Adenine as Epoxy Resin Hardener for Sustainable Composites Production with Recycled Carbon Fibers and Cellulosic Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Resins Formulations Preparation
2.1.2. Chopped Carbon Fiber Reinforced Composites Production
2.1.3. Chopped Natural Fiber Reinforced Composites Production
2.2. Methods
3. Results and Discussion
3.1. Investigation of Curing of CLR—Adenine Formulations
3.2. Study of Cross-Linking Reaction Mechanism
3.3. Production and Characterization of CLR—Adenine (CA) Composite Materials with Natural Fibers and Recyled Carbon Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pillain, B.; Viana, L.R.; Lefeuvre, A.; Jacquemin, L.; Sonnemann, G. Social life cycle assessment framework for evaluation of potential job creation with an application in the French carbon fiber aeronautical recycling sector. Int. J. Life Cycle Assess. 2019, 24, 1729–1742. [Google Scholar] [CrossRef]
- Koumoulos, E.P.; Trompeta, A.F.; Santos, R.M.; Martins, M.; Monterio dos Santos, C.; Iglesias, V.; Böhm, R.; Gong, G.; Chiminelli, A.; Verpoest, I.; et al. Research and Development in Carbon Fibers and Advanced High-Performance Composites Supply Chain in Europe: A Roadmap for Challenges and the Industrial Uptake. J. Compos. Sci. 2019, 3, 86. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.-M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgini, L.; Benelli, T.; Brancolini, G.; Mazzocchetti, L. Recycling of carbon fiber reinforced composites waste to close their Life Cycle in a Cradle-to-Cradle approach. Curr. Opin. Green Sustain. Chem. 2020, 26, 100368. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.; Dierkes, W.; Akkerman, R.; Brem, G. A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, A.C.; Pandit, P.; Gayatri, T.N.; Chavan, P.P.; Jadhav, N.C. Production of Green Composites from Various Sustainable Raw Materials. In Green Composites. Textile Science and Clothing Technology; Muthu, S., Ed.; Springer: Singapore, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Anil, N.N.; Shitij, C. Composites get greener. Mater. Today 2003, 6, 22–29. [Google Scholar] [CrossRef]
- Boutevin, B.; Caillol, S.; Burguiere, C.; Rapior, S.; Fulcrand, H.; Nouailhas, H. Novel Method for Producing Thermosetting Epoxy Resins. Patent Application No. WO 2010136725, May 2010. [Google Scholar]
- Nouailhas, H.; Aouf, C.; Le Guernevé, C.; Caillol, S.; Boutevin, B.; Fulcrand, H. Synthesis and properties of biobased epoxy resins. part 1. Glycidylation of flavonoids by epichlorohydrin. J. Polym. Sci. Part. A 2011, 49, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Fenn, D.; Webster, G.R.; McCollum, G.J. Modified Epoxy Resins Comprising the Reaction Product of a Biomass Derived Compound and an Epoxy Resin, and Aqueous Dispersions and Coatings Comprising Such Resins. U.S. Patent 7,812,101, 12 October 2010. [Google Scholar]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; La Scala, J.J.; Stanzione, J.F. Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Mauck, J.R.; Yadav, S.K.; Sadler, J.M.; La Scala, J.J.; Palmese, G.R.; Schmalbach, K.M.; Stanzione, J.F., III. Preparation and Characterization of Highly Bio-based Epoxy Amine Thermosets Derived from Lignocellulosics. Macrom. Chem. Phys. 2017, 218, 1700013. [Google Scholar] [CrossRef]
- Frias, C.F.; Serra, A.C.; Ramalho, A.; Coelho, J.F.J.; Fonseca, A.C. Preparation of fully biobased epoxy resins from soybean oil based amine hardeners. Ind. Crop. Prod. 2017, 109, 434–444. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Espinoza-Perez, J.D.; Nerenz, B.A.; Haagenson, D.M.; Chen, Z.; Ulven, C.A.; Wiesenborn, D.P. Comparison of curing agents for epoxidized vegetable oils applied to composites. Polym. Compos. 2011, 32, 1806–1816. [Google Scholar] [CrossRef]
- Knorr, D.B.; Yu, J.H.; Richardson, A.D.; Hindenlang, M.D.; McAninch, I.M.; La Scala, J.J.; Lenhart, J.L. Glass transition dependence of ultrahigh strain rate response in amine cured epoxy resins. Polymer 2012, 53, 5917–5923. [Google Scholar] [CrossRef]
- Guo, Q. Thermosets: Structure, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Schäfer, A.; Seibold, S.; Walter, O.; Döring, M. Novel high Tg flame retardancy approach for epoxy resins. Polym. Degrad. Stab. 2008, 93, 557–560. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef] [Green Version]
- François, C.; Pourchet, S.; Boni, G.; Rautiainen, S.; Samec, J.; Fournier, L.; Robert, C.; Thomas, C.M.; Fontaine, S.; Gaillard, Y.; et al. Design and synthesis of biobased epoxy thermosets from biorenewable resources. Comptes Rendus Chim. 2017, 20, 1006–1016. [Google Scholar] [CrossRef]
- Mattar, N.; Rios de Anda, A.; Vahabi, H.; Renard, E.; Langlois, V. Resorcinol-Based Epoxy Resins Hardened with Limonene and Eugenol Derivatives: From the Synthesis of Renewable Diamines to the Mechanical Properties of Biobased Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 13064–13075. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Wang, H.; Liu, B.; Liu, X.; Zhang, J.; Xian, M. Synthesis of biobased epoxy and curing agents using rosin and the study of cure reactions. Green Chem. 2008, 10, 1190–1196. [Google Scholar] [CrossRef]
- Quirino, R.L.; Monroe, K.; Fleischer, C.H., III; Biswas, E.; Kessler, M.R. Thermosetting polymers from renewable sources. Polym. Int. 2020. [Google Scholar] [CrossRef]
- Jia, P.; Ma, Y.; Kong, Q.; Xu, L.; Li, Q.; Zhou, Y. Progress in development of epoxy resin systems based on biomass resources. Green Mater. 2020, 8, 6–23. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; . Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.-J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Comp. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Jariwala, H.; Jain, P. A review on mechanical behavior of natural fiber reinforced polymer composites and its applications. J. Reinf. Plast. Comp. 2019, 38, 441–453. [Google Scholar] [CrossRef]
- Torres-Arellano, M.; Renteria-Rodríguez, V.; Franco-Urquiza, E. Mechanical Properties of Natural-Fiber-Reinforced Biobased Epoxy Resins Manufactured by Resin Infusion Process. Polymers 2020, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2020, 55, 829–892. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Benelli, T.; D’Angelo, E.; Leonardi, C.; Zattini, G.; Giorgini, L. Validation of carbon fibers recycling by pyro-gasification: The influence of oxidation conditions to obtain clean fibers and promote fiber/matrix adhesion in epoxy composites. Compos. A 2018, 112, 504–514. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Mazzocchetti, L.; Leonardi, C.; Zattini, G.; Minak, G.; Dolcini, E.; Cavazzoni, M.; Montanari, I.; Tosi, C. Recovery of carbon fibers from cured and uncured carbon fiber reinforced composites wastes and their use as feedstock for a new composite production. Polym. Compos. 2015, 36, 1084–1095. [Google Scholar] [CrossRef]
- Giorgini, L.; Leonardi, C.; Mazzocchetti, L.; Zattini, G.; Cavazzoni, M.; Montanari, I.; Tosi, C.; Benelli, T. Pyrolysis of fiberglass/polyester composites: Recovery and characterization of obtained products. FME Trans. 2016, 44, 405–414. [Google Scholar] [CrossRef]
- Netravali, A.N. Biodegradable and Sustainable Fibers; Woodhead Publishing Limited: Cambridge, UK, 2005. [Google Scholar]
- Sreenivas, H.T.; Krishnamurthy, N.; Arpitha, G.R. A comprehensive review on light weight kenaf fiber for automobiles. Int. J. Lightweight Mater. Manufact. 2020, 3, 328–337. [Google Scholar] [CrossRef]
- Wambua, P.; Ivens, J.; Verpoest, I. Natural fibres: Can they replace glass in fibre reinforced plastics? Comp. Sci. Technol. 2003, 63, 1259–1264. [Google Scholar] [CrossRef]
- Marczak, D.; Lejcuś, K.; Misiewicz, J. Characteristics of biodegradable textiles used in environmental engineering: A comprehensive review. J. Clean. Prod. 2020, 268, 122129. [Google Scholar] [CrossRef]
- Begum, S.; Fawzia, S.; Hashmi, M.S.J. Polymer matrix composite with natural and synthetic fibres. Adv. Mater. Process. Tech. 2020, 6, 547–564. [Google Scholar] [CrossRef]
- Agarwal, J.; Sahoo, S.; Mohanty, S.; Nayak, S.K. Progress of novel techniques for lightweight automobile applications through innovative eco-friendly composite materials: A review. J. Thermoplast. Comp. Mater. 2020, 33, 978–1013. [Google Scholar] [CrossRef]
- Zini, E.; Scandola, M. Green composites: An overview. Polym. Comp. 2011, 32, 1905–1915. [Google Scholar] [CrossRef]
- Singha, A.S.; Thakur, V.K. Fabrication and characterization of S. cilliare fibre reinforced polymer composites. Bull. Mater. Sci. 2009, 32, 49–58. [Google Scholar] [CrossRef]
- Kim, W.; Argento, A.; Lee, E.; Flanigan, C.; Houston, D.; Harris, A.; Mielewski, D.F. High strain-rate behavior of natural fiber- reinforced polymer composites. J. Compos. Mater. 2011, 46, 1051–1065. [Google Scholar] [CrossRef]
- Stamboulis, A.; Baillie, C.A.; Peijs, T. Effects of environmental conditions on mechanical and physical properties of flax fibers. Compos. Part. A 2001, 32, 1105–1115. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Benelli, T.; Zattini, G.; Maccaferri, E.; Brancolini, G.; Giorgini, L. Evaluation of carbon fibers structure and morphology after their recycling via pyro-gassification of CFRPs. AIP Conf. Proc. 2019, 2196, 020036. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Leonardi, C.; Mazzocchetti, L.; Zattini, G.; Cavazzoni, M.; Montanari, I.; Tosi, C. Efficient recovery of non-shredded tires via pyrolysis in an innovative pilot plant. Environ. Eng. Manag. J. 2015, 14, 1611–1622. [Google Scholar] [CrossRef]
- Ajinomoto, K. Preparation of Adenine by Fermentation. Patent Application No. JP12162879A, 1979. [Google Scholar]
- Li, Y.; Xiao, F.; Moon, K.S.; Wong, C.P. Novel Curing Agent for Lead-Free Electronics: Amino Acid. J. Polym. Sci. Pol. Chem. 2006, 44, 1020–1027. [Google Scholar] [CrossRef]
- Dong, H.; Zu, X.; Zheng, P.; Zhang, D. A rapid enzymatic assay for high-throughput screening of adenosine-producing strains. Microb. Biotechnol. 2015, 8, 230–238. [Google Scholar] [CrossRef]
- Zhang, C.; Du, S.; Liu, Y.; Xie, X.; Xu, Q.; Chen, N. Strategy for enhancing adenosine production under the guidance of transcriptional and metabolite pool analysis. Biotechnol. Lett. 2015, 37, 1361–1369. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Entropy Resins. Clear Laminating System. Available online: https://entropyresins.com/app/uploads/CLR-TDS.pdf (accessed on 19 December 2020).
- Entropy Resins. Super Sap. Available online: https://entropyresins.com/sustainability/super-sap/ (accessed on 19 December 2020).
- Mazzocchetti, L.; Merighi, S.; Benelli, T.; Giorgini, L. Evaluation of L-Tryptophan—Late Curing Agents Systems as Hardeners for Epoxy Resins. AIP Conf. Proc. 2018, 1981, 020170. [Google Scholar] [CrossRef]
- Entropy Resins. Life Cycle Assessment. Available online: https://entropyresins.com/sustainability/life-cycle-assessment/ (accessed on 19 December 2020).
- Tureček, F.; Chen, X. Protonated adenine: Tautomers, solvated clusters, and dissociation mechanisms. J. Am. Soc. Mass Spectrom. 2005, 16, 1713–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubczak, R.; Duliban, J. A study of the reaction of adenine with ethylene oxide or with ethylene carbonate. React. Funct. Polym. 2002, 52, 127–134. [Google Scholar] [CrossRef]
- Mazumdar, S.K. Composites Manufacturing; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mallik, P.K. Fiber- Reinforced Composites: Materials, Manufacturing, and Design; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Flemming, T.; Kress, G.; Flemming, M. A new aligned short-carbon-fiber-reinforced thermoplastic prepreg. Adv. Compos. Mater. 1996, 5, 151–159. [Google Scholar] [CrossRef]
- Feraboli, P.; Peitso, E.; Deleo, F.; Cleveland, T.; Stickler, P.B. Characterization of prepreg-based discontinuous carbon fiber/epoxy systems. J. Reinf. Plast. Compos. 2009, 28, 1191–1214. [Google Scholar] [CrossRef]
- Capela, C.; Oliveira, S.E.; Ferreira, J.A.M. Mechanical behavior of high dosage short carbon fiber reinforced epoxy composites. Fibers Polym. 2017, 18, 1200–1207. [Google Scholar] [CrossRef]
- Meredith, J.; Cozien-Cazuc, S.; Collings, E.; Carter, S.; Alsop, S.; Lever, J.; Coles, S.R.; Wood, B.M.; Kirwan, K. Recycled carbon fibre for high performance energy absorption. Compos. Sci. Technol. 2012, 72, 688–695. [Google Scholar] [CrossRef]
- Stoeffler, K.; Andjelic, S.; Legros, N.; Roberge, J.; Schougaard, S.B. Polyphenylene sulfide (PPS) composites reinforced with recycled carbon fiber. Compos. Sci. Technol. 2013, 29, 65–71. [Google Scholar] [CrossRef]
- Akonda, M.H.; Lawrence, C.A.; Weager, B.M. Recycled carbon fibre-reinforced polypropylene thermoplastic composites. Compos. A 2012, 43, 79–86. [Google Scholar] [CrossRef]
- Zattini, G.; Mazzocchetti, L.; Benelli, T.; Maccaferri, E.; Brancolini, G.; Giorgini, L. Mechanical Properties and Fracture Surface Analysis of Vinyl Ester Resins Reinforced with Recycled Carbon Fibres. Key Eng. Mater. 2020, 827, 110–115. [Google Scholar] [CrossRef]
- Asim, M.; Paridah, M.T.; Chandrasekar, M.; Shahroze, R.M.; Jawaid, M.; Nasir, M.; Siakeng, R. Thermal stability of natural fibers and their polymer composites. Iran. Polym. J. 2020, 29, 625–648. [Google Scholar] [CrossRef]
- Moosburger-Will, J.; Bauer, M.; Laukmanis, E.; Horny, R.; Wetjen, D.; Manske, T.; Schmidt-Stein, F.; Töpker, J.; Horn, S. Interaction between carbon fibers and polymer sizing: Influence of fiber surface chemistry and sizing reactivity. Appl. Surf. Sci. 2018, 439, 305–312. [Google Scholar] [CrossRef]
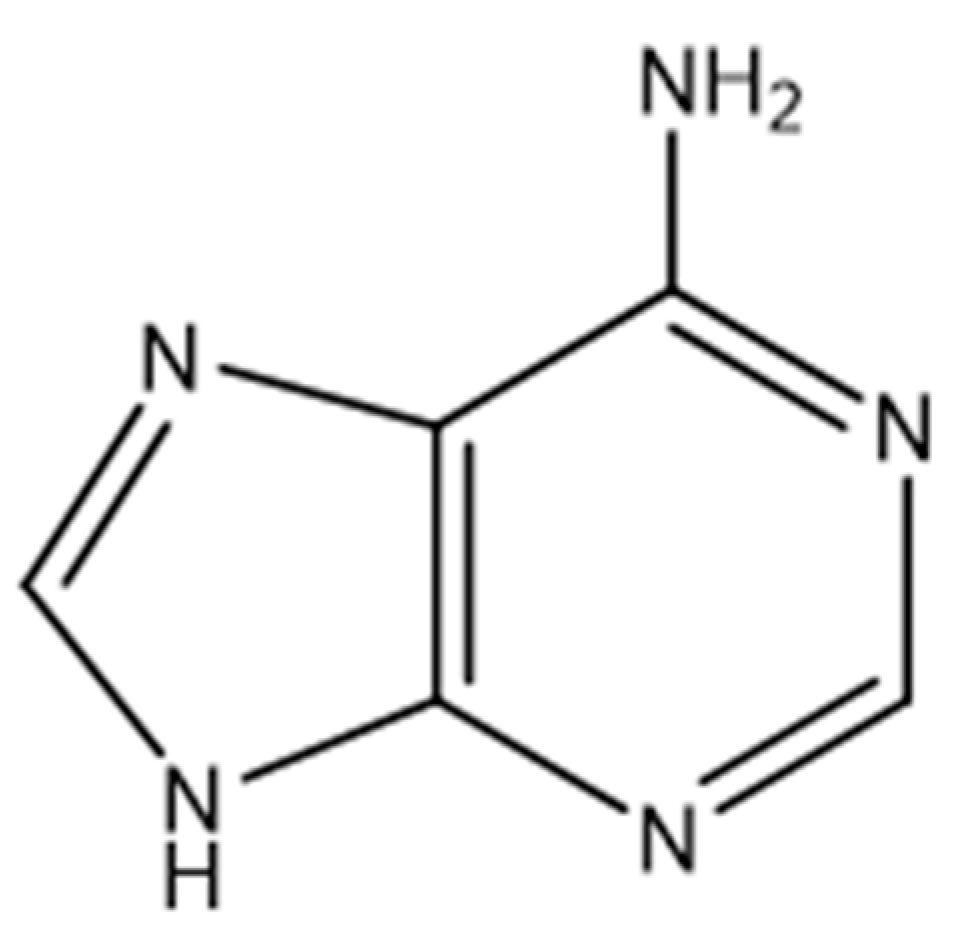



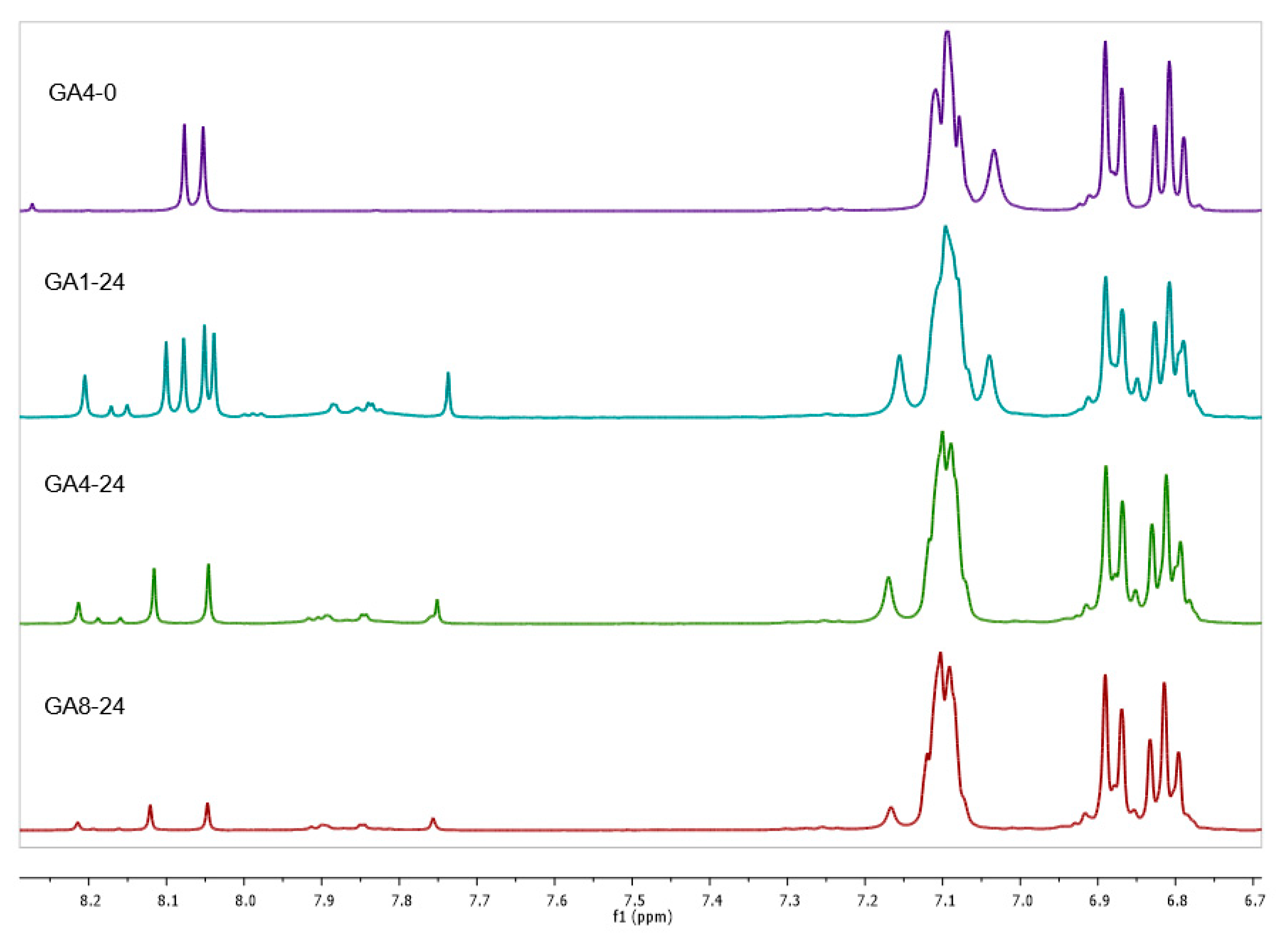



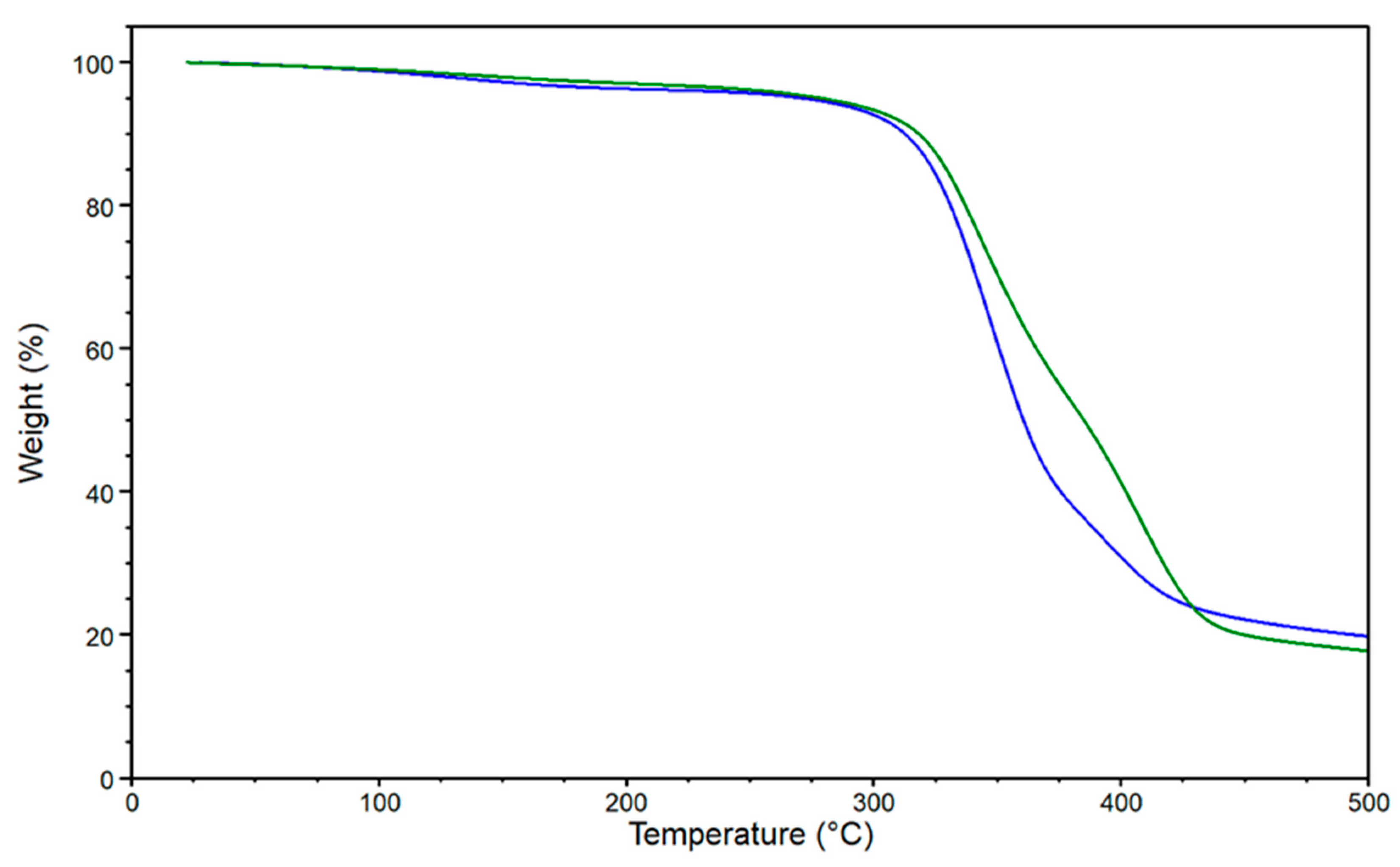
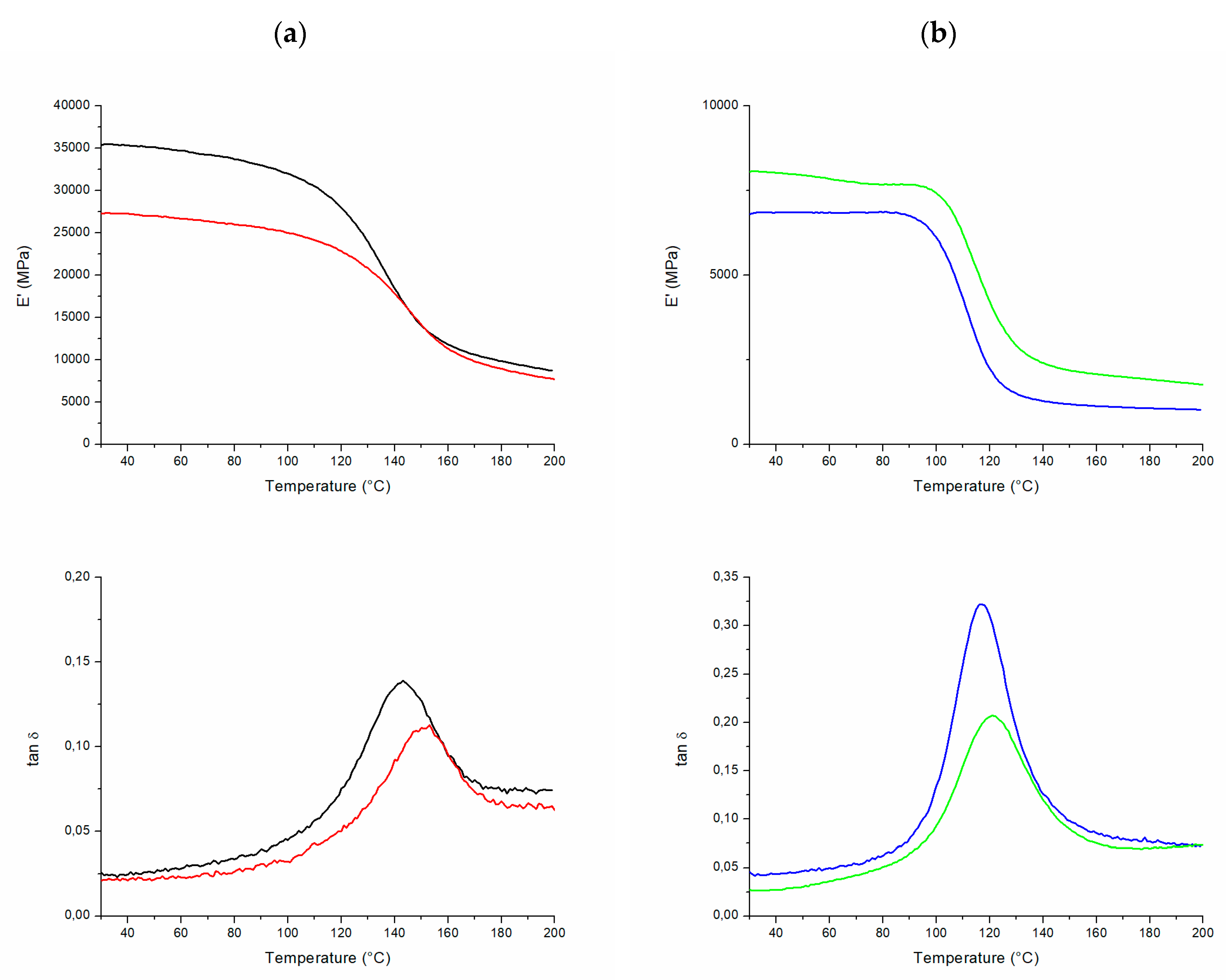
| Sample | CLR (w%) | Adenine (w%) | CLR (g) | Adenine (g) | CLR (mmol) | Adenine (mmol) | Biobased C Content (%) 1 | Biobased Mass Fraction (%) 2 |
|---|---|---|---|---|---|---|---|---|
| CA1 | 97.7 | 2.3 | 10 | 0.235 | 29.4 | 1.7 | 29.2 | 34.1 |
| CA2 | 95.5 | 4.5 | 10 | 0.471 | 29.4 | 3.5 | 29.8 | 34.8 |
| CA3 | 92.5 | 7.5 | 10 | 0.810 | 29.4 | 6.0 | 30.3 | 35.6 |
| Sample | ΔH (J/g) 1 | Tr max (°C) 2 | Tg (°C) 3 |
|---|---|---|---|
| CA1 | 316 | 217 | 123 |
| CA2 | 325 | 207 | 124 |
| CA3 | 350 | 142 | 132 |
| Sample | Tg (°C) | Tg (°C) | Tg (°C) |
|---|---|---|---|
| Tiso 160 °C 1 | Tiso 180 °C 1 | Tiso 200 °C 1 | |
| CA1 | / | 68 | 117 |
| CA2 | 57 | 152 | 142 |
| CA3 | 168 | 147 | 147 |
| Sample | Fiber Fraction 1 (w%) | ADD 2 (g/cm3) | E’ 3 (GPa) | E’ onset 3 (°C) | tan δ peak 3 (°C) |
|---|---|---|---|---|---|
| CA-vCF | 77 | 1.45 | 33.8 ± 1.6 | 119 | 144 |
| CA-rCF | 75 | 1.49 | 25.9 ± 1.5 | 120 | 147 |
| CA-FF | 70 | 1.15 | 7.2 ± 1.5 | 95 | 116 |
| CA-JF | 62 | 1.03 | 8.7 ± 2.1 | 101 | 120 |
| Sample | Weight Loss T < 110 °C (w%) | Td onset (°C) | Residue (w%) |
|---|---|---|---|
| CA-vCF | / | 359 ± 2 | 77 ± 2 |
| CA-rCF | / | 356 ± 2 | 69 ± 7 |
| CA-FF | 3 ± 1 | 316 ± 1 | 1 ± 1 |
| CA-JF | 3 ± 1 | 318 ± 2 | 1 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merighi, S.; Mazzocchetti, L.; Benelli, T.; Giorgini, L. Adenine as Epoxy Resin Hardener for Sustainable Composites Production with Recycled Carbon Fibers and Cellulosic Fibers. Polymers 2020, 12, 3054. https://doi.org/10.3390/polym12123054
Merighi S, Mazzocchetti L, Benelli T, Giorgini L. Adenine as Epoxy Resin Hardener for Sustainable Composites Production with Recycled Carbon Fibers and Cellulosic Fibers. Polymers. 2020; 12(12):3054. https://doi.org/10.3390/polym12123054
Chicago/Turabian StyleMerighi, Stefano, Laura Mazzocchetti, Tiziana Benelli, and Loris Giorgini. 2020. "Adenine as Epoxy Resin Hardener for Sustainable Composites Production with Recycled Carbon Fibers and Cellulosic Fibers" Polymers 12, no. 12: 3054. https://doi.org/10.3390/polym12123054





