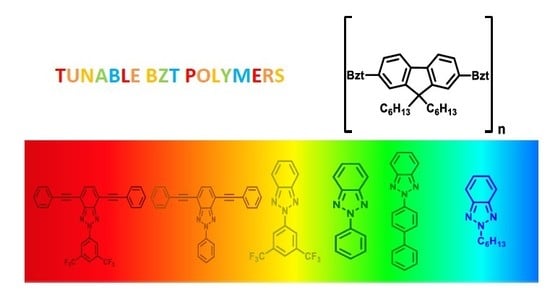
Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Monomer Synthesis
3.2. Polymers Synthesis
3.3. Photophysical Characterizacion
3.3.1. Optical Spectroscopy Study
3.3.2. Raman Spectroscopy Study
3.4. Fabrication and Characterization of OFETs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, M.Y.J. Recent Advances in Polymer Organic Light-Emitting Diodes (PLED) Using Non-conjugated Polymers as the Emitting Layer and Contrasting Them with Conjugated Counterparts. J. Electron. Mater. 2017, 46, 6246–6281. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Xiao, Y.; Han, G.; Lin, Y.J. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B.J. From Fullerene–Polymer to All-Polymer Solar Cells: The Importance of Molecular Packing, Orientation, and Morphology Control. Acc. Chem. Res. 2016, 49, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Li, M.; Hu, Z.; Wang, J.; Liu, F.; Li, N.; Brabec, C.; Janssen, R.; Bazan, G.; et al. Morphology Optimization via Side Chain Engineering Enables All-Polymer Solar Cells with Excellent Fill Factor and Stability. J. Am. Chem. Soc. 2018, 140, 8934–8943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, D.; Yu, D.; Wang, E. Conjugated Donor–Acceptor Terpolymers Toward High-Efficiency Polymer Solar Cells. Adv. Mater. 2019, 31, 1807019. [Google Scholar] [CrossRef] [PubMed]
- Genene, Z.; Mammo, W.; Wang, E.; Andersson, M.R. Recent Advances in n-Type Polymers for All-Polymer Solar Cells. Adv. Mater. 2019, 31, 1807275. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zheng, Y.; Yu, J. Polymer Dielectric in Organic Field-Effect Transistor. Prop. Appl. Polym. Dielectr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Kumari, N.; Nagamatsu, S.; Pandey, S. Recent Advances in Orientation of Conjugated Polymers for Organic Field-Effect Transistors. J. Mate. Chem. C 2019, 7, 13323–13351. [Google Scholar] [CrossRef]
- Gobalasingham, N.; Thompson, B. Direct arylation polymerization: A guide to optimal conditions for effective conjugated polymers. Prog. Polym. Sci. 2018, 83, 135–201. [Google Scholar] [CrossRef]
- Osaka, I.; Abe, T.; Shimawaki, M.; Koganezawa, T.; Takimiya, K. Naphthodithiophene-Based Donor–Acceptor Polymers: Versatile Semiconductors for OFETs and OPVs. ACS Macro Lett. 2012, 1, 437–440. [Google Scholar] [CrossRef]
- Paterson, A.; Singh, S.; Fallon, K.J.; Hodsden, T.; Han, Y.; Schroeder, B.C.; Bronstein, H.; Heeney, M.; McCulloch, I.; Anthopoulos, T.D. Recent Progress in High-Mobility Organic Transistors: A Reality Check. Adv. Mater. 2018, 30, 1801079. [Google Scholar] [CrossRef] [PubMed]
- Root, S.E.; Sagavatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef] [PubMed]
- Swager, T.M. 50th Anniversary Perspective: Conducting/Semiconducting Conjugated Polymers. A Personal Perspective on the Past and the Future. Macromolecules 2017, 50, 4867–4886. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hang, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar]
- Mei, J.; Bao, Z. Side Chain Engineering in Solution-Processable Conjugated Polymers. Chem. Mater. 2014, 26, 604–615. [Google Scholar] [CrossRef]
- Rochat, S.; Swager, T.M. Conjugated Amplifying Polymers for Optical Sensing Applications. ACS Appl. Mater. Interfaces 2013, 5, 4488–4502. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Ashraf, R.S.; Schroeder, P.; Angelo, P.D.; Watkins, S.E.; Song, K.; Anthopoulos, T.D.; McCulloch, I. Random benzotrithiophene-based donor–acceptor copolymers for efficient organic photovoltaic devices. Chem. Commun. 2012, 48, 5832–5834. [Google Scholar] [CrossRef] [Green Version]
- Burkhart, B.; Khylabich, P.P.; Thompson, B.C. Influence of the Acceptor Composition on Physical Properties and Solar Cell Performance in Semi-Random Two-Acceptor Copolymers. ACS Macro Lett. 2012, 1, 660–666. [Google Scholar] [CrossRef]
- Jung, J.W.; Liu, F.; Russell, T.P.; Jo, W.H. Semi-crystalline random conjugated copolymers with panchromatic absorption for highly efficient polymer solar cells. Energy Environ. Sci. 2013, 6, 3301–3307. [Google Scholar] [CrossRef]
- Sun, W.; Ma, Z.; Dang, D.; Zhu, W.; Andersson, M.R.; Zhang, F.; Wang, E. An alternating D–A1–D–A2 copolymer containing two electron-deficient moieties for efficient polymer solar cells. J. Mater. Chem. 2013, 1, 11141–11144. [Google Scholar] [CrossRef]
- Yuen, J.D.; Kumar, R.; Zakhidov, D.; Seifter, J.; Lim, B.; Heeger, A.J.; Wudl, F. Ambipolarity in Benzobisthiadiazole-Based Donor–Acceptor Conjugated Polymers. Adv. Mater. 2011, 23, 3780–3785. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Zoombelt, A.P.; Mathijssen, S.G.J.; Wienk, M.M.; Turbiez, M.; de Leeuw, D.M.; Janssen, R.A.J. Poly(diketopyrrolopyrrole−terthiophene) for Ambipolar Logic and Photovoltaics. J. Am. Chem. Soc. 2009, 131, 16616–16617. [Google Scholar] [CrossRef] [PubMed]
- Anthopoulos, T.D.; Setayesh, S.; Smits, E.; Cölle, M.; Cantatore, E.; de Boer, B.; de Leeuw, D.M. Air-Stable Complementary-like Circuits Based on Organic Ambipolar Transistors. Adv. Mater. 2006, 18, 1900–1904. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer−Fullerene Solar Cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zhang, Z.G.; Li, Y. Synthesis and applications of difluorobenzothiadiazole based conjugated polymers for organic photovoltaics. J. Am. Chem. Soc. 2011, 21, 3226–3233. [Google Scholar]
- Li, W.; Yan, L.; Zhou, H.; You, W. High Thermoelectric Performance and Enhanced Mechanical Stability of p-type Ge1–xSbxTe. Chem. Matter. 2015, 27, 6470–6476. [Google Scholar] [CrossRef]
- Casey, A.; Green, J.P.; Tuladhar, P.S.; Kirkus, M.; Han, Y.; Anthopoulos, T.D.; Heeney, M. Cyano substituted benzotriazole based polymers for use in organic solar cells. J. Mater. Chem. A 2017, 5, 6465–6470. [Google Scholar] [CrossRef] [Green Version]
- Torres-Moya, I.; Díaz-Ortiz, A.; Sánchez, L.; Orduna, J.; Blesa, M.J.; Carrillo, J.R.; Prieto, P. Tunable emission in aggregated T-Shaped 2H-Benzo[d][1,2,3]triazoles with waveguide behaviour. Dyes Pigments 2017, 142, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Torres-Moya, I.; Saikia, B.; Carrillo, J.R.; Prieto, P.; Steed, J.W. High thermal stability, pH responsive organogels of 2H-benzo[d]1,2,3-triazole derivatives as pharmaceutical crystallization media. CrystEngComm 2018, 21, 2135–2143. [Google Scholar] [CrossRef] [Green Version]
- Torres-Moya, I.; Arrechea-Marcos, I.; Tardío, C.; Carrillo, J.R.; Díaz-Ortiz, A.; López Navarrete, J.T.; Ruiz Delgado, M.C.; Prieto, P.; Ponce, R. D–A–D 2H-benzo[d][1,2,3]triazole derivatives as p-type semiconductors in organic field-effect transistors. RSC Adv. 2018, 8, 21879–21888. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Guilló, R.; Martínez-Tomé, M.J.; Kahveci, Z.; Torres, I.; Falco, A.; Mallavia, R.; Mateo, C.R. Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers 2018, 10, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekiz, F.; Rende, E.; Timur, S.; Toppare, L. A novel functional conducting polymer: Synthesis and application to biomolecule immobilization. J. Mater. Chem. 2012, 22, 22517–22525. [Google Scholar]
- Wettach, H.; Pasker, F.; Höger, S. Fluorescent Conjugated Polymers That Incorporate Substituted 2,1,3-Benzooxadiazole and 2,1,3-Benzothiadiazole Units. Macromolecules 2008, 41, 9513–9515. [Google Scholar] [CrossRef]
- Ma, C.; Pisula, W.; Weber, E.; Freng, X.; Müllen, K.; Bäverle, P. Dendritic Oligothiophenes Terminated with Tris(alkyloxy)phenylethynyl Tails: Synthesis, Physical Properties, and Self-Assembly. Chem. Eur. J. 2011, 17, 1507–1518. [Google Scholar] [CrossRef]
- Torres, I.; Carrillo, J.R.; Díaz-Ortiz, A.; Martín, R.; Gómez, M.V.; Stegemann, L.; Strassert, C.A.; Orduna, J.; Buendía, J.; Greciano, E.E.; et al. Self-assembly of T-shape 2H-benzo[d][1,2,3]-triazoles. Optical waveguide and photophysical properties. RSC Adv. 2016, 6, 36544–36553. [Google Scholar] [CrossRef]
- Pastor, M.J.; Torres, I.; Cebrián, C.; Carrillo, J.R.; Díaz-Ortiz, A.; Matesanz, E.; Buendía, J.; García, F.; Barberá, J.; Prieto, P.; et al. 4-Aryl-3,5-bis(arylethynyl)aryl-4H-1,2,4-triazoles: Multitasking Skeleton as a Self-Assembling Unit. Chem. Eur. J. 2015, 21, 1795–1802. [Google Scholar] [CrossRef]
- Vázquez-Guilló, R.; Falco, A.; Martínez-Tomé, M.J.; Mateo, C.R.; Herrero, M.A.; Vázquez, E.; Mallavia, R. Advantageous Microwave-Assisted Suzuki Polycondensation for the Synthesis of Aniline-Fluorene Alternate Copolymers as Molecular Model with Solvent Sensing Properties. Polymers 2018, 10, 215–235. [Google Scholar]
- Liu, B.; Bazan, G.C. Synthesis of cationic conjugated polymers for use in label-free DNA microarrays. Nat. Protoc. 2006, 1, 1698–1702. [Google Scholar] [CrossRef]
- Molina, R.; Gómez-Ruiz, S.; Montilla, F.; Salinas-Castillo, A.; Fernández-Arroyo, S.; del Mar Ramos, M.; Micol, V.; Mallavia, R. Progress in the Synthesis of Poly(2,7-Fluorene-alt-1,4-Phenylene), PFP, via Suzuki Coupling. Macromolecules 2009, 42, 5471–5477. [Google Scholar] [CrossRef]
- Neves, A.C.B.; Pinto, S.M.A.; Henriques, C.A.; Rosado, M.T.S.; Mallavia, R.; Burrows, H.D.; Pereira, M.M.; Calvete, M.J.F. Routes to synthesis of porphyrins covalently bound to poly(carbazole)s and poly(fluorene)s: Structural and computational studies on oligomers. J. Mol. Struct. 2012, 1029, 199–208. [Google Scholar] [CrossRef]
- Klaerner, G.; Mullen, R.D. Polyfluorene Derivatives: Effective Conjugation Lengths from Well-Defined Oligomers. Macromolecules 1998, 31, 2007–2009. [Google Scholar] [CrossRef]
- Kuhn, H. Uber das Absorptionsspektrum der Polyenel. Chim. Acta 1948, 31, 1780–1799. [Google Scholar] [CrossRef] [PubMed]
- Mori-Sanchez, P.; Cohen, A.J.; Yang, W. Localization and Delocalization Errors in Density Functional Theory and Implications for Band-Gap Prediction. Phys. Rev. Lett. 2008, 100, 14601. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.F.; Roldao, J.C.; Medina, B.B.; Lavarda, F.C.; Gierschner, J. Calculation of low bandgap homopolymers: Comparison of TD-DFT methods with experimental oligomer series. Chem. Phys. Lett. 2016, 645, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Wykes, M.; Milián Medina, B.; Gierschner, J. Computational engineering of low bandgap copolymers. Front. Chem. 2013, 1, 35. [Google Scholar] [CrossRef]
- Ponce, R.; Casado, J.; Hernández, V.; López, J.T.; Viruela, P.M.; Orti, E.; Takimiya, K.; Otsubo, T. On the biradicaloid nature of long quinoidal oligothiophenes: Experimental evidence guided by theoretical studies. Angew. Chem. Int. Ed. 2007, 46, 9057–9061. [Google Scholar] [CrossRef]
- Zerbi, G.; Veronelli, M.; Martina, S.; Schlüter, A.D.; Wegner, G. π-Electron delocalization in conformationally distorted oligopyrroles and ploypyrrole. Adv. Mater. 1994, 6, 385–388. [Google Scholar] [CrossRef]
- Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman spectroscopy of polyconjugated molecules and materials: Confinement effect in one and two dimensions. Math. Phys. Eng. Sci. 2004, 362, 2425–2459. [Google Scholar] [CrossRef]
- Size, S.M.; Lee, M.K. Semiconductor Devices: Physics and Technology, 3rd ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Ponce, R.; Facchetti, A.; Marks, T.J. High-k Organic, Inorganic, and Hybrid Dielectrics for Low-Voltage Organic Field-Effect Transistors. Chem. Rev. 2010, 110, 205–239. [Google Scholar]
- Sun, Y.; Liu, Y.; Zhu, D. Advances in organic field-effect transistors. J. Mater. Chem. 2005, 15, 53–65. [Google Scholar] [CrossRef]












| Compound | Mua | N# b | Mwc | Mnd | ne | PDI f |
|---|---|---|---|---|---|---|
| P1a | 536 | 6 | 10,771 | 3699 | 7 | 2.91 |
| P1b | 526 | 6 | 19,442 | 7333 | 14 | 2.65 |
| P1c | 662 | 6 | 9505 | 5001 | 8 | 1.90 |
| P1d | 602 | 6 | 6239 | 3298 | 5.5 | 1.89 |
| P2b | 726 | 12 | 6455 | 1605 | 2.5 | 4.02 |
| P2c | 862 | 12 | 7377 | 3879 | 4.5 | 1.90 |
| Compound | Abs a | Em | Φ b | Egap c | HOMO | LUMO | Egapd | ||
|---|---|---|---|---|---|---|---|---|---|
| λmax1 | λmax2 | λonset | λmax | (eV) | (eV) | ||||
| P1a | 279 | 410 | 462 | 468 | 0.64 | 2.69 | −4.85 | −1.96 | 2.93 |
| P1b | 275 | 423 | 460 | 493 | 0.62 | 2.70 | −4.94 | −2.12 | 2.82 |
| P1c | 318 | 441 | 503 | 497 | 0.53 | 2.46 | −5.20 | −2.49 | 2.71 |
| P1d | 341 | 429 | 461 | 493 | 0.54 | 2.69 | −4.94 | −2.19 | 2.75 |
| P2b | 335 | 399 | 469 | 520 | 0.58 | 2.64 | −5.07 | −2.38 | 2.69 |
| P2c | 332 | 401 | 0481 | 574 | 0.51 | 2.58 | −5.21 | −2.68 | 2.53 |
| Compound | C=C/C-C Stretching (cm−1) | C≡C Stretching (cm−1) |
|---|---|---|
| P1a | 1582 | - |
| P1b | 1580 | - |
| P1c | 1579 | - |
| P1d | 1580 | - |
| P2b | 1578 | 2205 |
| P2c | 1572 | 2201 |
| Compound | Treatment | Annealing (°C) | ION/IOFF | VT (V) | µ (cm2·V−1·s−1) |
|---|---|---|---|---|---|
| P1a | HMDS | 180 | 3.94 × 103 | −47 | 2.23 × 10−4 |
| P1b | HMDS | 240 | 1.11 × 101 | −32 | 3.11 × 10−4 |
| P1c | None | 240 | 1.57 × 102 | −10 | 2.38 × 10−4 |
| P1d | HMDS | 180 | 3.36 × 102 | −50 | 2.15 × 10−4 |
| P2c | OTS | 100 | 6.79 × 102 | −85 | 1.79 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Moya, I.; Vázquez-Guilló, R.; Fernández-Palacios, S.; Carrillo, J.R.; Díaz-Ortiz, Á.; López Navarrete, J.T.; Ponce Ortiz, R.; Ruiz Delgado, M.C.; Mallavia, R.; Prieto, P. Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties. Polymers 2020, 12, 256. https://doi.org/10.3390/polym12020256
Torres-Moya I, Vázquez-Guilló R, Fernández-Palacios S, Carrillo JR, Díaz-Ortiz Á, López Navarrete JT, Ponce Ortiz R, Ruiz Delgado MC, Mallavia R, Prieto P. Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties. Polymers. 2020; 12(2):256. https://doi.org/10.3390/polym12020256
Chicago/Turabian StyleTorres-Moya, Iván, Rebeca Vázquez-Guilló, Sara Fernández-Palacios, José Ramón Carrillo, Ángel Díaz-Ortiz, Juan Teodomiro López Navarrete, Rocío Ponce Ortiz, Mari Carmen Ruiz Delgado, Ricardo Mallavia, and Pilar Prieto. 2020. "Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties" Polymers 12, no. 2: 256. https://doi.org/10.3390/polym12020256
APA StyleTorres-Moya, I., Vázquez-Guilló, R., Fernández-Palacios, S., Carrillo, J. R., Díaz-Ortiz, Á., López Navarrete, J. T., Ponce Ortiz, R., Ruiz Delgado, M. C., Mallavia, R., & Prieto, P. (2020). Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties. Polymers, 12(2), 256. https://doi.org/10.3390/polym12020256