Facile Preparation of Flame Retardant Cotton Fabric via Adhesion of Mg(OH)2 by the Assistance of Ionic Liquid
Abstract
1. Introduction
2. Experimental
2.1. Synthesis and Purification of IL and MH/IL Suspension
2.2. Swelling of CF in IL
2.3. Spray of MH/IL Suspension and Adhesion of MH onto CF
2.4. Characterization and Analysis
3. Results and Discussion
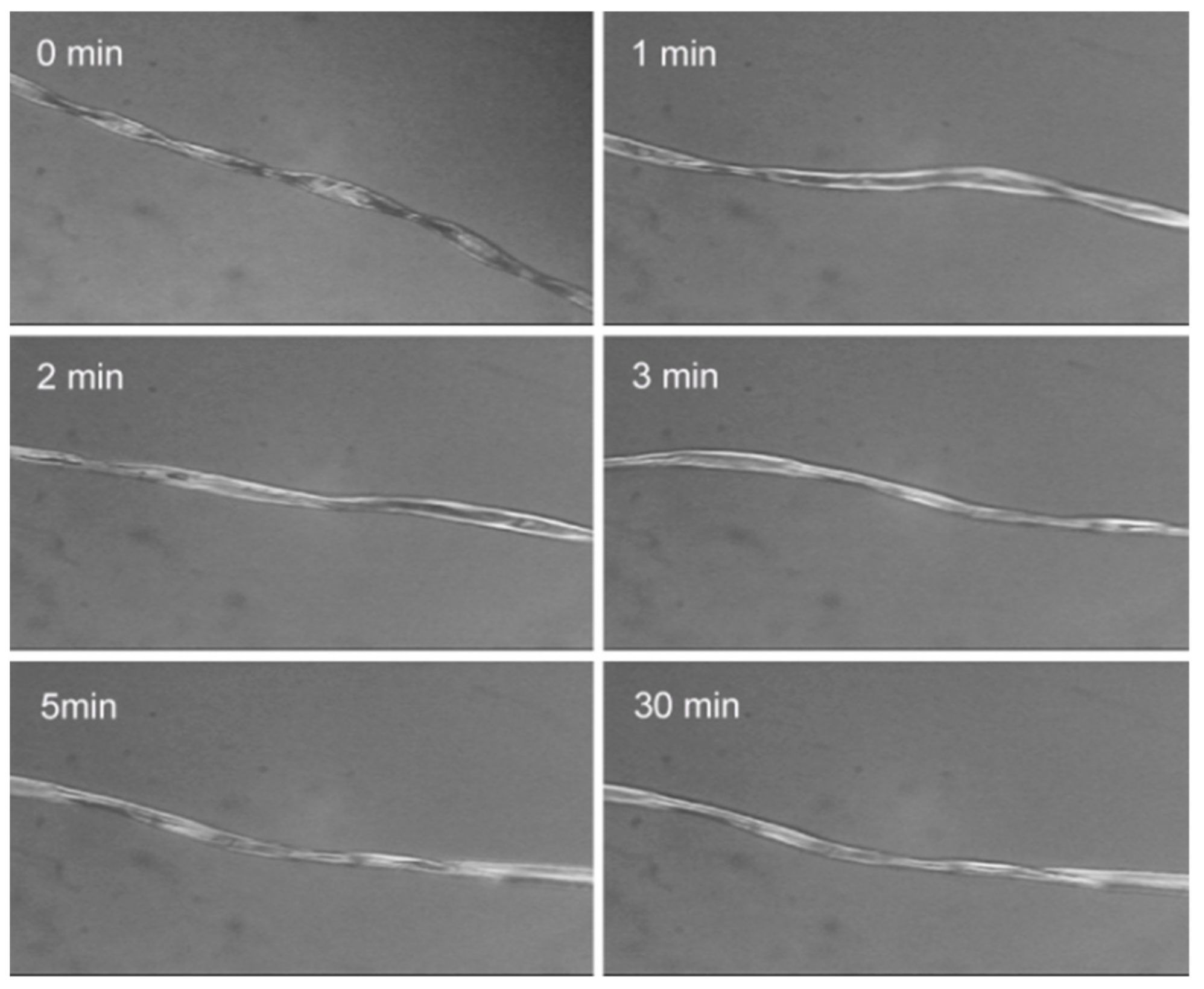
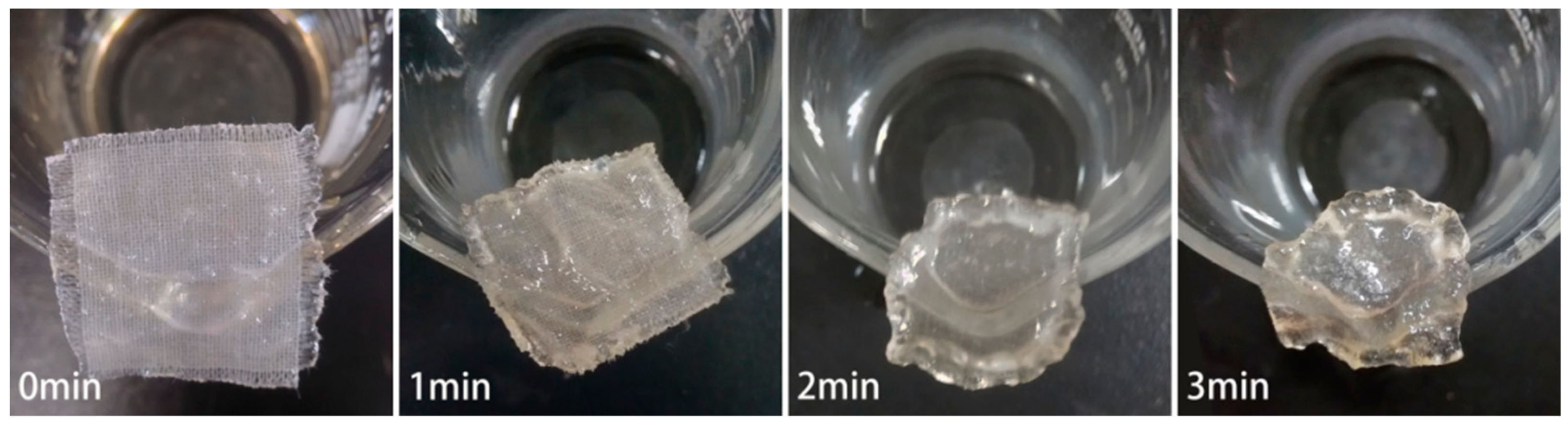
3.1. CF Swelling in IL
3.2. Effect of Adhesion Conditions on Flame Retardancy of CF
3.3. Effect of Heating Methods on Flame Retardancy of CF
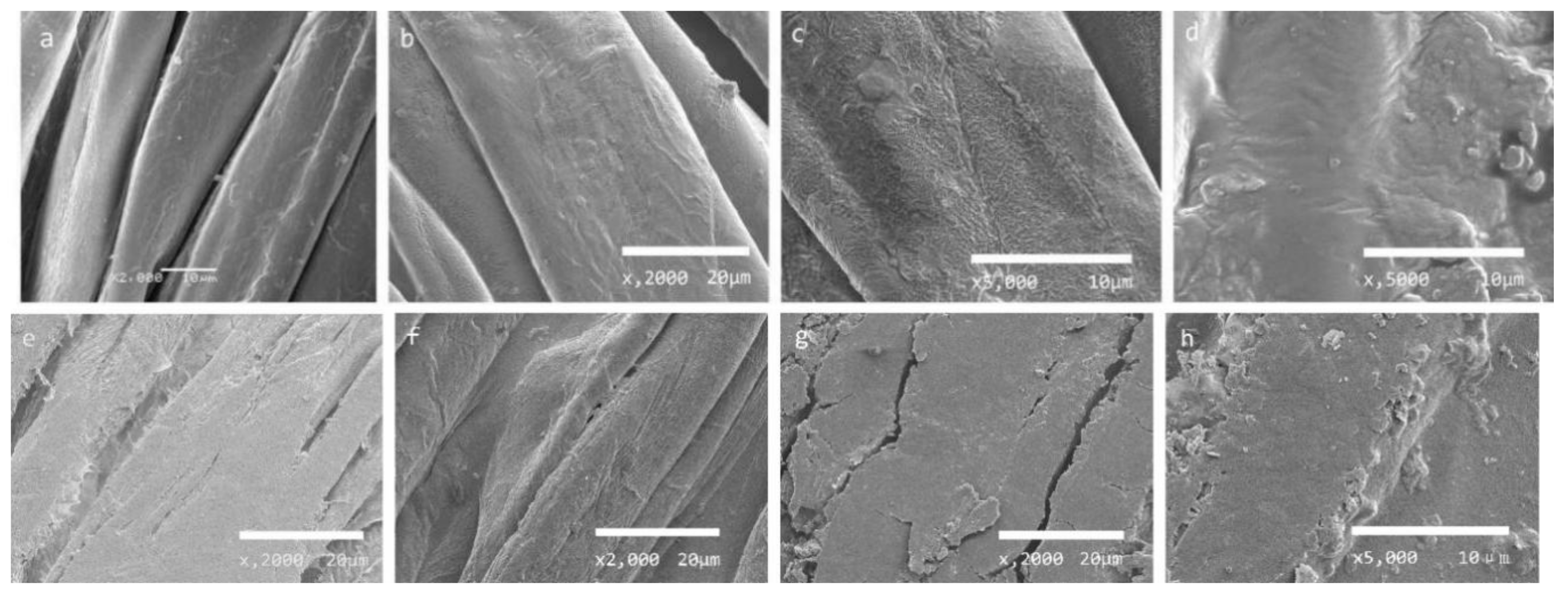
3.4. SEM Analysis
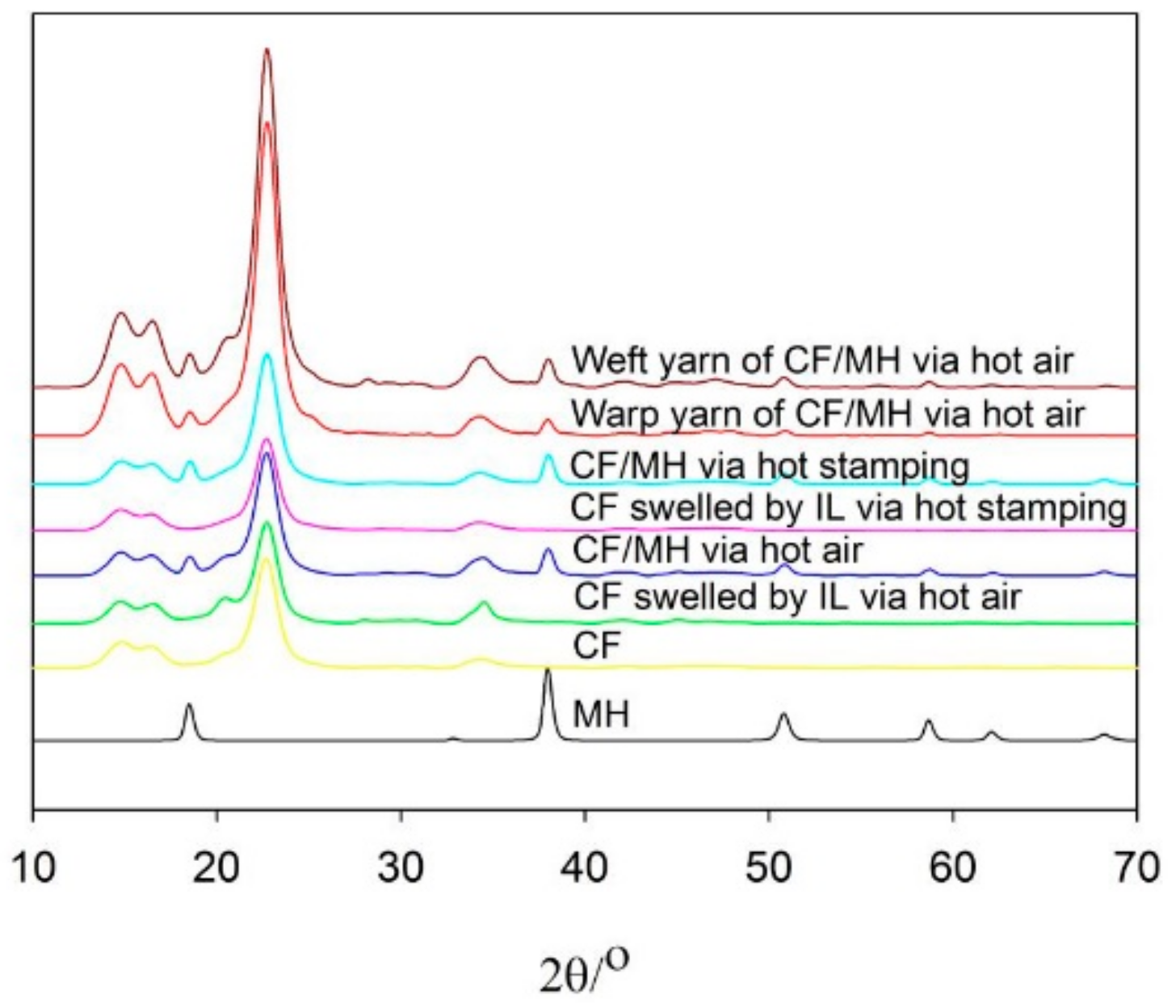
3.5. XRD Analysis
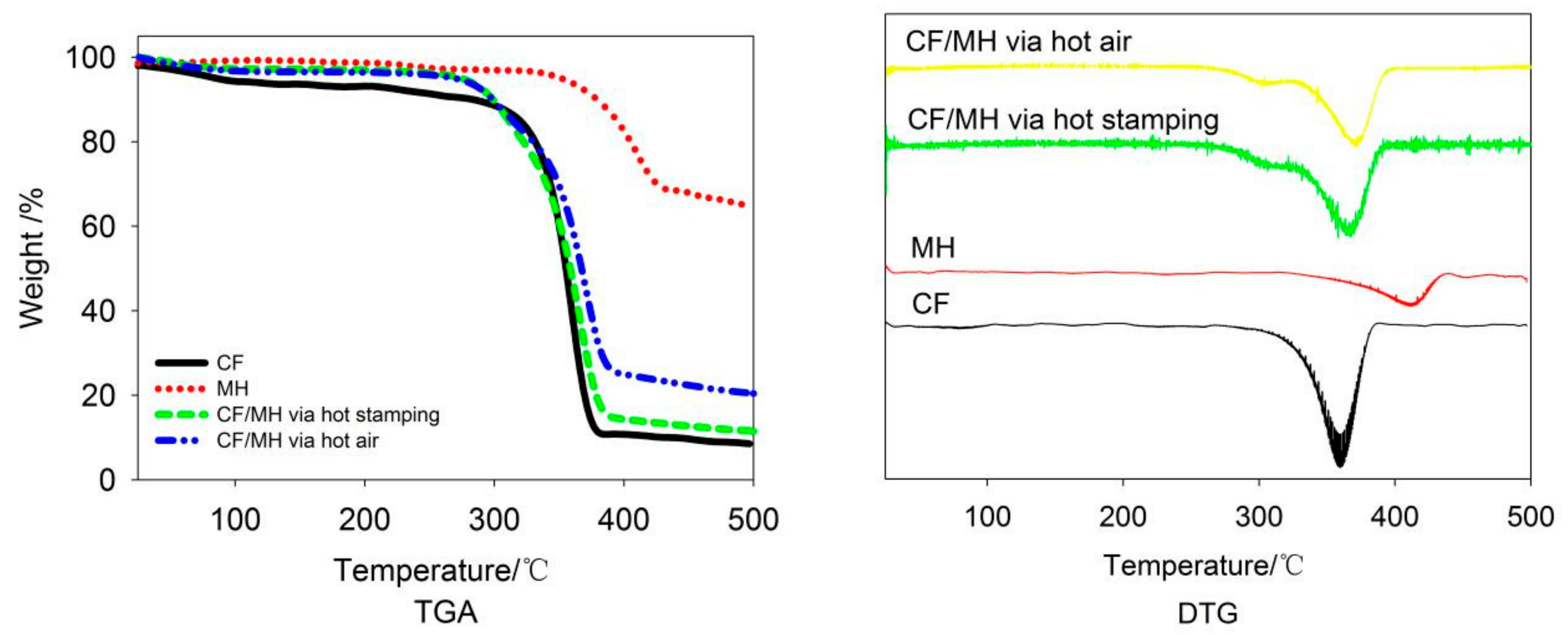
3.6. Thermal Properties
3.7. Combustion Behavior
3.8. Physical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- White, L.A. Preparation and thermal analysis of cotton-clay nanocomposites. J. Appl. Polym. Sci. 2004, 92, 2125–2131. [Google Scholar] [CrossRef]
- Mohsin, M.; Ahmad, S.W.; Khatri, A.; Zahid, B. Performance enhancement of fire retardant finish with environment friendly bio cross-linker for cotton. J. Clean. Prod. 2013, 51, 191–195. [Google Scholar] [CrossRef]
- Lam, Y.L.; Kan, C.W.; Yuen, C.W.M. Objective measurement of hand properties of plasma pre-treated cotton fabrics subjected to flame-retardant finishing catalyzed by zinc oxide. Fiber Polym. 2014, 15, 1880–1886. [Google Scholar] [CrossRef]
- El-Shafei, A.; Elshemy, M.; Abou-Okeil, A. Eco-friendly finishing agent for cotton fabrics to improve flame retardant and antibacterial properties. Carbohydr. Polym. 2015, 118, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, L.; Xu, Y.; Zhang, C.; Zhang, F.; Zhang, G. Highly efficient flame-retardant and soft cotton fabric prepared by a novel reactive flame retardant. Cellulose 2019, 26, 4225–4240. [Google Scholar] [CrossRef]
- Wang, X.; Romero, M.Q.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Intumescent multilayer hybrid coating for flame retardant cotton fabrics based on layer-by-layer assembly and sol–gel process. RSC Adv. 2015, 5, 10647–10655. [Google Scholar] [CrossRef]
- Giuseppe, R.; Angela, C.; Valentina, T.; Giuseppina, I.; Giulio, M. Thermal and flame retardant behavior of cotton fabrics treated with a novel nitrogen-containing carboxyl-functionalized organophosphorus system. Carbohydr. Polym. 2018, 196, 348–358. [Google Scholar]
- Zheng, D.; Zhou, J.; Zhong, L.; Zhang, F.; Zhang, G. A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 2016, 23, 2211–2220. [Google Scholar] [CrossRef]
- Chan, S.Y.; Si, L.; Lee, K.I.; Ng, P.F.; Chen, L.; Yu, B. A novel boron–nitrogen intumescent flame retardant coating on cotton with improved washing durability. Cellulose 2017, 25, 843–857. [Google Scholar] [CrossRef]
- Xie, K.; Gao, A.; Zhang, Y. Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr. Polym. 2013, 98, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Abou-Okeil, A.; El-Sawy, S.M.; Abdel-Mohdy, F.A. Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohydr. Polym. 2013, 92, 2293–2298. [Google Scholar] [CrossRef]
- Li, Q.L.; Wang, X.L.; Wang, D.Y.; Wang, Y.Z.; Feng, X.N.; Zheng, G.H. Durable flame retardant finishing of pet/cotton blends using a novel pva-based phosphorus-nitrogen polymer. J. Appl. Polym. Sci. 2011, 122, 342–353. [Google Scholar] [CrossRef]
- Li, Q.L.; Wang, X.L.; Wang, D.Y.; Xiong, W.C.; Zhong, G.H.; Wang, Y.Z. A novel organophosphorus flame retardant: Synthesis and durable finishing of poly(ethylene terephthalate)/cotton blends. J. Appl. Polym. Sci. 2010, 117, 3066–3074. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Mao, Z. Controlled growth of magnesium hydroxide crystals and its effect on the high-temperature properties of cotton/magnesium hydroxide composites. Vacuum 2013, 95, 1–5. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame retardant, heat insulating cellulose aerogels from waste cotton fabrics by in situ formation of magnesium hydroxide nanoparticles in cellulose gel nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lv, L. Preparation of Mg(OH)2 hybrid pigment by direct precipitation and graft onto cellulose fiber via surface-initiated atom transfer radical polymerization. Appl. Surf. Sci. 2016, 363, 189–196. [Google Scholar] [CrossRef]
- Cheng, G.; Varanasi, P.; Arora, R.; Stavila, V.; Simmons, B.A.; Kent, M.S. Impact of ionic liquid pretreatment conditions on cellulose crystalline structure using 1-ethyl-3-methylimidazolium acetate. J. Phys. Chem. B 2012, 116, 10049–10054. [Google Scholar] [CrossRef]
- Kim, M.H.; An, S.; Won, K.; Kim, H.J.; Lee, S.H. Entrapment of enzymes into cellulose–biopolymer composite hydrogel beads using biocompatible ionic liquid. J. Mol. Catal. B Enzym. 2012, 75, 68–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Miao, J.; Maeda, N.; Frey, D.; Linhardt, R.J.; Simmons, T.J. Uniform nanoparticle coating of cellulose fibers during wet electrospinning. J. Mater. Chem. A 2014, 2, 15029–15034. [Google Scholar] [CrossRef]
- Lucas, M.; Macdonald, B.A.; Wagner, G.L.; Joyce, S.A.; Rector, K.D. Ionic liquid pretreatment of poplar wood at room temperature: Swelling and incorporation of nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ono, T. Ionic liquid assisted enzymatic delignification of wood biomass: A new ‘green’ and efficient approach for isolating of cellulose fibers. Biochem. Eng. J. 2012, 60, 156–160. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Siriprom, W.; Kuha, P.; Kongsriprapan, S.; Teanchai, K. Studying methylcellulose-base edible films properties by XRD, EDXRF and FTIR. Adv. Mater. Res. 2014, 979, 319–322. [Google Scholar] [CrossRef]
- Siriprom, W.; Witit-Anun, N.; Choeysuppaket, A.; Ratana, T. Characterization of cellulose/calcium carbonate biocomposite film. Key Eng. Mater. 2016, 675–676, 209–212. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Newman, R.H.; Staiger, M.P. Structure–property relationship of all-cellulose composites. Compos. Sci. Technol. 2009, 69, 1225–1230. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Wang, D.; Zhang, R.; Liu, G.; Cheng, G. Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour. Technol. 2014, 153, 379–382. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, R.; Zhang, X. Application of Cone Calorimeter in Combustion Performance Evaluation of Flame Retardant Cotton Fabrics. J. Beijing Inst. Cloth. Technol. 2018, 38, 13–17. [Google Scholar]

| Spraying Temperature/Heating Time/Heating Temperature/Tension | Adhesion Ratio of MH/% | Warp Shrinkage Ratio/% | Weft Shrinkage Ratio/% | Afterflame Time/s | Afterglow Time/s |
|---|---|---|---|---|---|
| CF | 0 | 0 | 0 | 4.2 | 40.8 |
| 36 °C/10 min/105 °C | 4.00 | 9.71 | 38.83 | 8.9 | 16.1 |
| 39 °C/10 min/105 °C | 9.89 | 11.43 | 40.95 | 7.1 | 10.6 |
| 41 °C/10 min/105 °C | 9.74 | 12.57 | 42.86 | 11.1 | 10.0 |
| 39 °C/3 min/95 °C | 4.20 | 0 | 4.72 | 1.2 | 15.7 |
| 39 °C/6 min/95 °C | 7.99 | 1.09 | 15.24 | 2.4 | 3.5 |
| 39 °C/10 min/95 °C | 9.38 | 0.84 | 31.78 | 6.6 | 2.1 |
| 39 °C/10 min/105 °C/under no tension | 10.13 | 20.28 | 42.37 | 9.0 | 13.6 |
| Samples | Adhesion Ratio of MH/% | Warp Shrinkage Ratio/% | Weft Shrinkage Ratio/% | Afterflame Time/s | Afterglow Time/s | |
|---|---|---|---|---|---|---|
| 90 °C | hot air | 7.86 | 0 | 16.35 | 4.6 | 7.9 |
| 95 °C | 9.38 | 0.84 | 31.78 | 6.6 | 2.1 | |
| 105 °C | 9.89 | 11.43 | 40.95 | 7.1 | 10.6 | |
| 80 °C | hot stamping | 6.28 | 0.83 | 1.90 | 3.2 | 4.0 |
| 90 °C | 7.36 | 1.40 | 2.13 | 2.0 | 3.0 | |
| 95 °C | 8.02 | 2.00 | 2.86 | 2.5 | 4.6 | |
| 105 °C | 10.33 | 2.86 | 3.46 | 2.8 | 5.2 | |
| Sample | Weight Loss/% | Maximum Decomposition Temperature/°C |
|---|---|---|
| MH | 35.4 | 411.3 |
| CF | 91.4 | 359.5 |
| CF/MH via hot air | 79.7 | 371.0 |
| CF/MH via hot stamping | 88.5 | 367.2 |
| Samples | Bending Rigidity/cN.mm−1 | Activity Ratio/% | Breaking Strength/N | Breaking Elongation Ratio/% |
|---|---|---|---|---|
| CF | 0.95 | 52.44 | 593 | 12.9 |
| CF/MH via hot air | 1.09 | 45.31 | 787 | 13.7 |
| CF/MH via hot stamping | 2.15 | 17.90 | 662 | 12.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, X.; Li, J.; Chen, R.; Wei, J. Facile Preparation of Flame Retardant Cotton Fabric via Adhesion of Mg(OH)2 by the Assistance of Ionic Liquid. Polymers 2020, 12, 259. https://doi.org/10.3390/polym12020259
Ma J, Wang X, Li J, Chen R, Wei J. Facile Preparation of Flame Retardant Cotton Fabric via Adhesion of Mg(OH)2 by the Assistance of Ionic Liquid. Polymers. 2020; 12(2):259. https://doi.org/10.3390/polym12020259
Chicago/Turabian StyleMa, Jinli, Xiao Wang, Jing Li, Ru Chen, and Ju Wei. 2020. "Facile Preparation of Flame Retardant Cotton Fabric via Adhesion of Mg(OH)2 by the Assistance of Ionic Liquid" Polymers 12, no. 2: 259. https://doi.org/10.3390/polym12020259
APA StyleMa, J., Wang, X., Li, J., Chen, R., & Wei, J. (2020). Facile Preparation of Flame Retardant Cotton Fabric via Adhesion of Mg(OH)2 by the Assistance of Ionic Liquid. Polymers, 12(2), 259. https://doi.org/10.3390/polym12020259





