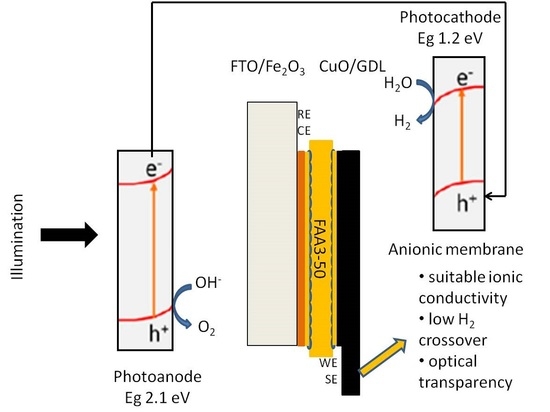
Anionic Exchange Membrane for Photo-Electrolysis Application
Abstract
:1. Introduction
- Suitable ionic conductivity in the range of 10–50 mS cm−1.
- Low gas crossover. Hydrogen/oxygen permeation smaller than 10−8 mol cm−2 min−1.
- High water permeation to allow for proper water management between cathode and anode according to the main conduction mechanism.
- Availability of ionomer dispersions to allow for an extension of the ionomer/oxide interface.
- Robust mechanical and thermal properties. Capability to sustain high temperatures (up to 85 °C).
- Optical transparency in the useful range of wavelengths.
- Substantial photo-response of the semiconductor electrodes in contact with the polymer material.
- Semiconductor corrosion mitigation properties.
- Low cost and wide availability of the base components.
2. Materials and Methods
2.1. Membrane
2.2. Ionomer Preparation
2.3. Determination of Ion-Exchange Capacity (IEC)
- (1)
- drying the membrane in an oven at 50 °C for 2 h under vacuum condition (1000 mbar);
- (2)
- immersing the dried sample in 0.1 M NaNO3 for 48 h at room temperature, afterwards removing the membrane from the solution;
- (3)
- adding 5 mL of 0.1 M AgNO3 and 5 drops of Fe(NO3)3 (11 wt.%), used as an indicator;
- (4)
- back-titration with 0.1 M KSCN until the equivalent point.
- (1)
- immersing the dried sample in alkaline solution at room temperature for a specific time, after removing the membrane from the solution and washing to remove the excess of hydroxide ions;
- (2)
- drying the membrane in oven at 50 °C for 2 h under vacuum (1000 mbar);
- (3)
- immersing the membrane in HCl 0.01 M for 24 h at room T, then removing the membrane from the solution;
- (4)
- back-titration with NaOH 0.01 M.
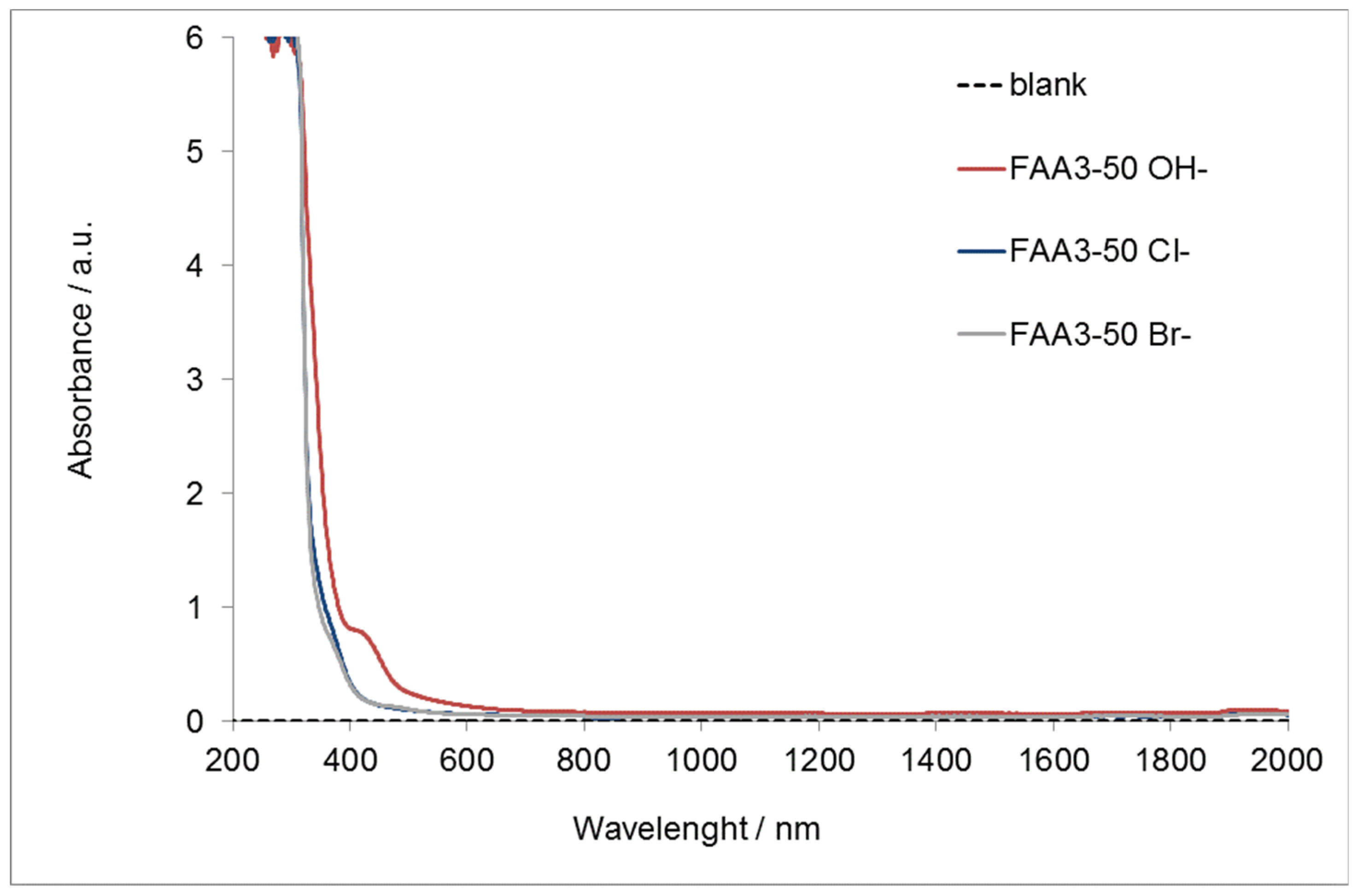
2.4. UV-Vis-NIR Detection
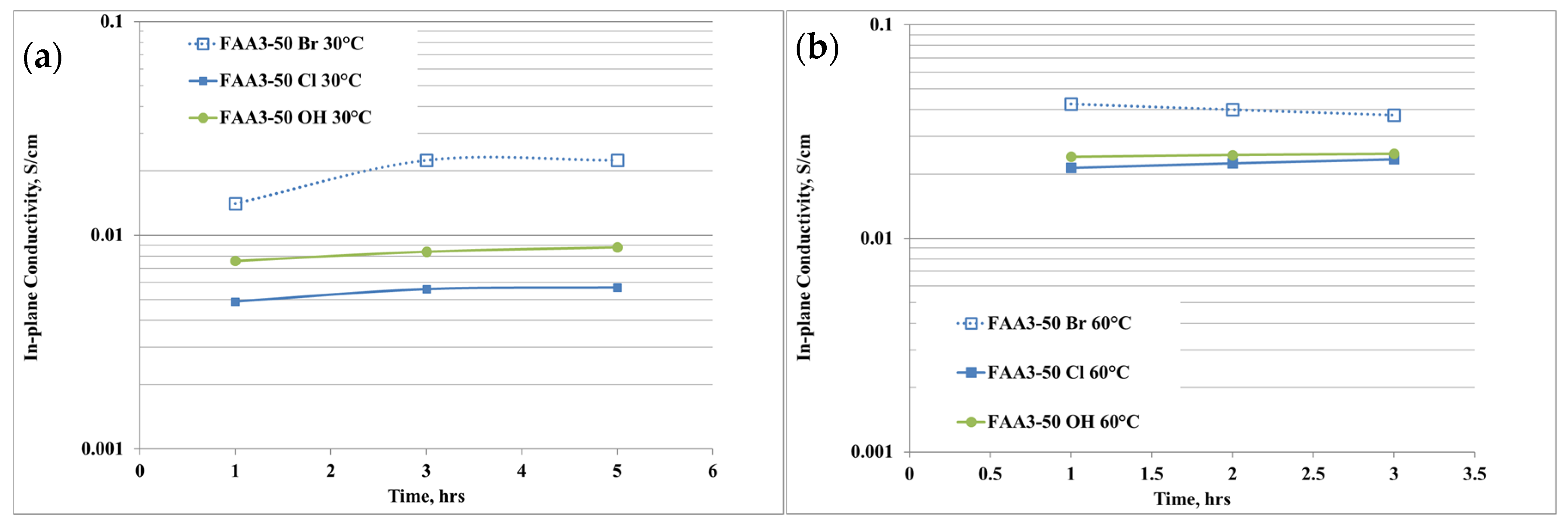
2.5. In-Plane Anion Conductivity Measurements
2.6. Electro-Chemical Characterizations
2.6.1. Assessment of Hydrogen Crossover
2.6.2. Photo-Electro-Chemical Test
3. Results and Discussion
3.1. Membrane Characterization
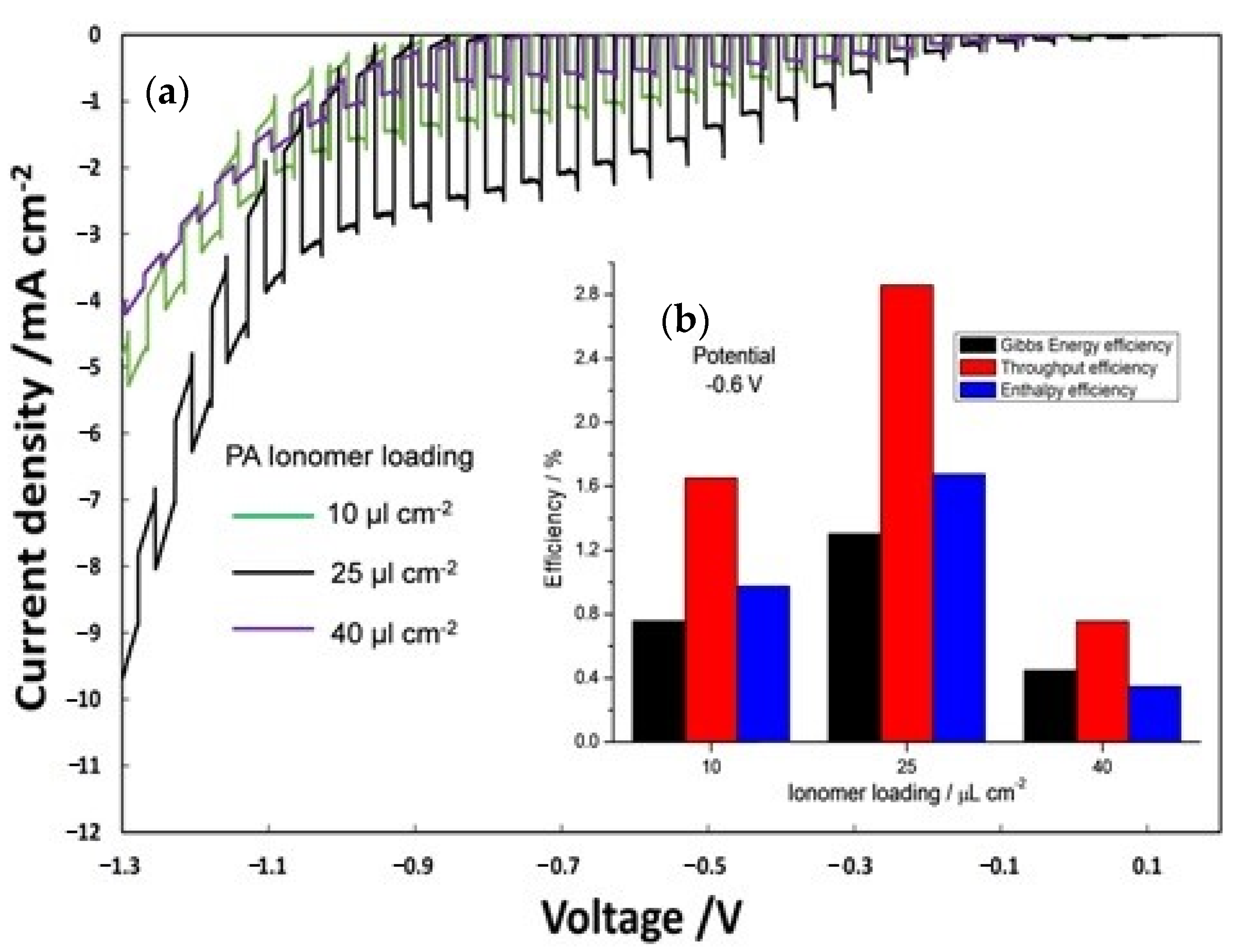
3.2. Photo-Electro-Chemical Test for Membrane Assessment in Full-Cell Configuration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.; Wilberforce, T.; Olabi, A.G. Environmental impacts of solar energy systems: A review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, C.; Tian, Z.; Sun, J. Solar-driven integrated energy systems: State of the art and challenges. J. Power Sources 2020, 478, 228762. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tavner, P.J.; Crabtree, C.J.; Feng, Y.; Qiu, Y. Wind turbine condition monitoring: Technical and commercial challenges. Wind Energ. 2014, 17, 673–693. [Google Scholar] [CrossRef] [Green Version]
- Dhar, A.; Naeth, M.A.; Jennings, P.D.; El-Din, M.G. Perspectives on environmental impacts and a land reclamation strategy for solar and wind energy systems. Sci. Total Environ. 2020, 718, 134602. [Google Scholar] [CrossRef]
- Burton, N.A.; Padilla, R.V.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]
- Truffer, B.; Bratrich, C.; Markard, J.; Peter, A.; Wüest, A.; Wehrli, B. Green Hydropower: The contribution of aquatic science research to the promotion of sustainable electricity. Aquat. Sci. 2003, 65, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Buhmann, P.; Moormann, C.; Westrich, B.; Pralle, N.; Friedemann, W. Tunnel geothermics—A German experience with renewable energy concepts in tunnel projects. Geomech. Energy Environ. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Bayer, P.; De Paly, M.; Beck, M. Strategic optimization of borehole heat exchanger field for seasonal geothermal heating and cooling. Appl. Energy 2014, 136, 445–453. [Google Scholar] [CrossRef]
- Kment, Š.; Sivula, K.; Naldoni, A.; Sarmah, S.P.; Kmentov, H.; Kulkarni, M.; Rambabu, Y.; Schmuki, P.; Zboril, R. FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog. Mater. Sci. 2020, 110, 100632. [Google Scholar] [CrossRef]
- Adamopoulos, P.M.; Papagiannis, I.; Raptis, D. Photoelectrocatalytic Hydrogen Production Using a TiO2/WO3 Bilayer Photocatalyst in the Presence of. Ethanol as a fuel. Catalysts 2019, 9, 976. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, A.; Chu, D.; Zhang, C.; Du, Y.; Huang, J.; Yang, P. High Performance of Manganese Porphyrin Sensitized p-Type CuFe2O4 Photocathode for Solar Water Splitting to Produce Hydrogen in a Tandem Photoelectrochemical Cell. Catalysts 2018, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Sanchez de la Cruz, P.T.; Irikura, K.; Lachgar, A.; Cardoso, J.C.; Cavero, H.A.; Zanoni, M.V.B. Preparation of FTO/CU2O Electrode Protected by PEDOT:PSS and Its Better Performance in the Photoelectrocatalytic Reduction of CO2 to Methanol. Electrocatalysis 2020, 11, 546–554. [Google Scholar] [CrossRef]
- Qin, L.; Ma, G.; Wang, L.; Tang, Z. Atomically precise metal nanoclusters for (photo)electroreduction of CO2: Recent advances, challenges and opportunities. J. Energy Chem. 2020, 57, 359–370. [Google Scholar] [CrossRef]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic Water Splitting: Significance of Cocatalysts, Electrolyte, and Interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
- Kuang, Y.; Yamada, T.; Domen, K. Surface and Interface Engineering for Photoelectrochemical Water Oxidation. Joule 2017, 1, 290–305. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Domen, K. Development of Sunlight Driven Water Splitting Devices towards Future Artificial Photosynthetic Industry. Chem. Eng. 2018, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Yang, M.; Zhang, S.; Fang, P. Protic Imidazolium Polymer as Ion Conductor for Improved Oxygen Evolution Performance. Polymers 2019, 11, 1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, J.H. Applications of proton exchange membrane fuel cell systems. Renew. Sustain. Energy Rev. 2007, 11, 1720–1738. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Sebastiàn, D.; Làzaro, M.J.; Aricò, A.S.; Baglio, V. Methanol-Tolerant M–N–C Catalysts for Oxygen Reduction Reactions in Acidic Media and Their Application in Direct Methanol Fuel Cells. Catalysts 2018, 8, 650. [Google Scholar] [CrossRef] [Green Version]
- Sebastiàn, D.; Serov, A.; Artyushkova, K.; Gordon, J.; Atanassov, P.; Aricò, A.S.; Baglio, V. High Performance and Cost-Effective Direct Methanol Fuel Cells: Fe-N-C Methanol-Tolerant Oxygen Reduction Reaction Catalysts. ChemSusChem 2016, 9, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Lo Vecchio, C.; Serov, A.; Romero, H.; Lubers, A.; Zulevi, B.; Aricò, A.S.; Baglio, V. Commercial platinum group metal-free cathodic electrocatalysts for highly performed direct methanol fuel cell applications. J. Power Sources 2019, 437, 226948. [Google Scholar] [CrossRef]
- Berger, A.; Segalman, R.A.; Newman, J. Material requirements for membrane separators in a water-splitting photoelectrochemical cell. Energy Environ. Sci. 2014, 7, 1468–1476. [Google Scholar] [CrossRef]
- Aricò, A.S.; Girolamo, M.; Siracusano, S.; Sebastian, D.; Baglio, V.; Schuster, M. Polymer Electrolyte Membranes for Water Photo-Electrolysis. Membranes 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Cots, A.; Bonete, P.; Sebastian, D.; Baglio, V.; Aricò, A.S.; Gomez, R. Toward tandem solar cells for water splitting using polymer electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 25393–25400. [Google Scholar] [CrossRef]
- Vecchio, C.L.; Trocino, S.; Zignani, S.C.; Baglio, V.; Carbone, A.; Garcia, M.I.D.; Contreras, M.; Gómez, R.; Aricò, A.S. Enhanced Photoelectrochemical Water Splitting at Hematite Photoanodes by Effect of a NiFe-Oxide co-Catalyst. Catalysts 2020, 10, 525. [Google Scholar] [CrossRef]
- Trocino, S.; Lo Vecchio, C.; Zignani, S.C.; Carbone, A.; Saccà, A.; Baglio, V.; Gòmez, R.; Aricò, A.S. Dry Hydrogen Production in a Tandem Critical Raw Material-FreeWater Photoelectrolysis Cell Using a Hydrophobic Gas-Diffusion Backing Layer. Catalysts 2020, 10, 1319. [Google Scholar] [CrossRef]
- Carbone, A.; Zignani, S.C.; Gatto, I.; Trocino, S.; Aricò, A.S. Assessment of the FAA3-50 polymer electrolyte in combination with a NiMn2O4 anode catalyst for anion exchange membrane water electrolysis. Int. J. Hydrol. Energy 2020, 45, 9285–9292. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Gatto, I.; Saccà, A.; Patti, A.; Bella, G.; Cordaro, M. Development of Polymeric Membranes Based on Quaternized Polysulfones for AMFC Applications. Polymers 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Siracusano, S.; Baglio, V.; Stassi, A.; Merlo, L.; Moukheiber, E.; Aricò, A.S. Performance analysis of short-side-chain Aquivions perfluorosulfonic acid polymer for proton exchange membrane water electrolysis. J. Memb. Sci. 2014, 466, 1–7. [Google Scholar] [CrossRef]
- Gatto, I.; Saccà, A.; Baglio, V.; Aricò, A.S.; Oldani, C.; Merlo, L.; Carbone, A. Evaluation of hot pressing parameters on the electrochemical performance of MEAs based on Aquivion® PFSA membranes. J. Energy Chem. 2019, 35, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar]
- DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 1 November 2020).

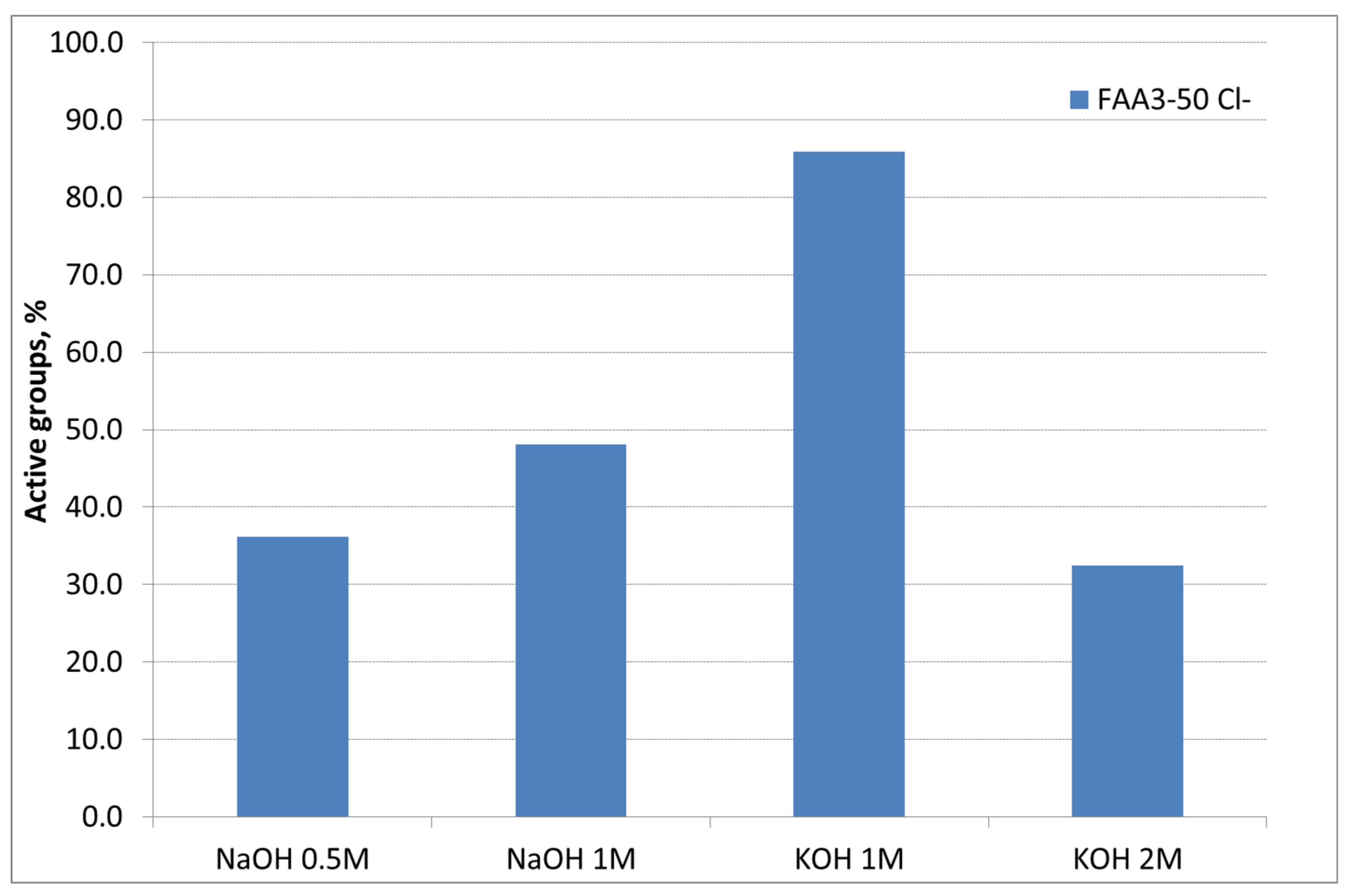


| Membrane Counter-Ion | IEC, meq/g | Active Groups, % |
|---|---|---|
| Br− | 2.28 | 120 |
| Cl− | 1.85 | 100 |
| OH− | 1.59 | 86 |
| T, °C | H2 Crossover, mA/cm2 | H2 Crossover, mol/s cm2 | H2 Crossover, ml/s cm2 | H2 Crossover, % |
|---|---|---|---|---|
| 40 | 0.64 | 3.32E−09 | 7.43E−05 | 0.037 |
| 50 | 0.56 | 2.90E−09 | 6.50E−05 | 0.035 |
| 60 | 0.68 | 3.52E−09 | 7.90E−05 | 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Vecchio, C.; Carbone, A.; Trocino, S.; Gatto, I.; Patti, A.; Baglio, V.; Aricò, A.S. Anionic Exchange Membrane for Photo-Electrolysis Application. Polymers 2020, 12, 2991. https://doi.org/10.3390/polym12122991
Lo Vecchio C, Carbone A, Trocino S, Gatto I, Patti A, Baglio V, Aricò AS. Anionic Exchange Membrane for Photo-Electrolysis Application. Polymers. 2020; 12(12):2991. https://doi.org/10.3390/polym12122991
Chicago/Turabian StyleLo Vecchio, Carmelo, Alessandra Carbone, Stefano Trocino, Irene Gatto, Assunta Patti, Vincenzo Baglio, and Antonino Salvatore Aricò. 2020. "Anionic Exchange Membrane for Photo-Electrolysis Application" Polymers 12, no. 12: 2991. https://doi.org/10.3390/polym12122991
APA StyleLo Vecchio, C., Carbone, A., Trocino, S., Gatto, I., Patti, A., Baglio, V., & Aricò, A. S. (2020). Anionic Exchange Membrane for Photo-Electrolysis Application. Polymers, 12(12), 2991. https://doi.org/10.3390/polym12122991