Feasibility of Manufacturing Strand-Based Wood Composite Treated with β-Cyclodextrin–Boric Acid for Fungal Decay Resistance
Abstract
:1. Introduction
2. Materials and Methods
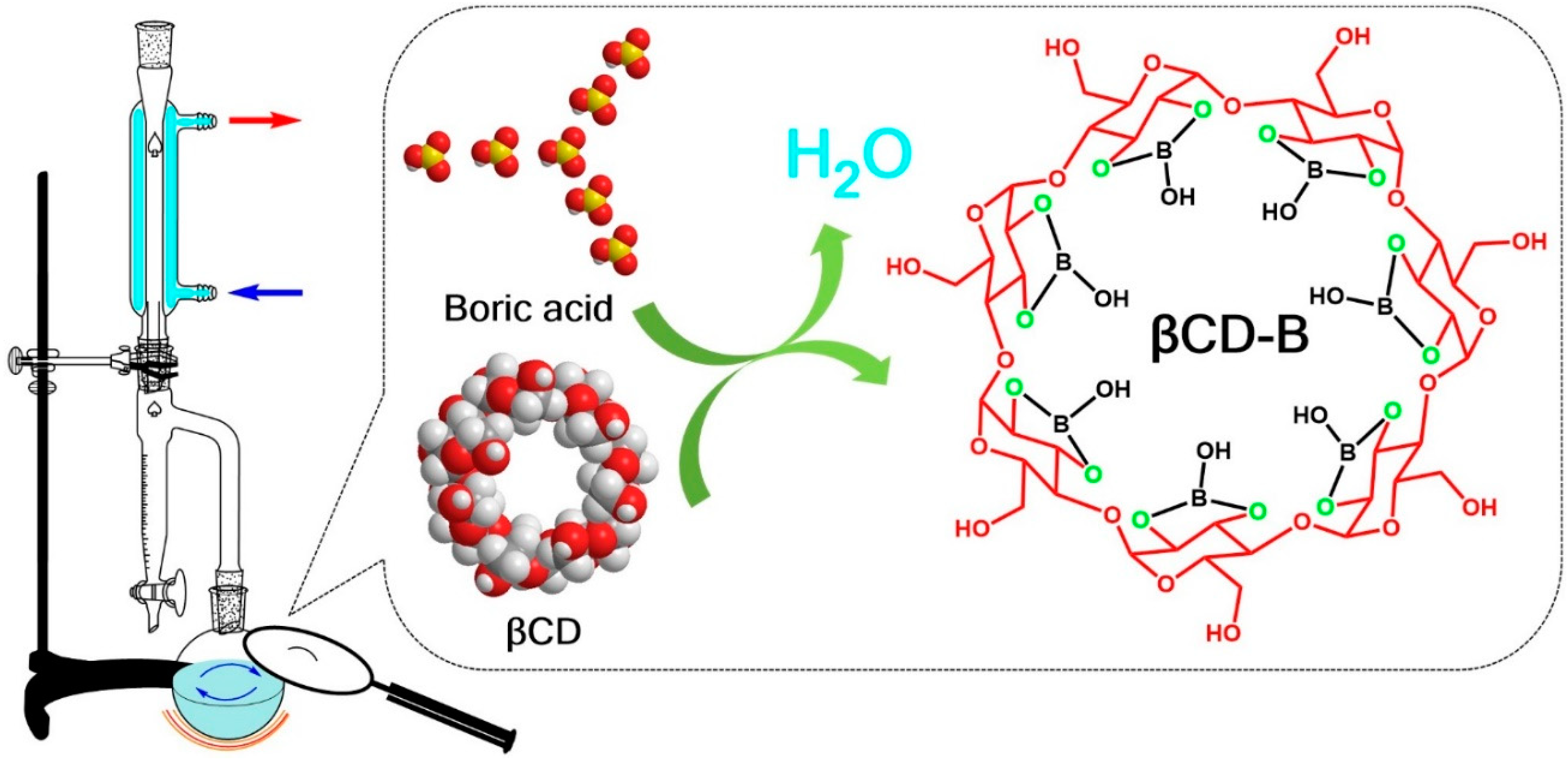
2.1. Preparation of βCD–B Complex
2.2. Confirmation of βCD–B Complex
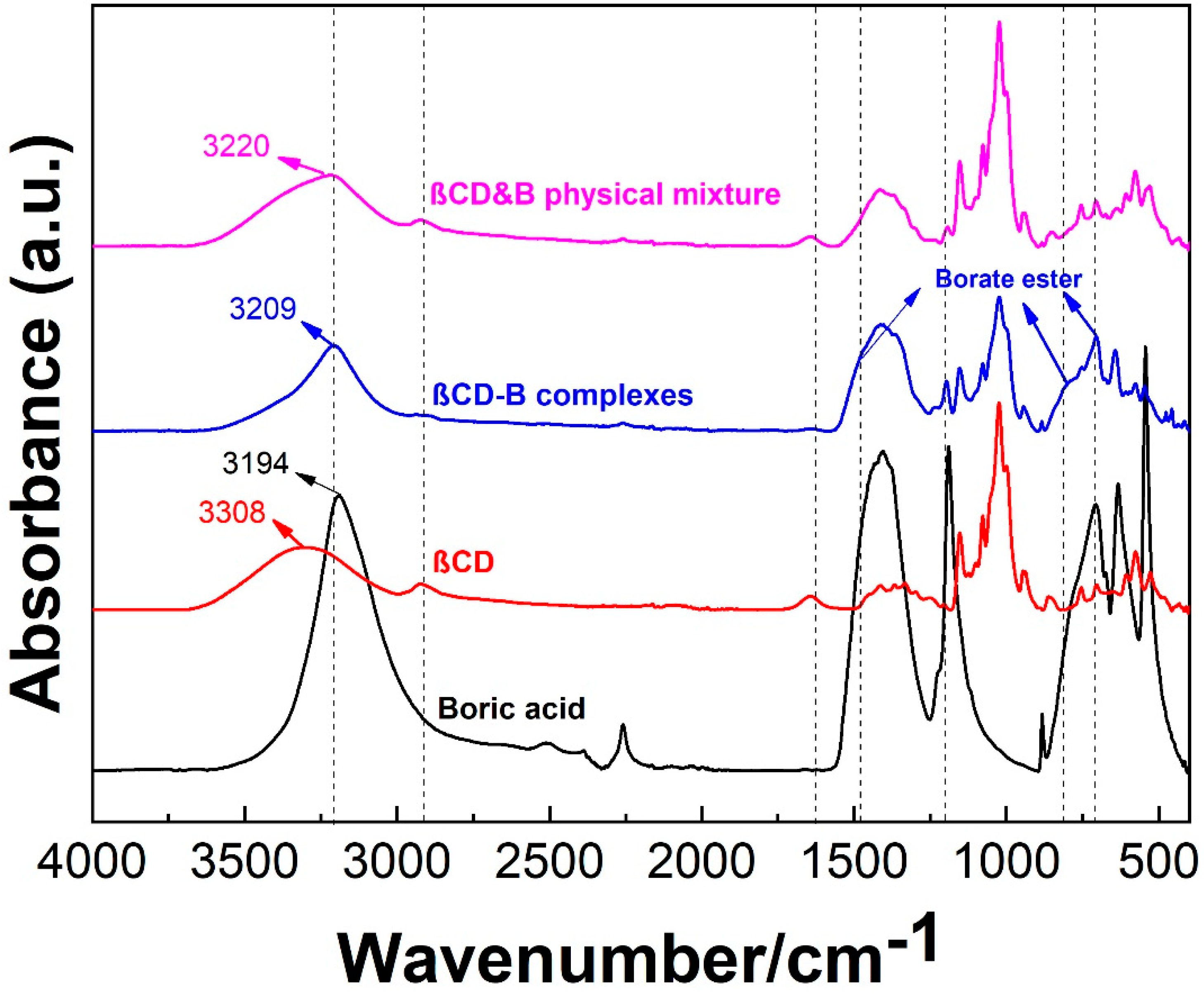
2.2.1. Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
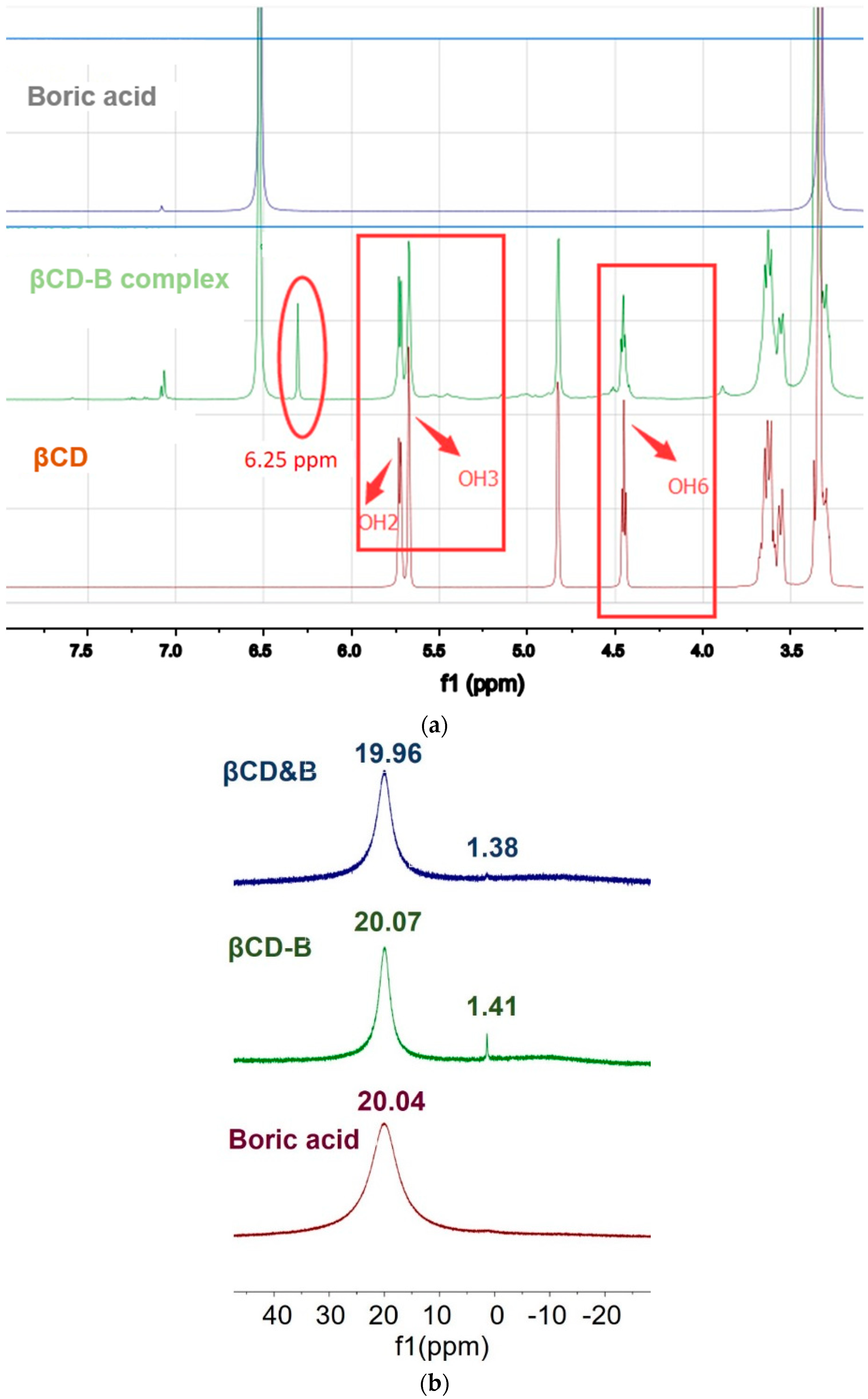
2.2.2. Nuclear Magnetic Resonance Analysis
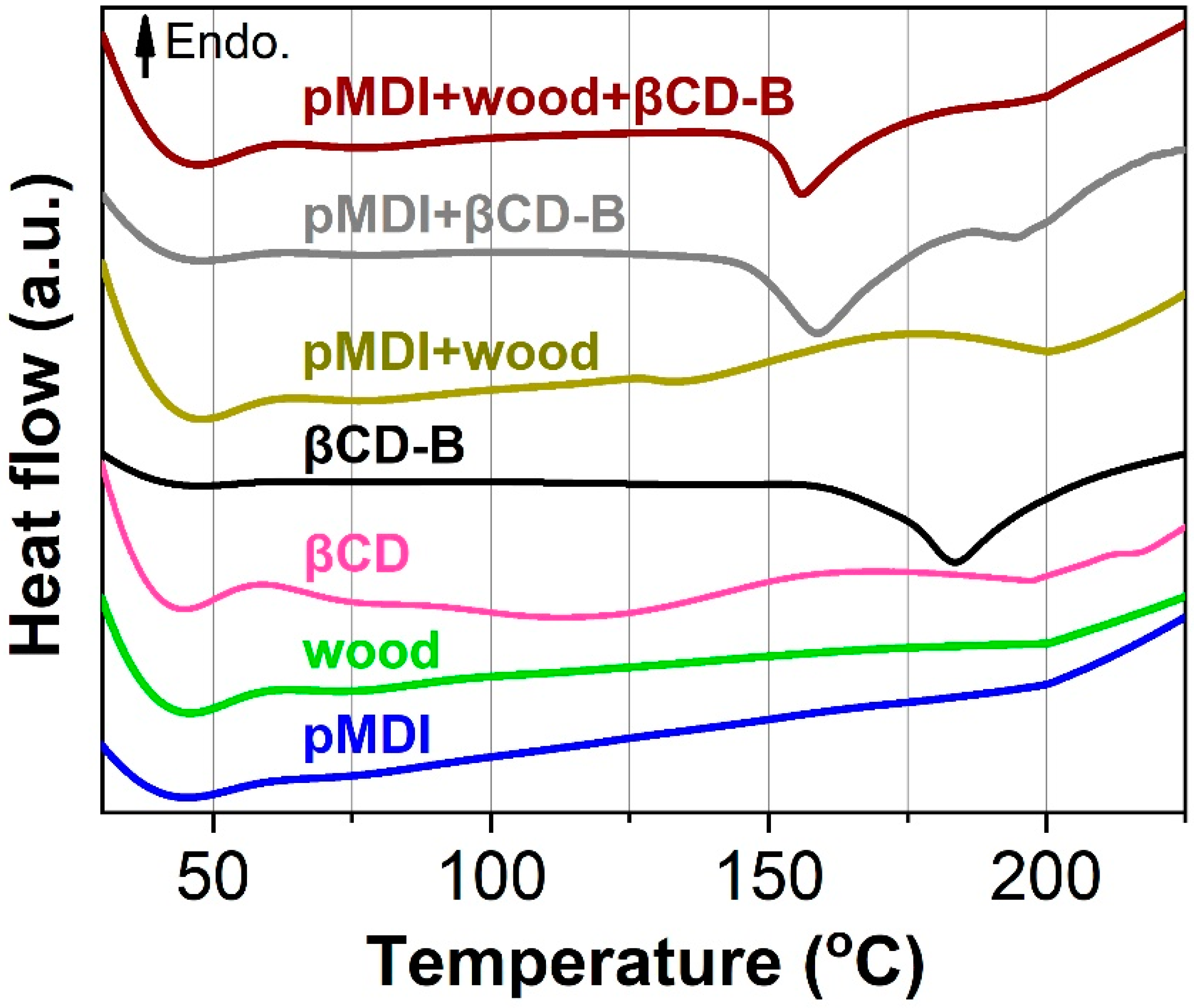
2.2.3. Differential Scanning Calorimetry (DSC) Analysis
2.3. Preparation of Oriented Strand Board (OSB)
2.4. Internal Bond Strength and Fungal Resistance of OSB
2.5. Statistical Analysis
3. Results and Discussion
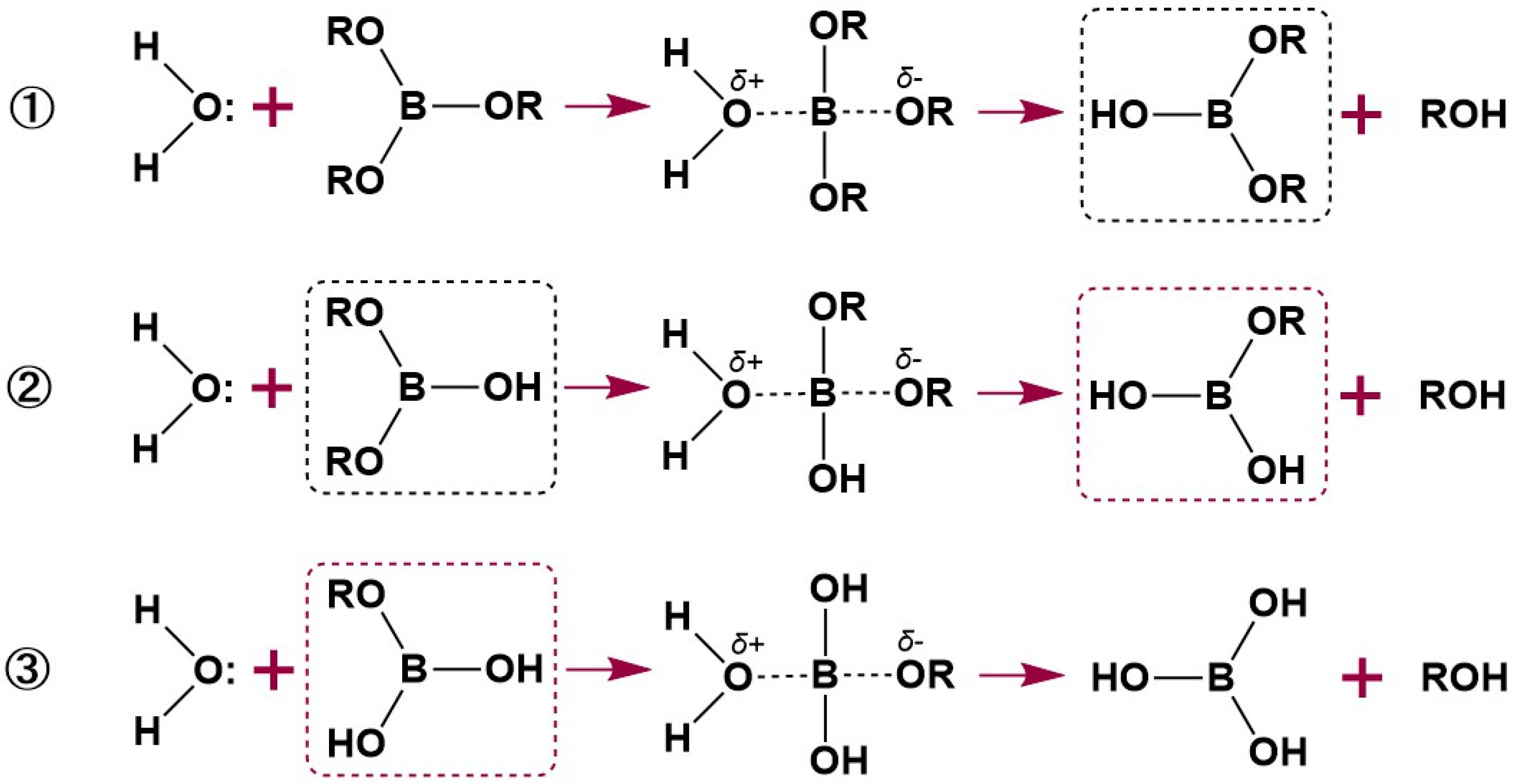
3.1. Formation of βCD–B Complex
3.2. Curing Behavior of Polymeric Methylene Diphenyl Diisocyanate (pMDI), Wood, βCD-B Complex and Their Mixtures
3.3. Vertical Density Profile (VDP) and Internal Bonding of the Panel
3.4. Decay Resistance of βCD–B Complex Against Brown-Rot Fungi
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, D.J.; Tascioglu, C.; Wålinder, M.E.P. Wood Composite Protection. In Wood Deterioration and Preservation; ACS Publications: Washington, DC, USA, 2003; pp. 399–419. [Google Scholar] [CrossRef]
- Goodnight, C.J. Toxicity of Sodium Pentachlorophenate and Pentachlorophenol to Fish. Ind. Eng. Chem. 1942, 34, 868–872. [Google Scholar] [CrossRef]
- Hall, A.H. Chronic Arsenic Poisoning. Toxicol. Lett. 2002, 128, 69–72. [Google Scholar] [CrossRef]
- Williams, L.H. Borate Wood-Protection Compounds: A Review of Research and Commercial Use. APT Bull. 1996, 27, 46–51. [Google Scholar] [CrossRef]
- Freeman, M.H.; Mcintyre, C.R.; Associates, M. A Critical and Comprehensive Review of Boron in Wood Preservation. In Proceedings of the American Wood Protection Association, San Antonio, TX, USA, 19–21 April 2009; pp. 279–294. [Google Scholar]
- Cabrera, Y.; Morrell, J.J. Effect of Wood Moisture Content and Rod Dosage on Boron or Fluoride Movement through Douglas-Fir Heartwood. For. Prod. J. 2009, 59, 93–97. [Google Scholar]
- Caldeira, F. Boron in Wood Preservation: A Review in Its Physico-Chemical Aspects. Silva Lusit. 2010, 18, 179–196. [Google Scholar]
- Obanda, D.N.; Shupe, T.F.; Barnes, H.M. Reducing Leaching of Boron-Based Wood Preservatives—A Review of Research. Bioresour. Technol. 2008, 99, 7312–7322. [Google Scholar] [CrossRef]
- Tsunoda, K. Preservative Properties of Vapor-Boron-Treated Wood and Wood-Based Composites. J. Wood Sci. 2001, 47, 149–153. [Google Scholar] [CrossRef]
- Manning, M.J. Wood Protection Processes for Engineered Wood Products. In Proceedings of the Enhancing the Durability of Lumber and Engineered Wood Products, FPS Symposium Proceedings, Kissimme, FL, USA, 11–13 February 2002; Forest Products Society: Madison, WI, USA, 2002. [Google Scholar]
- Laks, P.E. The Effects of Sodium Octaborate Tetrahydrate and Zinc Borate on the Properties of Isocyanate-Bonded Waferboard. In Proceedings of the Adhesives and Bonded Wood Products Symposium, Seattle, WA, USA, 19–21 November 1991; pp. 144–157. [Google Scholar]
- Szejtli, J. Cyclodextrin Technology; Topics in Inclusion Science; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar] [CrossRef]
- Bhat, S.; Chandrasekaran, S. Oxygenation of Alkenes with T-BuOOH Catalysed by β-Cyclodextrin Borate. Tetrahedron Lett. 1996, 37, 3581–3584. [Google Scholar] [CrossRef]
- Baur, R.; Macholdt, H.-T. Cyclooligosaccharide-Boron Complex. US Patent 6083653, 4 July 2000. [Google Scholar]
- Cai, L.; Lim, H.; Nicholas, D.D.; Kim, Y. Bio-Based Preservative Using Methyl-β-Cyclodextrin-Essential Oil Complexes for Wood Protection. Int. J. Biol. Macromol. 2020, 147, 420–427. [Google Scholar] [CrossRef]
- Cai, L.; Lim, H.; Kim, Y.; Jeremic, D. β-Cyclodextrin-Allyl Isothiocyanate Complex as a Natural Preservative for Wood Products. 2019; Submitted. [Google Scholar]
- Cai, L.; Lim, H.; Kim, Y.; Jeremic, D. β-Cyclodextrin-Allyl Isothiocyanate Complex as a Natural Preservative for Strand-Based Wood Composites. 2019; Submitt. to Elsevier. [Google Scholar]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and Characterization of Novel β-Cyclodextrin Based Copolymer Materials. Carbohydr. Res. 2011, 346, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Kida, T.; Miyawaki, K.; Fukuda, Y.; Kato, E.; Nakano, T.; Akashi, M. Adsorption Capability of Urethane-Crosslinked Heptakis (2, 6-Di-O-Methyl)-β-Cyclodextrin Polymers toward Polychlorobiphenyls in Nonpolar Organic Media. Polym. J. 2015, 47, 443. [Google Scholar] [CrossRef]
- ASTM International. ASTM D1037-12 Standard Test Methods for Evaluating Properties of Wood-Base Fiber and Particle Panel Materials; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar] [CrossRef]
- AWPA. E11-16: Standard Method for Accelerated Evaluation of Preservative Leaching. In AWPA Book of Standard; American Wood Protection Association: Birmingham, AL, USA, 2016; pp. 418–420. [Google Scholar]
- AWPA. E10-16: Laboratory Method for Evaluating the Decay Resistance of Wood-Based Materials against Pure Basidiomycete Cultures: Soil/Block Test. In AWPA Book of Standard; American Wood Protection Association Standard: Birmingham, AL, USA, 2016; pp. 448–458. [Google Scholar]
- Jun, L.; Shuping, X.; Shiyang, G. FT-IR and Raman Spectroscopic Study of Hydrated Borates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 519–532. [Google Scholar] [CrossRef]
- Kemelbekov, U.; Luo, Y.; Orynbekova, Z.; Rustembekov, Z.; Haag, R.; Saenger, W.; Praliyev, K. IR, UV and NMR Studies of β-Cyclodextrin Inclusion Complexes of Kazcaine and Prosidol Bases. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 181–190. [Google Scholar] [CrossRef]
- Li, X.N.; Zheng, G.; Yang, H.X. Study on Synthesis and Properties of a Novel Borate Ester Surfactant. In Advanced Materials Research; Trans Tech Publications: Switzerland, 2010; Volume 129, pp. 857–861. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Siddiqui, M.T.; Haq, Q.M.R.; Ahmad, S. Synthesis and Characterization of Surface-Active Antimicrobial Hyperbranched Polyurethane Coatings Based on Oleo-Ethers of Boric Acid. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Staroszczyk, H. Microwave-Assisted Boration of Potato Starch. Polimery 2009, 54, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Aries, R.S. Process and Product of Reacting Boric Acid with Isocyanates. US Patent 2931831, 5 April 1960. [Google Scholar]
- Ashida, K. Boronium Complexes as Polymer Catalysts. UK Patent GB2161475A, 15 January 1986. [Google Scholar]
- Lucey, M.F. Electronic Component with Radiation-Hardenable Coating. US Patent 4282269, 4 August 1981. [Google Scholar]
- Wang, S.; Winistorferl, P.M. Density Formation Under Dynamic Conditions. Wood Fiber Sci. 2000, 32, 220–238. [Google Scholar]
- Wong, E.D. Effects of Mat Moisture Content and Press Closing Speed on the Formation of Density Profile and Properties of Particleboard. J. Wood Sci. 1998, 44, 287–295. [Google Scholar] [CrossRef]
- Wong, E.D.; Zhang, M.; Wang, Q.; Kawai, S. Formation of the Density Profile and Its Effects on the Properties of Particleboard. Wood Sci. Technol. 1999, 33, 327–340. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Saeed, A.M.; Donthula, S.; Majedi Far, H.; Rewatkar, P.M.; Kaiser, H.; Robertson, J.D.; Lu, H.; Churu, G. Nanoporous Polyurea from a Triisocyanate and Boric Acid: A Paradigm of a General Reaction Pathway for Isocyanates and Mineral Acids. Chem. Mater. 2015, 28, 67–78. [Google Scholar] [CrossRef]
- BSI. BS EN 300:2006. In Oriented Strand Boards (OSB). Definitions, Classification and Specifications; British Standards Institution: London, UK, 2006. [Google Scholar]
- Xu, X.; Lee, S.; Wu, Y.; Wu, Q. Borate-Treated Strand Board from Southern Wood Species: Resistance against Decay and Mold Fungi. BioResources 2013, 8, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Freitag, C.; Morrell, J.J. Development of Threshold Values for Boron and Fluoride in Non-Soil Contact Applications. For. Prod. J. 2005, 55, 97–101. [Google Scholar]
- Evans, P.D.; Lube, V.; Averdunk, H.; Limaye, A.; Turner, M.; Kingston, A.; Senden, T.J. Visualizing the Microdistribution of Zinc Borate in Oriented Strand Board Using X-Ray Microcomputed Tomography and SEM-EDX. J. Compos. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.G. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications. Boronic Acids Prep. Appl. Org. Synth. Med. 2006, 1, 1–99. [Google Scholar]
- Steinberg, H.; Hunter, D.L. Preparation and Rate of Hydrolysis of Boric Acid Esters. Ind. Eng. Chem. 1957, 49, 174–181. [Google Scholar] [CrossRef]
- Matsumi, N.; Naka, K.; Chujo, Y. Extension of π-Conjugation Length via the Vacant p-Orbital of the Boron Atom. Synthesis of Novel Electron Deficient π-Conjugated Systems by Hydroboration Polymerization and Their Blue Light Emission. J. Am. Chem. Soc. 1998, 120, 5112–5113. [Google Scholar] [CrossRef]
- Efhamisisi, D.; Thevenon, M.; Hamzeh, Y.; Pizzi, A.; Karimi, A.; Pourtahmasi, K. Tannin-Boron Complex as a Preservative for 3-Ply Beech Plywoods Designed for Humid Conditions. Holzforschung 2017, 71, 249–258. [Google Scholar] [CrossRef]
- Tondi, G.; Wieland, S.; Lemenager, N.; Petutschnigg, A.; Pizzi, A.; Thevenon, M.F. Efficacy of Tannin in Fixing Boron in Wood: Fungal and Termite Resistance. BioResources 2012, 7, 1238–1252. [Google Scholar]

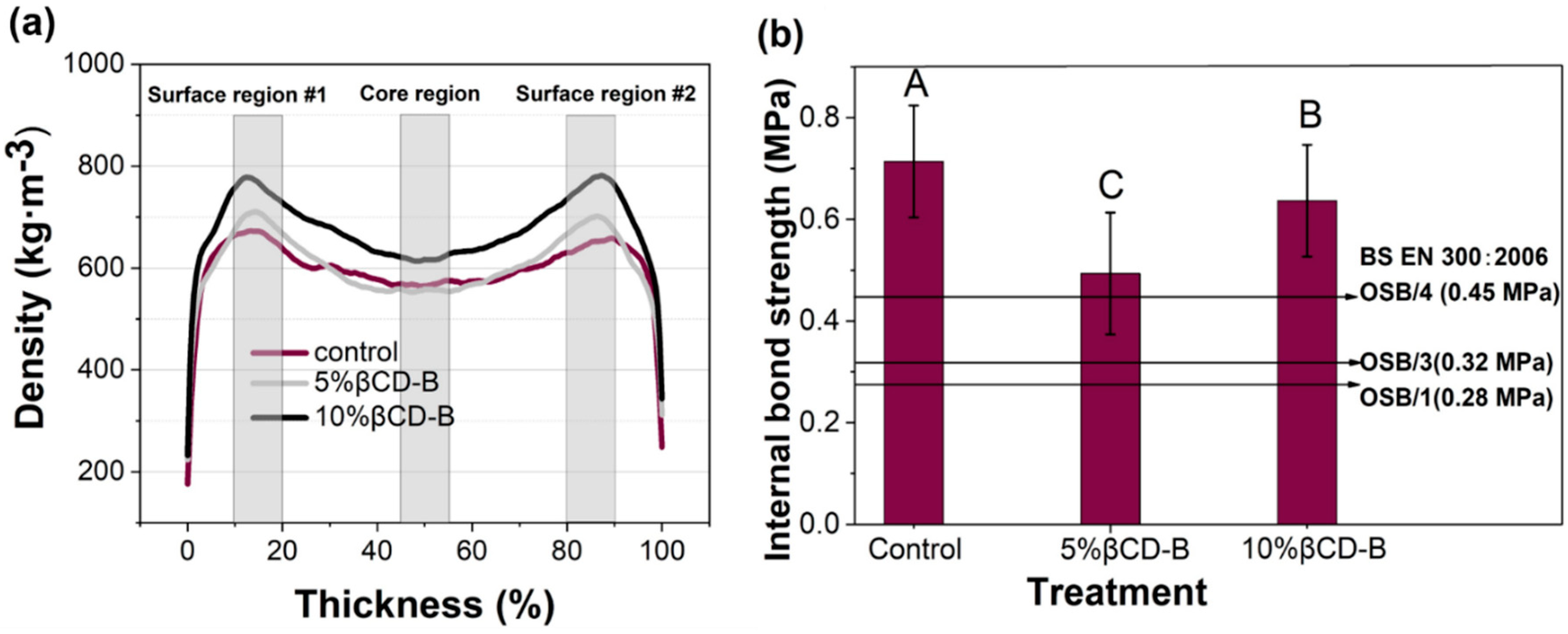

| Preservatives Level (%) | Average Density ± Standard Error (kg/m3) | ||
|---|---|---|---|
| Surface Region #1 | Core Region | Surface Region #2 | |
| 0 * | 665 ± 24 (C) | 562 ± 28 (A) | 652 ± 30 (C) |
| 5 | 712 ± 33 (D) | 568 ± 24 (A) | 711 ± 30 (D) |
| 10 | 770 ± 36 (E) | 606 ± 26 (B) | 774 ± 40 (E) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Lim, H.; Fitzkee, N.C.; Cosovic, B.; Jeremic, D. Feasibility of Manufacturing Strand-Based Wood Composite Treated with β-Cyclodextrin–Boric Acid for Fungal Decay Resistance. Polymers 2020, 12, 274. https://doi.org/10.3390/polym12020274
Cai L, Lim H, Fitzkee NC, Cosovic B, Jeremic D. Feasibility of Manufacturing Strand-Based Wood Composite Treated with β-Cyclodextrin–Boric Acid for Fungal Decay Resistance. Polymers. 2020; 12(2):274. https://doi.org/10.3390/polym12020274
Chicago/Turabian StyleCai, Lili, Hyungsuk Lim, Nicholas C. Fitzkee, Bojan Cosovic, and Dragica Jeremic. 2020. "Feasibility of Manufacturing Strand-Based Wood Composite Treated with β-Cyclodextrin–Boric Acid for Fungal Decay Resistance" Polymers 12, no. 2: 274. https://doi.org/10.3390/polym12020274
APA StyleCai, L., Lim, H., Fitzkee, N. C., Cosovic, B., & Jeremic, D. (2020). Feasibility of Manufacturing Strand-Based Wood Composite Treated with β-Cyclodextrin–Boric Acid for Fungal Decay Resistance. Polymers, 12(2), 274. https://doi.org/10.3390/polym12020274







