Thin Film Composite Forward Osmosis Membrane with Single-Walled Carbon Nanotubes Interlayer for Alleviating Internal Concentration Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization
2.4. Intrinsic Transport Property and FO Performance Measurement
3. Results
3.1. SWCNTs Interlayer Characterization
3.2. TFC FO Membrane Characterization
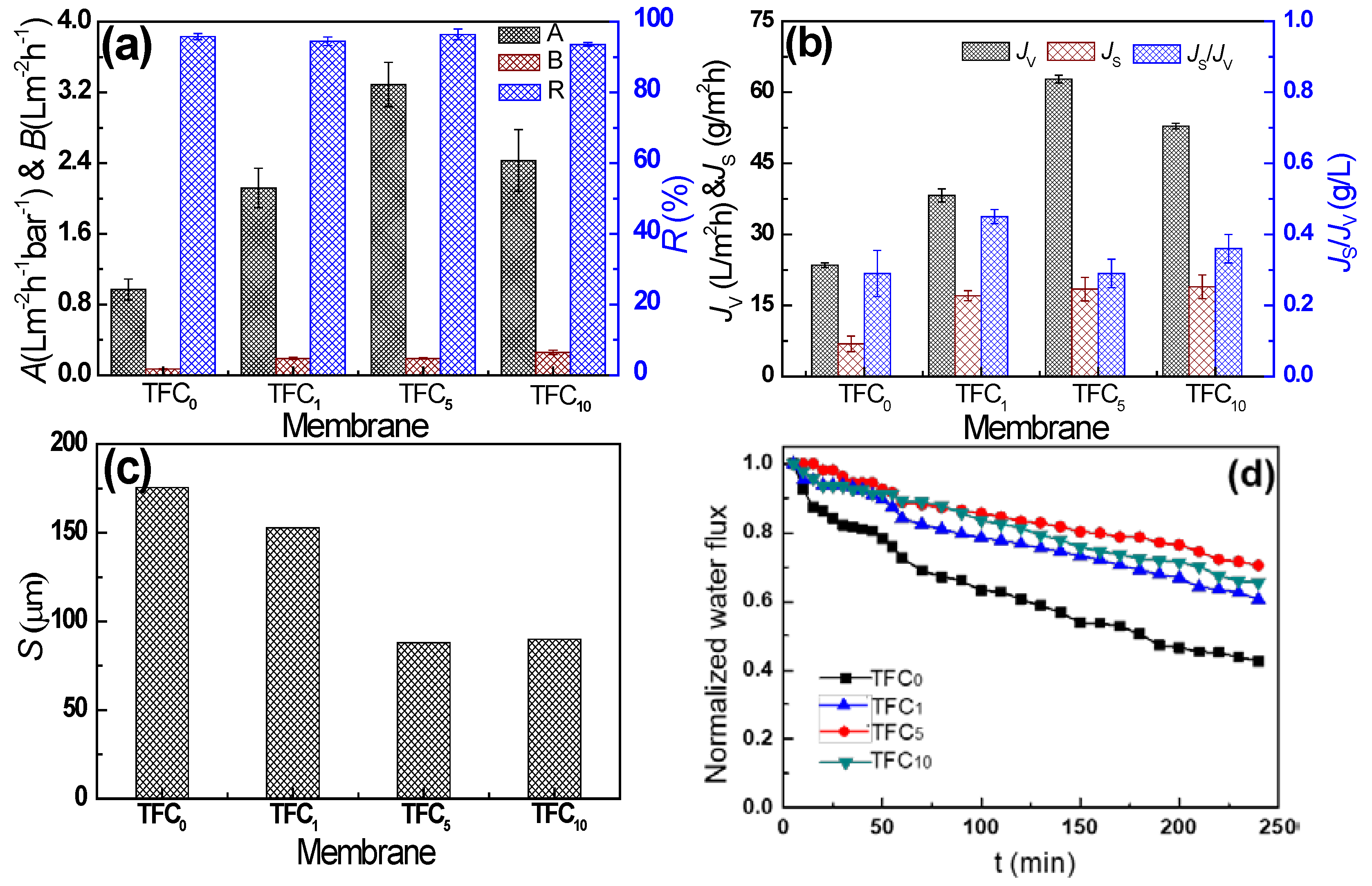
3.3. Intrinsic Separation Properties and FO Performance of TFC Membranes
3.4. Comparisons of Intrinsic Separation Properties and FO Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cath, T.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Klaysom, C.; Cath, T.Y.; Depuydt, T.; Vankelecom, I.F.J. Forward and pressure retarded osmosis: Potential solutions for global challenges in energy and water supply. Chem. Soc. Rev. 2013, 42, 6959–6989. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.; Chen, G.; Chung, W.; Shon, H. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Ulbricht, M. Nano-sized metal organic framework to improve the structural properties and desalination performance of thin film composite forward osmosis membrane. J. Membr. Sci. 2017, 531, 59–67. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Wen, X.; Huang, X. Feasibility of applying forward osmosis to the simultaneous thickening, digestion, and direct dewatering of waste activated sludge. Bioresour. Technol. 2012, 113, 207–213. [Google Scholar] [CrossRef]
- Holloway, R.W.; Childress, A.E.; Dennett, K.E.; Cath, T.Y. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4114. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. Desalination by ammonia–carbon dioxide forward osmosis: Influence of draw and feed solution concentrations on process performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. A novel ammonia—Carbon dioxide forward (direct) osmosis desalination process. Desalination 2005, 174, 1–11. [Google Scholar] [CrossRef]
- Wang, K.; Teoh, M.; Nugroho, A.; Chung, T. Integrated forward osmosis–membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Jin, X.; Shan, J.; Wang, C.; Wei, J.; Tang, C. Rejection of pharmaceuticals by forward osmosis membranes. J. Hazard Mater. 2012, 227-228, 55–61. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. J. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.; Weber, M.; Maletzko, C.; Warzelhan, V. The role of sulphonated polymer and macrovoid-free structure in the support layer for thin-film composite (TFC) forward osmosis (FO) membranes. J. Membr. Sci. 2011, 383, 214–223. [Google Scholar] [CrossRef]
- Han, G.; Chung, T.; Toriida, M.; Tamai, S. Thin-film composite forward osmosis membranes with novel hydrophilic supports for desalination. J. Membr. Sci. 2012, 423-424, 543–555. [Google Scholar] [CrossRef]
- Mehta, G.; Loeb, S. Internal polarization in the porous substructure of a semipermeable membrane under pressure-retarded osmosis. J. Membr. Sci. 1978, 4, 261–265. [Google Scholar] [CrossRef]
- Alsvik, I.; Hägg, M. Pressure Retarded Osmosis and Forward Osmosis Membranes: Materials and Methods. Polymers 2013, 5, 303–327. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Liu, Y.; Jian, M.; Gao, L.; Wang, H.; Zhang, X. Thin-Film Nanocomposite Forward-Osmosis Membranes on Hydrophilic Microfiltration Support with an Intermediate Layer of Graphene Oxide and Multiwall Carbon Nanotube. ACS Appl. Mater. Inter. 2018, 10, 34464–34474. [Google Scholar] [CrossRef]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Choi, H.; Son, M.; Choi, H. Integrating seawater desalination and wastewater reclamation forward osmosis process using thin-film composite mixed matrix membrane with functionalized carbon nanotube blended polyethersulfone support layer. Chemosphere 2017, 185, 1181–1188. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Karan, A.; Jiang, Z.; Livingston, A.G. Su—10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 6241, 1347–1351. [Google Scholar] [CrossRef]
- Wu, M.; Lv, Y.; Yang, H.; Liu, L.; Zhang, X.; Xu, Z. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Gao, S.; Zhang, F.; Zhang, W.; Liu, Z.; Jin, J. Single-Walled Carbon Nanotube Film Supported Nanofiltration Membrane with a Nearly 10 nm Thick Polyamide Selective Layer for High-Flux and High-Rejection Desalination. Small 2016, 12, 5034–5041. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, Y.; Boo, C.; Liu, Z.; Li, J.; Deng, L.; An, X. High-Performance Thin-Film Composite Membrane with an Ultrathin Spray-Coated Carbon Nanotube Interlayer. Environ. Sci. Technol. Lett. 2018, 5, 243–248. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, J.; Xu, J.; Shan, B. Switchable oil/water separation with efficient and robust Janus nanofiber membranes. Carbon 2017, 115, 477–485. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, W.; Zhang, F.; Liu, X.; Wang, D.; Jin, J.; Jiang, L. Ultrafast Separation of Emulsified Oil/Water Mixtures by Ultrathin Free-Standing Single-Walled Carbon Nanotube Network Films. Adv. Mater. 2013, 25, 2422–2427. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.S.; Amy, G. Developing thin-film-composite forward osmosis membranes on the PES/SPSf substrate through interfacial polymerization. AICHE J. 2012, 58, 770–781. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Xu, L.; Xu, J.; Shan, B.; Wang, X.; Gao, C. Novel thin-film composite membranes via manipulating the synergistic interaction of dopamine and m-phenylenediamine for highly efficient forward osmosis desalination. J. Mater. Chem. A 2017, 5, 7920–7932. [Google Scholar] [CrossRef]
- Lv, L.; Xu, J.; Shan, B.; Gao, C. Concentration performance and cleaning strategy for controlling membrane fouling during forward osmosis concentration of actual oily wastewater. J. Membr. Sci. 2017, 523, 15–23. [Google Scholar] [CrossRef]
- Xu, J.; Li, P.; Jiao, M.; Shan, B.; Gao, C. Effect of Molecular Configuration of Additives on the Membrane Structure and Water Transport Performance for Forward Osmosis. ACS Sustain. Chem. Eng. 2016, 4, 4433–4441. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High Performance Thin-Film Composite Forward Osmosis Membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Mustafa, A.; Kusworo, T.D.; Busairi, A.; Ismail, A.F. The Effect of Functionalization Carbon Nanotubes (CNTs) on the Performance of PES-CNTs Mixed Matrix Membrane. Int. J. Sci. Eng. 2010, 1, 15–20. [Google Scholar]
- Liu, X.; Ng, H.Y. Fabrication of layered silica–polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Ong, R.C.; Chung, T.; de Wit, J.S.; Helmer, B.J. Novel cellulose ester substrates for high performance flat-sheet thin-film composite (TFC) forward osmosis (FO) membranes. J. Membr. Sci. 2015, 473, 63–71. [Google Scholar] [CrossRef]
- Lu, X.; Arias Chavez, L.H.; Romero-Vargas Castrillón, S.; Ma, J.; Elimelech, M. Influence of Active Layer and Support Layer Surface Structures on Organic Fouling Propensity of Thin-Film Composite Forward Osmosis Membranes. Environ. Sci. Technol. 2015, 49, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Klaysom, C.; Hermans, S.; Gahlaut, A.; Van Craenenbroeck, S.; Vankelecom, I.F.J. Polyamide/Polyacrylonitrile (PA/PAN) thin film composite osmosis membranes: Film optimization, characterization and performance evaluation. J. Membr. Sci. 2013, 445, 25–33. [Google Scholar] [CrossRef]
- Choi, W.; Jeon, S.; Kwon, S.J.; Park, H.; Park, Y.; Nam, S.; Lee, P.; Lee, J.; Choi, J.; Hong, S.; et al. Thin film composite reverse osmosis membranes prepared via layered interfacial polymerization. J. Membr. Sci. 2017, 527, 121–128. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, Q.; Ji, Y.; An, Q.; Gao, C. A novel route for surface zwitterionic functionalization of polyamide nanofiltration membranes with improved performance. J. Membr. Sci. 2015, 490, 311–320. [Google Scholar] [CrossRef]
- Tang, C.; Kwon, Y.; Leckie, J. Probing the nano- and micro-scales of reverse osmosis membranes—A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Technol. lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X. A novel reduced graphene oxide/carbon nanotube hollow fiber membrane with high forward osmosis performance. Desalination 2019, 451, 117–124. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, S.; Fang, Z.; Ng, D.; Xie, C.; Wang, H.; Xie, Z. Thin-Film Composite Membrane with Interlayer Decorated Metal–Organic Framework UiO-66 toward Enhanced Forward Osmosis Performance. Ind. Eng. Chem. Res. 2019, 58, 195–206. [Google Scholar] [CrossRef]
- Choi, H.; Shah, A.A.; Nam, S.; Park, Y.; Park, H. Thin-film composite membranes comprising ultrathin hydrophilic polydopamine interlayer with graphene oxide for forward osmosis. Desalination 2019, 449, 41–49. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Ren, Z.; Shi, W.; Zhang, Z.; Xu, Y.; Gao, S.; Cui, F. High performance thin-film composite (TFC) forward osmosis (FO) membrane fabricated on novel hydrophilic disulfonated poly(arylene ether sulfone) multiblock copolymer/polysulfone substrate. J. Membr. Sci. 2016, 520, 529–539. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C. Inhibiting the concentration polarization of FO membranes based on the wettable microporous supporting layer and the enhanced dense skin layer. J. Appl. Polym. Sci. 2017, 134, 45133. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Liu, C. A novel TFC-type FO membrane with inserted sublayer of carbon nanotube networks exhibiting the improved separation performance. Desalination 2017, 413, 176–183. [Google Scholar] [CrossRef]
- Shah, A.A.; Cho, Y.H.; Choi, H.; Nam, S.; Kim, J.F.; Kim, Y.; Kim, Y.; Park, Y.; Park, H. Facile integration of halloysite nanotubes with bioadhesive as highly permeable interlayer in forward osmosis membranes. J. Ind. Eng. Chem. 2019, 73, 276–285. [Google Scholar] [CrossRef]
- Hung, W.; Chiao, Y.; Sengupta, A.; Lin, Y.; Wickramasinghe, S.R.; Hu, C.; Tsai, H.; Lee, K.; Lai, J. Tuning the interlayer spacing of forward osmosis membranes based on ultrathin graphene oxide to achieve desired performance. Carbon 2019, 142, 337–345. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Straub, A.P.; Romero-Vargas Castrillon, S.; Elimelech, M. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J. Membr. Sci. 2013, 444, 523–538. [Google Scholar] [CrossRef]

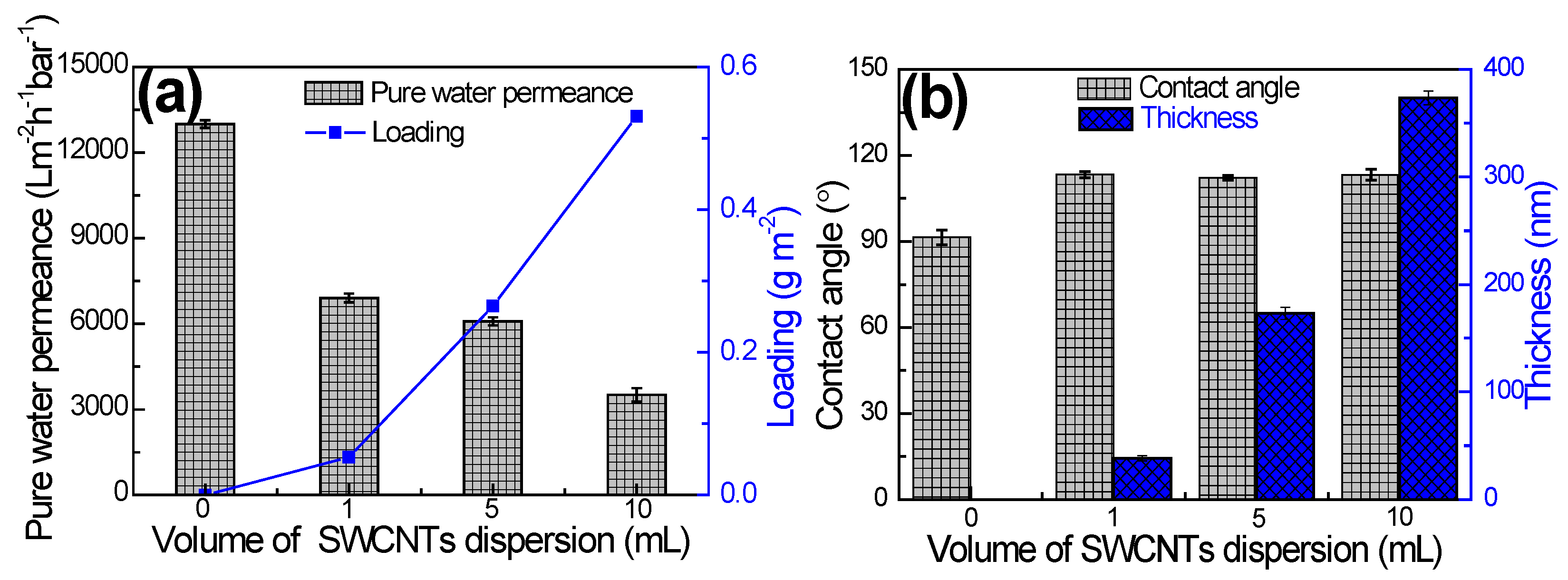
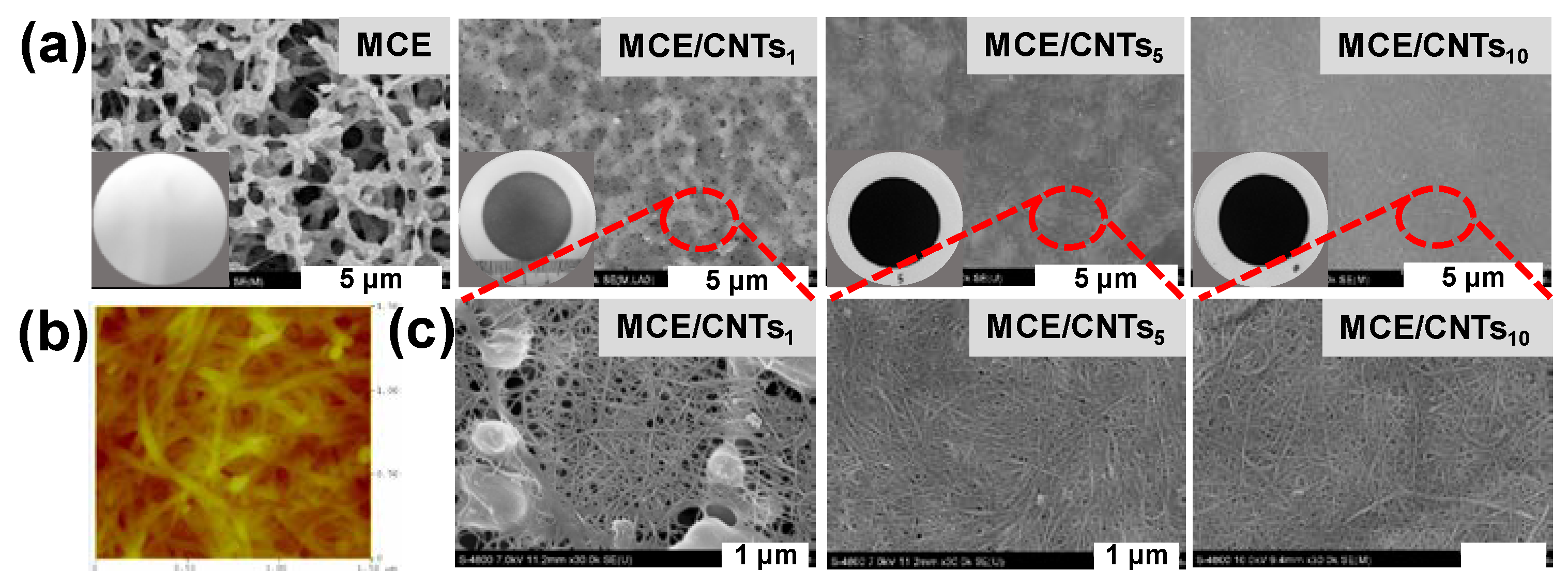
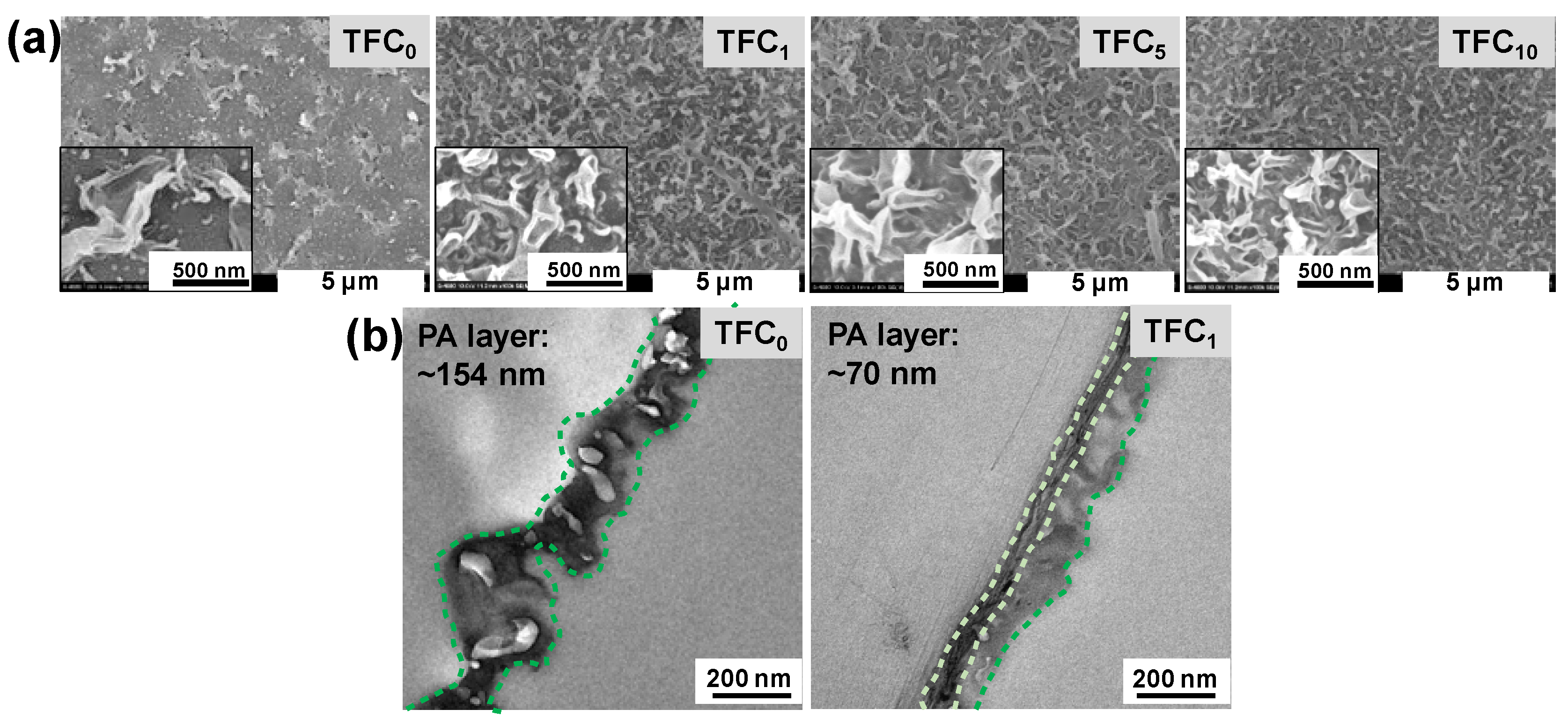

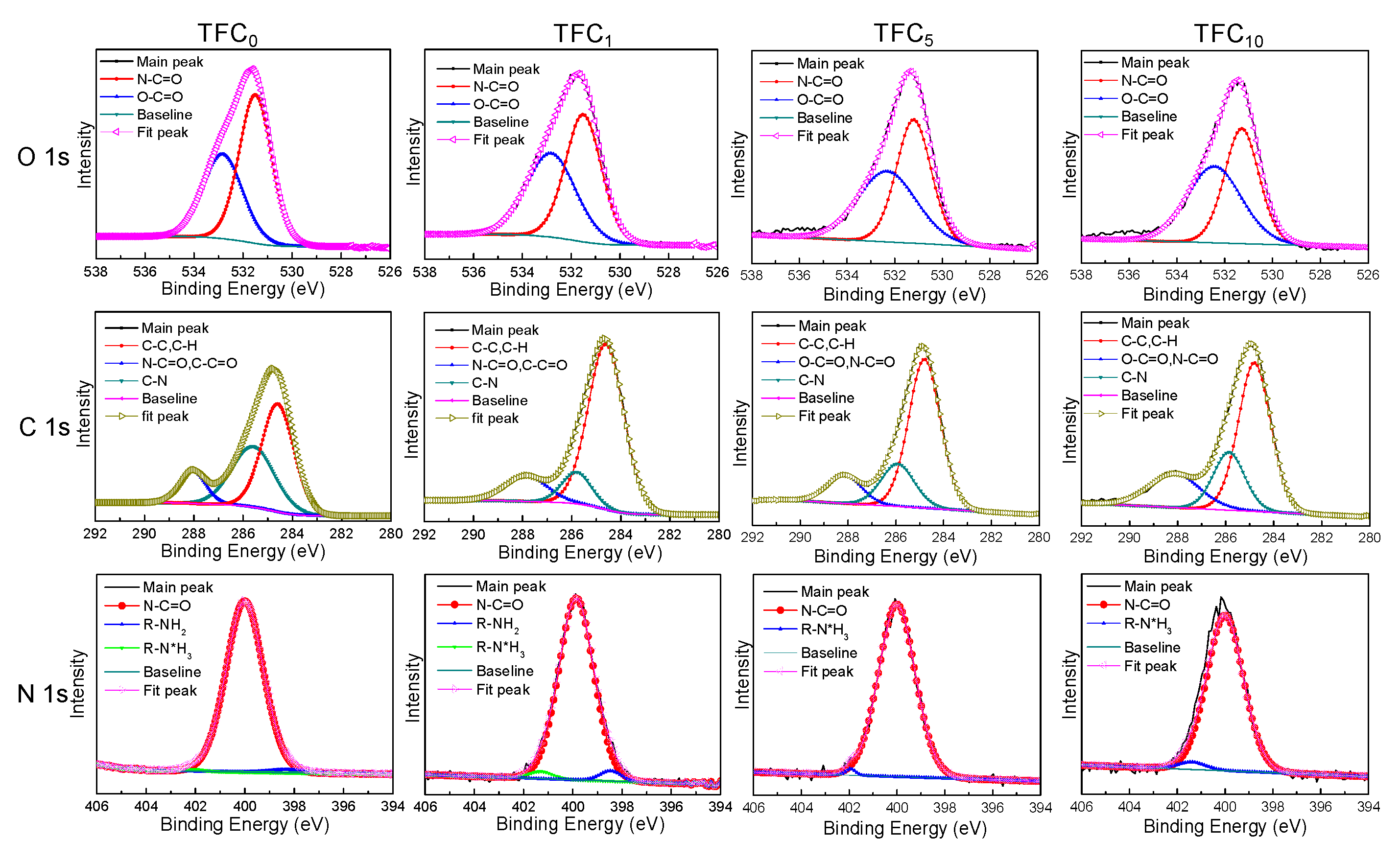
| Membrane | Jv (L m−2 h−1) | Js (g m−2h−1) | Js/Jv (g L−1) | A (L m−2h-1bar−1) | B (L m−2 h−1) | A/B (bar−1) | S (µm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TFC membrane with SWCNTs interlayer on MCE MF membrane | 62.8 | 19.4 | 0.29 | 3.3 c | 0.19 c | 17.3 | 88 c | This work |
| TFC membrane with PDA coated CNTs interlayer on PES MF support layer | 31.0 | 0.6 | 0.02 | 2.0 d | 0.05 d | 39.0 | 197 d | [28] |
| RGO layer on CNTs hollow fiber support layer | 22.6 a | 1.6 a | 0.07 | 2.1 e | 0.05 e | 41.4 | 202 e | [47] |
| TFC membrane with interlayer decorated metal−organic framework UiO-66 on PSf support layer | 11.0 | 2.9 | 0.27 | 4.5 f | 0.81 f | 5.5 | 741 f | [48] |
| TFC membrane with CNTs interlayer with carboxyl groups on PVDF support layer | 24.0 b | 5.9 b | 0.25 | 1.3 g | 0.54 g | 2.3 | 392 g | [53] |
| TFC membrane with GO/MWCNTs interlayer on PES MF support layer | 17.2 | 3.7 | 0.22 | [27] | ||||
| TFC membrane with PDA/GO interlayer on PSf support layer | 24.3 | 3.8 | 0.16 | [49] | ||||
| TFC membrane with PDA/ halloysite nanotubes (HNT) interlayer on PSf support layer | 26.9 | 4.0 | 0.15 | [54] | ||||
| TFC membrane with GO interlayer on PVDF support layer | 17.5 | 1.0 | 0.06 | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Li, S.; Xu, J.; Gao, C. Thin Film Composite Forward Osmosis Membrane with Single-Walled Carbon Nanotubes Interlayer for Alleviating Internal Concentration Polarization. Polymers 2020, 12, 260. https://doi.org/10.3390/polym12020260
Tang Y, Li S, Xu J, Gao C. Thin Film Composite Forward Osmosis Membrane with Single-Walled Carbon Nanotubes Interlayer for Alleviating Internal Concentration Polarization. Polymers. 2020; 12(2):260. https://doi.org/10.3390/polym12020260
Chicago/Turabian StyleTang, Yuanyuan, Shan Li, Jia Xu, and Congjie Gao. 2020. "Thin Film Composite Forward Osmosis Membrane with Single-Walled Carbon Nanotubes Interlayer for Alleviating Internal Concentration Polarization" Polymers 12, no. 2: 260. https://doi.org/10.3390/polym12020260
APA StyleTang, Y., Li, S., Xu, J., & Gao, C. (2020). Thin Film Composite Forward Osmosis Membrane with Single-Walled Carbon Nanotubes Interlayer for Alleviating Internal Concentration Polarization. Polymers, 12(2), 260. https://doi.org/10.3390/polym12020260






