3.1. ZnO–Vermiculite–Chlorhexidine Nanofiller Characterization
Natural Mg–vermiculite (V), determined to have narrow monomodal particle size distributions, with a mean diameter
d43 = 13.48 µm, specific surface area (SSA) of 32.02 m
2·g
−1 and ξ-potential value of −60.0 mV, was used as the starting material. The V particles had irregular shape with a smooth surface and round and deformed edges (
Figure 1). The structural formula of the V sample was calculated on the basis of elemental chemical analysis: (Si
6.32Al
1.58Ti
0.1) (Mg
4.75Ca
0.34Fe
0.91) O
20 (OH)
4 (Ca
0.04 K
0.38).
The ZnO/V nanocomposite particles exhibited homogenous ZnO nanoparticle growth on the vermiculite particle’s edges and surfaces (
Figure 1). The agglomeration rate of the ZnO/V nanocomposite particles was confirmed with a value of the surface charge, ξ-potential value, of −20.6 mV. On the basis of the particle size analyses, it was found that the sonochemical process and heat treatment caused a reduction in the V particles with diameter d
43 = 9.07 µm and with SSA = 20.23 m
2·g
−1. From the elemental chemical analysis, the presence of 21.09 wt% of the ZnO nanoparticles in the ZnO/V particles was confirmed.The ZnO/V_CH nanocomposite particles were formed by the vermiculite particles with a very smooth surface and sharp edges, at the ends of which ZnO nanoparticles were preserved (
Figure 1). The ZnO/V_CH particles exhibited narrow monomodal particle size distributions, with mean diameter d
43 = 10.97 µm, an SSA of 24.30 m
2·g
−1 and a ξ-potential value of +23.5 mV. The ultrasound intercalation of CH led to a decrease in ZnO composition from 21.09 wt% (ZnO/V) to 16.33 wt% (ZnO/V_CH), which was supported by the organic carbon content having the value of 27.47 wt%.
X-ray diffraction (XRD) analysis confirmed the successful preparation of the ZnO/V and ZnO/V_CH nanocomposite samples. The XRD pattern of V (
Figure 1) shows that the vermiculite phase (ICDD PDF card no. 01-074-1732) at
2θ = 6.09°, 19.21°, 27.16°, 33.19° and 60.11° corresponds to
d-values of 1.449, 0.461, 0.328, 0.263 and 0.154 nm, and an admixture phase of tremolite (ICDD PDF card no. 00-013-0437) at
2θ = 10.52° and 28.46° corresponds to
d = 0.840 and 0.313 nm. The reflection at
2θ = 8.75° with
d = 1.010 nm belongs to the dehydrated phase of the V structure after calcination at 350 °C. The XRD pattern of ZnO/V shows a shift of V’s basal reflections to 7.29°
2θ, with
d = 1.212 nm. This was caused by the cationic exchange of interlayer materials for ZnO during nanocomposite preparation. The reflections of the hexagonal wurtzite structure of ZnO (PDF card no. 01-079-2205) at
2θ = 31.87°, 34.46°, 36.35°, 47.58°, 56.66°, 62.90°, 66.38°, 68.05° and 69.25° correspond to
d-values of 0.281, 0.260, 0.247, 0.191, 0.162, 0.148, 0.138 and 0.136 nm, confirming the ZnO nanoparticles on V’s surface.
The XRD pattern of ZnO/V_CH shows, in addition to the reflections of V and ZnO, new reflections at
2θ = 2.88°, 3.94° and 5.12°, with
d-values of 3.060, 2.239 and 1.722 nm, respectively, and 1.119 nm as a result of CH intercalation into the V interlayer [
21,
22]. Reflections of non-intercalated CH on the V surface are also observed.
The ZnO crystallite sizes calculated according to Scherrer’s equation [
23] based on (100) reflection (2
θ = 31.78°) are 14.33 nm for ZnO/V and 20.31 nm for ZnO/V_CH.
The FTIR spectrum of the initial V (
Figure 2a) shows a band at 3673 cm
−1 in the O–H stretching region attributed to the Mg
3OH unit, together with absorption at 684 cm
−1 belonging to the O–H bending vibration; these bands suggest a trioctahedral character for V [
24]. The absorption observed at 3411 cm
−1 corresponds to O–H stretching vibrations of absorbed water, and those at 1640 cm
−1, to the O–H bending vibration of absorbed water. The intense band at 999 cm
−1 was assigned to Si–O stretching vibrations, together with the Si–O bending vibration at 451 cm
−1 [
24]. In the FTIR spectrum of ZnO/V (
Figure 2b), the band at 1440 cm
−1 is the overlap of both peaks for C-H and C=O bonds originating from acetate; a further stretching mode of vibration of the C=O bond is observed at 882 cm
−1 [
25]. The main Zn–O stretching band is overlapped by the Si–O bending vibration of V. The FTIR spectrum of ZnO/V_CH (
Figure 2c) shows bands at 3311, 3199 and 3120 cm
−1 corresponding to NH stretching vibrations of secondary amine and imine functional groups of CH; further bands at 2937 and 2855 cm
−1 belong to asymmetric and symmetric C–H stretching bands of CH. The C–N stretching vibration of an imine group appears at 1635 cm
−1. The bands occurring in the 1580–1490 cm
−1 interval are due to a N–H bending vibration of secondary amine and imine groups. The absorption at 1416 cm
−1 belongs to the C–C stretching vibrations of an aromatic ring. Finally, the bands at 824 and 725 cm
−1 belong to the C–H out-of-plane deformation vibrations of 1,4-disubstituted aromatic rings and C-C rocking vibration of methylene groups, respectively [
26,
27].
3.2. LDPE Nanocomposite Plate Characterization
The XRD pattern of the LDPE nanocomposite plates (
Figure 3) shows a semicrystalline structure for LDPE with the reflections of the crystalline region at 2
θ = 21.35°, 23.71°, 29.83° and 36.20° corresponding to
d = 0.416,
d = 0.375,
d = 0.299 and
d = 0.248 nm and also the amorphous region (A) between 15 and 25° 2
θ [
28]. The XRD pattern of the samples with nanofillers does not show significant changes in the LDPE reflections.
The LDPE nanocomposite samples with the ZnO/V nanofiller show V reflections with
d-values of 1.277–1.265 and 1.473–1.481 nm, which means a partially rehydrated V structure. ZnO reflections with
d-values of 0.281, 0.260 and 0.248 nm are observed (
Figure 3A).
The LDPE nanocomposite samples with the ZnO/V_CH nanofiller show very low intense V reflections with
d-values of 1.230–1.240 and 1.471–1.481 nm corresponding to the rehydrated V structure. However, the reflections 3.060, 2.239 and 1.722 nm disappeared in the LDPE nanocomposites with ZnO/V_CH nanofiller, which could mean a partially exfoliated V structure in the LDPE and the formation of a nanocomposite structure. ZnO and CH reflections are observed in the XRD patterns (
Figure 3B).
The FTIR spectrum of the original LDPE (
Figure 4a) shows two intense peaks at 2915 and 2848 cm
−1 assigned to C–H asymmetric and symmetric stretching vibrations of methylene groups CH
2. A further two bands of the doublet 1473 and 1463 cm
−1 belong to the bending deformation of methylene CH
2 groups in crystalline and amorphous domains, respectively [
26,
27,
29,
30]. The band at 1377 cm
−1 (
Figure 4b) is assigned to the CH
3 symmetric deformation vibration, the peaks at 1369 and 1354 cm
−1 are both assigned to the wagging deformation vibration of CH
2, and 1303 cm
−1 is assigned to twisting deformation vibration. The weak peak at 1745 cm
−1 (
Figure 4a) together with that at 1043 cm
−1 (
Figure 4b) is correlated with the presence of the carbonyl C=O absorption and CH
2–O vibration of the ester group, which is probably created by the natural aging of LDPE [
30]. Finally, the peaks of the doublet 729 and 718 cm
−1 (
Figure 4c) are assigned to CH
2 rocking deformation vibrations in crystalline and amorphous domains. In the case of LDPE_ZnO/V nanocomposites, we can observe in the FTIR spectra characteristic bands for the ZnO/V nanofiller. The shoulder on the intensive peak at 1471 cm
−1 (
Figure 4a) around 1439 cm
−1 is the overlap of both peaks for C–H and C=O bonds originating from zinc acetate; a further stretching mode of vibration of a C=O bond is observed at 882 cm
−1 (
Figure 4b) for 3 and 5 wt% of the ZnO/V nanofiller. The bands around 535 and 464 cm
−1 (
Figure 4c) correspond to Zn–O stretching bonds. Although we did not observe these bands in the spectrum of the ZnO/V nanofiller itself (
Figure 5) due to overlapping by more intense Si–O bending vibrations, after the incorporation into the LDPE matrix, it is possible that some changes in the nanofiller structural arrangement occurred and these bands are observable.
Figure 5 shows, as well as the previous
Figure 4 spectrum of the original LDPE, further spectra of the LDPE_ZnO/V_CH nanocomposites. In this case, besides the LDPE characteristic bands, we can again observe vibrations of the ZnO/V_CH nanofiller. Especially, some vibrations of very dominant CH from the nanofiller are shown in the LDPE nanocomposites’ spectra at 1490 cm
−1 (
Figure 5a), attributed to the N–H bending vibration of secondary amine or imine groups, and 823 cm
−1 (
Figure 5c), which belongs to the C–H out-of-plane deformation vibrations of 1,4-disubstituted aromatic rings of CH.
The surface topology of the LDPE and LDPE nanocomposite plates was investigated using scanning electron microscope (SEM) and atomic force microscope (AFM) measurements.
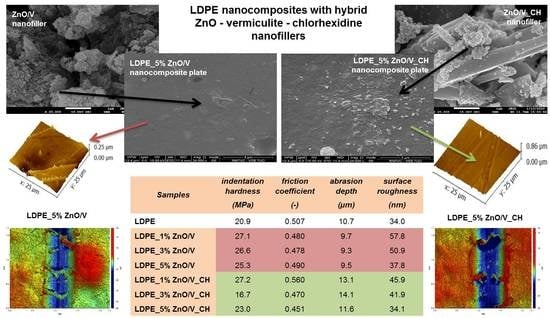
Figure 6 shows the cross-sectional SEM images representing the arrangement of the visible ZnO/V and ZnO/V_CH particles on the LDPE nanocomposite plates.
The original LDPE nanocomposite plates show a smooth surface with sporadically occurring holes and grooves as a result of the sample handling.
From the SEM images (
Figure 6) are evident the better incorporation and distribution of the ZnO/V nanofillers in the LDPE matrices’ volume than those of the ZnO/V_CH nanofillers. The LDPE_3% ZnO/V nanocomposite plate shows complete incorporation and the most homogeneous distribution of the ZnO/V nanofiller in the LDPE matrix. The ZnO/V nanofiller particles are uniformly distributed, and there are some areas of variable size and shape. Cavities smaller than 0.5 µm (also visible in the AFM image,
Figure 7) are visible at the places of the agglomerates. The LDPE_3% ZnO/V nanocomposite plate was an optimized sample in terms of technological preparation. The 5% of ZnO/V nanofillers were also uniformly distributed, but they created agglomerates in the LDPE plate. The ZnO/V nanofillers were horizontally oriented, and it is evident that the nanofiller presence on the surface of the LDPE matrix (part of it was sticking out from the LDPE surface) contributed to the formation of the surface cavities and holes around them. The surface of the LDPE_1% ZnO/V nanocomposite plate was wavy, and sporadically, there were ZnO/V nanofiller particles with non-uniform shapes and sizes. At the same time, very small, spherical nanoparticles were visible on the surfaces of the LDPE_1% ZnO/V and LDPE_3% ZnO/V nanocomposite plates as a result of the agglomeration of nanoparticles from the conductive layer sputtering process during the surface preparation for SEM analysis.
From the SEM images (
Figure 6) is evident the increased size of the weight fraction of the ZnO/V_CH nanofiller in the LDPE plates, where the ZnO/V_CH particles stuck out of the LDPE surface. In the case of the LDPE_1% ZnO/V_CH and LDPE_3% ZnO/V_CH nanocomposite plates, mainly ZnO/V_CH particles with an average size of 2 µm in the form of thin plates with sharp edges were anchored on the surface. The LDPE_5% ZnO/V_CH nanocomposite plate was represented by ZnO/V_CH particles with different particle size ranging from 0.5 to 2 µm, whereas particles bigger than 2 µm were partially embedded in the LDPE matrix and on the surface formed a sharp protrusion. On the LDPE_1% ZnO/V_CH and LDPE_3% ZnO/V_CH nanocomposite plates, surface defects were not observed; only in the case of the LDPE_5% ZnO/V_CH nanocomposite plate were short cracks (no longer that 2 µm) occasionally visible outside the area of the occurrence of the ZnO/V_CH nanofillers.
It can be stated that the predominant larger fractions (5–13 µm) of the ZnO/V and ZnO/V_CH nanofillers were incorporated inside the LDPE matrix, while smaller fractions (less than 4 µm) were anchored on the surface of the LDPE matrices, depending on the concentration amount.
The topology changes of the LDPE plates are shown in the 3D AFM images (
Figure 7). Variations in morphology were calculated via the average roughness (Ra, measured by profile/linear analysis) and root mean square roughness (RMS) values (
Table 1). All were obtained from the six measurements at different locations of the LDPE plates for each sample. The standard deviations are provided in parentheses.
From the 3D AFM images, it is evident that the LDPE nanocomposite plates reached maximum heights at around 0.25 µm (LDPE_5% ZnO/V and LDPE_3% ZnO/V_CH samples), 0.70 µm (LDPE_3% ZnO/V and LDPE_1% ZnO/V_CH samples) and 0.86 µm (LDPE_1% ZnO/V and LDPE_5% ZnO/V_CH samples). The highest measured values correspond with the perpendicular orientation (LDPE_1% ZnO/V sample) and the presence of agglomerates (LDPE_5% ZnO/V_CH samples) of the nanofillers on the LDPE surface, as has already been observed in the SEM images. The smoothest surfaces were characterized on the LDPE_1% ZnO/V, LDPE_3% ZnO/V and LDPE_5% ZnO/V_CH nanocomposite plates. In all the AFM images are evident maximal peaks that correspond to the presence of powdered gold from the covering of the LDPE plates. These could have only slightly affected the resulting numerical roughness values.
The roughnesses, with the lowest values of 21.7 nm (Ra) and 34.0 nm (RMS), were measured and evaluated for the original LDPE plate (
Table 1). The ZnO/V and ZnO/V_CH nanofillers caused an increase in the Ra roughness values in the range of values from 24.68 nm (for the LDPE_5% ZnO/V_CH sample) to 29.3 nm (for the LDPE_3% ZnO/V sample). As a remote value of the measurement can be considered the average roughness of 36.45 nm (Ra) for the LDPE_1% ZnO/V sample, which was caused by the perpendicular orientation of the ZnO/V nanofillers on the surface of the LDPE matrix, as can be seen from the AFM image (
Figure 7). For this reason, the mean values of the root mean square roughness (RMS) can be considered more objective, because they were evaluated from the total scanned area of the LDPE surface. Additionally, these values increased (RMS) in the range from 34.1 nm (for LDPE_5% ZnO/V_CH) to 50.9 nm (for LDPE_3% ZnO/V), respectively, and 57.8 nm for LDPE_1% ZnO/V. It is evident that with an increasing mass percentage of the nanofillers (ZnO/V and ZnO/V_CH) in the LDPE matrix, the roughness values decreased.
3.3. Antimicrobial Activity of the PVAc Nanocomposite Plates
The antimicrobial activity of the prepared LDPE nanocomposites was studied using the microbial fingerprints technique. Briefly, 25 μL of microbial suspension with a density of 10
5 CFU/mL was spread on 2.5 cm
2 surfaces of the tested square plates of LDPE nanocomposites, which meant that the original density of the suspension on the square plates dropped by one logarithmic order. The pure LDPE was used as the control sample. The average numbers of fungal colony forming units (CFU) at various time intervals are shown in
Table 2.
The ZnO/V and ZnO/V_CH nanofillers themselves exhibited high antibacterial activity against
S. aureus and
P. aeruginosa after 30 min, with a long-lasting effect persisting up to 5 days, with a dependence on the zinc oxide and chlorhexidine concentrations in the vermiculite structure [
31]. By incorporating them into the LDPE matrix, the antibacterial activity was changed slightly.
In the case of action against S. aureus, we can observe slightly improving efficacy over time with the samples LDPE_3% ZnO/V and LDPE_5% ZnO/V. All the other samples had fluctuating efficacies, without time and concentration dependence. The bactericidal effect was not confirmed.
We obtained very good results with all the LDPE nanocomposites against
P. aeruginosa after 72 h of action, when we noted a decrease in CFU up to four logarithmic orders, and after 96 h, the number of CFU even decreased nearly to zero values. Surprisingly, there was no difference between the samples with and without the presence of CH. These results correspond with the antibacterial activity published in [
27].
3.4. Tribo-Mechanical Properties of the LDPE Nanocomposite Plates
The tribological behaviour of the LDPE samples was evaluated by static wear tests performed against steel balls using a ball-on-disc micro tribometer for two different conditions—a 2 N loading force and speed of 5 Hz (test named F2N_v5), and a 1 N loading force and speed of 1 Hz (test named F1N_v1). Both tests were realised for 5 min. The resulting friction coefficients (COF, average values measured during the test based on five repetitions of each test were evaluated, with the minimum standard deviations ±0.05) and abrasion depths (standard deviations ±0.3 µm) are summarized in
Table 3. The representative profilometry images from F5N_v5 are shown in
Figure 8.
During the first 10 s of the tribological measurements (considered as the run-in period), the COF values always increased to the maximum, for all the LDPE samples. The highest COF value, 0.675, was measured for LDPE_3% ZnO/V_CH, followed by 0.665 for LDPE_5% ZnO/V and LDPE_3% ZnO/V, 0.64 for LDPE_5% ZnO/V_CH and LDPE_1% ZnO/V_CH, and 0.590 for LDPE and LDPE_1% ZnO/V. Then, the COF values decreased gradually and after 150 s reached a steady value. They correspond with the theory published in [
32], when after the run-in period, the contact surface of the tested balls became smoother, and prominent asperities of the surface were flattened or removed. The tribological plots (COF vs. time) are not listed here. The COF values measured at the end of the measuring time are shown in
Table 3.
The COF values of the LDPE nanocomposites from both wear tests were in the range, in terms of average values, of 0.451 to 0.560, compared to the original 0.454 to 0.507 for the natural LDPE plate. The COF values of the ZnO/V and ZnO/V_CH nanofillers in the LDPE matrix slightly increased during the F1N_v1 test (to a maximum COF value of 0.560 for LDPE_1% ZnO/V_CH) compared to the F2N_v5 test. In both tests, with an increasing concentration of the ZnO/V_CH nanofiller in the LDPE nanocomposite, the COF value slightly decreased, while in the ZnO/V nanofiller, this trend was the opposite.
From the tribological values, it is evident that the friction coefficient (COF) is a factor that expresses the surface characteristics of the LDPE nanocomposites and is higher for rougher surfaces (COM vs. RMS values). This fact is also confirmed by the measured values of the abrasion depth after the tribological test, which indicate the abrasion resistance, the ability of a surface to resist being worn away, upon rubbing or friction on the LDPE surface defect created after contact with the steel ball.
The abrasion depths of the wear obtained for the natural LDPE were around 11 µm (measured only after the F2N_v5 test). The average wear depth of the tracks for the ZnO/V nanofiller slightly decreased from 9.7 to 9.3 µm. Bigger abrasion depths were obtained for the ZnO/V_CH nanofillers, ranging from 11.6 to 14.1 µm, the biggest abrasion depth of 14.1 µm being measured for the LDPE_3% ZnO/V_CH nanocomposite plate. These results indicate that the LDPE_ZnO/V nanocomposite plates exhibited better wear resistance. They are also in good agreement with the profilometry images (
Figure 8), where are clearly evident structurally inhomogeneous areas with a lot of debris in the wear track. They are caused by the destruction of the samples relatively soft in comparison with the hard steel ball. This fact is also evident on the LDPE_ZnO/V_CH plates, where after the frictional tests, the material was displaced at the wear track interface. It is reflected on the images of the optical profilometer by a distinctive red colour in contrast to the blue colour of the wear track.
From the tribological results, it can be assumed that LDPE nanocomposites with ZnO/V nanofillers show better tribological properties due to higher COF (and RMS) values and lower values of abrasion depth, regardless of the degree of loading during the tribological test. The LDPE_ZnO/V nanocomposite plates better resisted frictional failure, the ZnO/V nanoparticles absorbing some of the slip, leaving less to migrate to the surface of the LDPE. On the contrary, the ZnO/V_CH nanofillers generated lower COF values and created deeper wear on the surface of the LDPE boards. This fact may be due to the presence of ZnO/V_CH nanofillers situated on the surface of the LDPE nanocomposite plates, where the predominantly layered structure of vermiculite particles and presence of the CH nanoparticles (organic chains) can act as lubricant agents and thus soften the surface of the LDPE plates.
The mechanical properties of the LDPE plates were determined using the indentation hardness (HIT) and Vickers microhardness (HV); the maximum achieved impression depth (hm) was measured, and the results are summarized in
Table 4. For the purpose of providing reliable microhardness values corresponding to the total hardness of the nanocomposites, the imprints under each load should be larger in size than the dimensions of the dispersed ZnO/V and ZnO/V_CH nanofillers (around 10 µm). It is known that the mean diagonal length of the imprints varies in the range of 100–200 μm. The measured values of the maximum impression depth (hm) ranged from 31.8 to 34.8 µm. The highest value of 41.9 µm was measured for the LDPE_3% ZnO/V_CH nanocomposite plate. This means that the imprints were always greater than the dimensions of the dispersed nanofillers. It is evident that both nanofillers led to a slight increase in the HIT and HV values.
The HIT value increased from 20.9 MPa (for the original LDPE) to 27.2 MPa (for LDPE_1% ZnO/V_CH) and 27.2 MPa (for LDPE_1% ZnO/V_CH), and the HIT value decreased with increasing concentrations of both nanofillers. These values corresponded with the Vickers microhardness values, where the original 2.0 HV for LDPE increased to 2.6 HV for LDPE_1% ZnO/V and also LDPE_1% ZnO/V_CH.
Different microhardness values were measured for the PVDF_3% ZnO/V_CH, where the microhardness reached significantly lower values of 1.6 HV and 16.7 MPa (HIT value). This indicates significantly softer/plastic behaviour. This also corresponds to the maximum impression depth (hm = 41.9 MPa), which was significantly deeper than for the other PVDF nanocomposite samples.