PVdF (10 wt%)]—15 wt% Zn (CF3SO3)2 IL 7 wt% (i.e., best conducting sample) shows by SEM the presence of uniformly distributed spherulites with numerous dark boundaries. Conductivity dependence vs. temperature exhibit curved plots, which is strongly associate with the segmental motion of polymer chain |
| PEO/PVDF-HFP/EC/PC | Zn(Tf)2/ ZnO | 1 M Zn(Tf)2/ 10% | 3∙10−3 | 30 | - | [174] | - | Raman shows that ZnO nanoparticles are present in the gelled polymer matrix as a separate phase. The temperature dependence σ vs. 1/T of the ionic conductivity of nanocomposite films showed VTF behavior. The gel electrolyte system EC–PC–Zn(Tf)2 immobilized in PVdF-HFP offers an acidic character. |
| PE/PVDF-HFP/EC/ PEGDME | Zn(Tf)2/ Zn(TFSI)2 | 0.5 M Zn(TFSI)2 | 4.7∙10−4 | 25 | - | [175] | - | (PEGDMEs), methyl capped short-chain PEO possess excellent thermal and chemical stability. Blending PEGDME with a small amount of EC has large beneficial effects on the ionic conductivity. It is likely that a solid electrolyte interface (SEI) film forms at the surface of zinc, as in the case of lithium |
| PEO-PPO-PEO Pluronic Hydrogel (PHE) | ZnSO4/ Li2SO4 | 0.25 M ZnSO4/ 0.25 M Li2SO4 | 6.33∙10−3 | 25 | - | [176] | Zn/LMO Zn/LFP | Perfect wetting of the electrodes, especially in the low temperature range. High initial open-circuit voltage (1.60 V) of the Zn/LMO cell, close to the thermodynamic voltage (1.71 V). Li+—intercalation/de-intercalation processes on the cathode side upon cycling. After crack due to bending or By a cooling-recovery procedure, PHE reversibly turned into its fluid phase rewetting the electrode in situ in 5 min. |
| Starch | KOH | 6 M | 4.34∙10−3 | RT | - | [177] | - | - |
| Cellulose/PAA/ gelatin | KOH | 0.4% | 0.097 | RT | - | [178] | - | Pristine NFC hydrogel had 10−6–10−7 S/cm−1. With KOH NFC hydrogel became brittle, and not stable. Adding PAA and gelatin, more stable GPEs are attained. |
| QA-functionalized nanocellulose/ GO | KOH | 1 M (sk.) | 58.8∙10−3 | 70 | - | [179] | Zn-air | According to the XRD results and activation energies, two types of ion transport including Grotthuss mechanism and vehicle mechanism exist. The water uptake of the QAFCGO membrane and performance stability in a zinc-air battery is higher than those of the A201 membrane. |
| Cellulose nanofibres | ZnCl2/ NH4Cl | 2 M ZnCl2/ 3 M NH4Cl | 16.4∙10−3 | - | - | [180] | Zn-ion | Graphite papers as flexible substrates for anodes. Superior battery performance related to the nanostructured PANI grown on lens paper. The nanostructured PANI facilitates electron and ion transport as well as its use during electrochemical process. |
| Functionalized DMOAP Cellulose nanofibres | KOH | 1 M | 21.2∙10−3 | - | - | [181] | Zn-air | Superior hydroxide-ion conduction and water retention of the membrane as well as low anisotropic swelling. Improved cycling stability of the battery, compared to commercial alkaline AEM (A201). Self-purging of carbonates in zinc-air batteries in CO2 rich atmosphere. |
| BC/PVA | Zn(Ac)2/ KOH | 6.0 M KOH/0.2 M Zn(Ac)2 | 80.8∙10−3 | RT | 0.951 | [182] | Zn-air | High crystallinity, high purity, and high hygroscopicity of bacterial cellulose (BCs). Load-bearing percolating dual network, these BC/PVA composite membranes exhibit superior mechanical strength and toughness. |
| Gelatin/Borax | ZnSO4/ MnSO4 | 4∙10−2 mol ZnSO4 4∙10−3 mol MnSO4 | 20∙10−3 | - | - | [183] | Zn-ion | Shape memory wire battery Zn-ion battery (SMWB) using Nitinol (NT). Twined yarns of SS/MnO2/PANI and Nitinol/Zn. Gelatin/Borax GPE showed similar performance respect to the liquid one. |
| Gelatin | KOH | 0.1 M | 3.1∙10−3 | - | - | [184] | Zn-air | Nonprecious metal catalyst (NPMC) based on silk fibroin for metal/nitrogen/carbon (M/N/C) with high catalytic activity for the ORR. Cable-type flexible ZAB with a spiral zinc anode. |
| κ-carrageenan/ rice paper | ZnSO4/ MnSO4 | 2 M ZnSO4/ 0.1 M MnSO4 | 33.2∙10−3 | RT | - | [185] | Zn-ion | After 300 bending cycles, 95% capacity was retained. |
| PNiPAM/CMC | Zn(Tf)2 | 0–30% | 0.17∙10−3 | RT | 35.6 | [186] | Zn-ion | PNiPAM suppress dendrite formation. Most CMC/PNiPAM blend exhibited better mechanical properties than that of CMC. Thermo-responsive macromolecular transition from a hydrophilic to a hydrophobic structure at 30–35 °C |
| PVA/chitosan/ EC | NH4NO3 | 40% | 1.6∙10−3 | RT | - | [187] | Zn/H+ battery | Conductivity–temperature plot is Arrhenian type. Proton battery. ZnSO4 added to zinc anode. |
| Chitosan/PDDA/GA | KOH | 2 M | 24∙10−3 | RT | 25.30 | [188] | Zn-air | Glutareldehide crooslinking. Good stability in 8 M KOH at 80 °C. Used in fuel cell, capacitor, and zinc-air battery. |
| Guar ammonium salt/GA/PCL | KOH | 2 M | 0.123 | 90 | [189] | Zn-air | Low anisotropic swelling degree, outstanding mechanical strength, and excellent thermal stability. Binary cross-linking strategy to prepare highly conductive alkaline anion polymer electrolyte. Discharge time and capacity are superior to that the A201 membrane | |
| Gelatin/ NaAlginate/GA (GAME) | ZnSO4 | 2 M ZnSO4 | 3.7∙10−2 | 2.14 | 2.14 | [190] | Zn-ion | Good interfacial contact between electrodes and GAME. 3D cross-linked IPN network with rich functional groups provides good ionic conductivity. Elasticity and toughness for a better resistance to Zn dendrite attack. |
| Poly ε-caprolactone | Zn(Tf)2 | 25% | 8.8∙10−6 | 25 | - | [191] | Zn-MnO2 | PCL (polycaprolactone) becomes a smoother one due to the addition of salt as observed from SEM. Good electrochemical stability window of 3.7 V with an excellent reversibility |
| PAN/PC/EC | Zn(Tf)2 | ≈0.6 M | 2.7∙10−3 | 27 | - | [192] | Zn-ion | The mass ratio of (PC-EC) to PAN is approximately 5:1. The logσ vs. 1/T relationship follows an Arrhenius-type behavior at all compositions. |
| Silica | Li2SO4/ ZnSO4 | 2 M Li2SO4/ 1 M ZnSO4 | 60∙10−3 | RT | - | [193] | Zn/LiMn2O4 | The required quantities of silica materials are in the range of 4–15 wt%. 10–12% higher cyclability compared with the performance of the batteries using the conventional liquid. |
| Fumed silica | ZnSO4 | 2 M ZnSO4 | 8.1∙10−3 | RT | - | [194] | Zn-ion | Zn dendrite growth is thoroughly eliminated. |
| Gelatin/PAM | ZnSO4/ MnSO4 | 2 M ZnSO4/ 0.1 M MnSO4 | 1.76∙10−2 | RT | 7.76 | [12] | Zn-ion | Flexible ZIB can be tailored to any desired shape to meet the demands of high-level integration. ZIB did not catch fire even after being exposed to fire for more than 5 min. Reliable power source that can work under a variety of severe conditions, such as being bent, hammered, punctured, cut, sewed, washed in water, and set on fire |
| Gelatin | ZnSO4/ MnSO4 | 2 M ZnSO4/ 0.1 M MnSO4 | 5.68∙10−3 | RT | 1.25 | [12] | ||
| Gelatin | ZnSO4/ Li2SO4 | 0.5 M ZnSO4/ 0.5 M Li2SO4 | 6.1∙10−3 | - | 0.11 | [87] | Zn/LiMn2O4 | In situ coating of the GHE on the electrodes. Solidifies into a strong film, provides mechanical strength to suppress Zn dendrite formation. Fast cooling rate leads to more orderly localized gelatin molecules and a more robust electrolyte |
| Xanthan gum | ZnSO4/ MnSO4 | 3 M ZnSO4/ 0.1 M MnSO4 | 16.5∙10−3 2.5∙10−3 | −8 °C | - | [64] | Zn-ion | A high-salt tolerant, water-soluble polysaccharide. Conductivity at −8 °C, suggesting its ability of working at low temperatures. Gum electrolyte has a long-term stability. |
| Polymer/Average MW (kDa) | |
|---|---|
Chitosan/100–800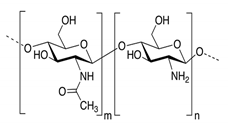 | κ-Carrageenan/200–800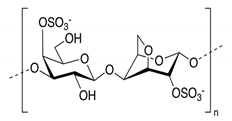 |
Carboxymethyl cellulose sodium salt/250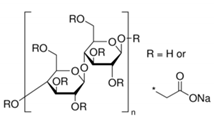 | Sodium Alginate/900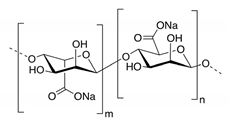 |
Gelatin/20–220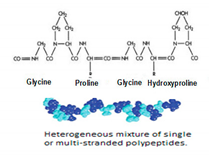 | Cellulose/200–20,000 |
Lignine/600–180,000 | Xanthan gum/2000 |
Guar gum/50–8000 | Agar-Agar/8–100 |
Starch/300 | Methyl cellulose/10–220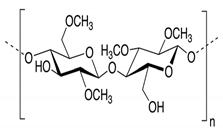 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorca, S.; Santos, F.; Fernández Romero, A.J. A Review of the Use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles. Polymers 2020, 12, 2812. https://doi.org/10.3390/polym12122812
Lorca S, Santos F, Fernández Romero AJ. A Review of the Use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles. Polymers. 2020; 12(12):2812. https://doi.org/10.3390/polym12122812
Chicago/Turabian StyleLorca, Sebastián, Florencio Santos, and Antonio J. Fernández Romero. 2020. "A Review of the Use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles" Polymers 12, no. 12: 2812. https://doi.org/10.3390/polym12122812
APA StyleLorca, S., Santos, F., & Fernández Romero, A. J. (2020). A Review of the Use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles. Polymers, 12(12), 2812. https://doi.org/10.3390/polym12122812






