Ultra-Stable UiO-66 Involved Molecularly Imprinted Polymers for Specific and Sensitive Determination of Tyramine Based on Quartz Crystal Microbalance Technology
Abstract
1. Introduction
2. Material and Methods
2.1. Instruments and Reagents
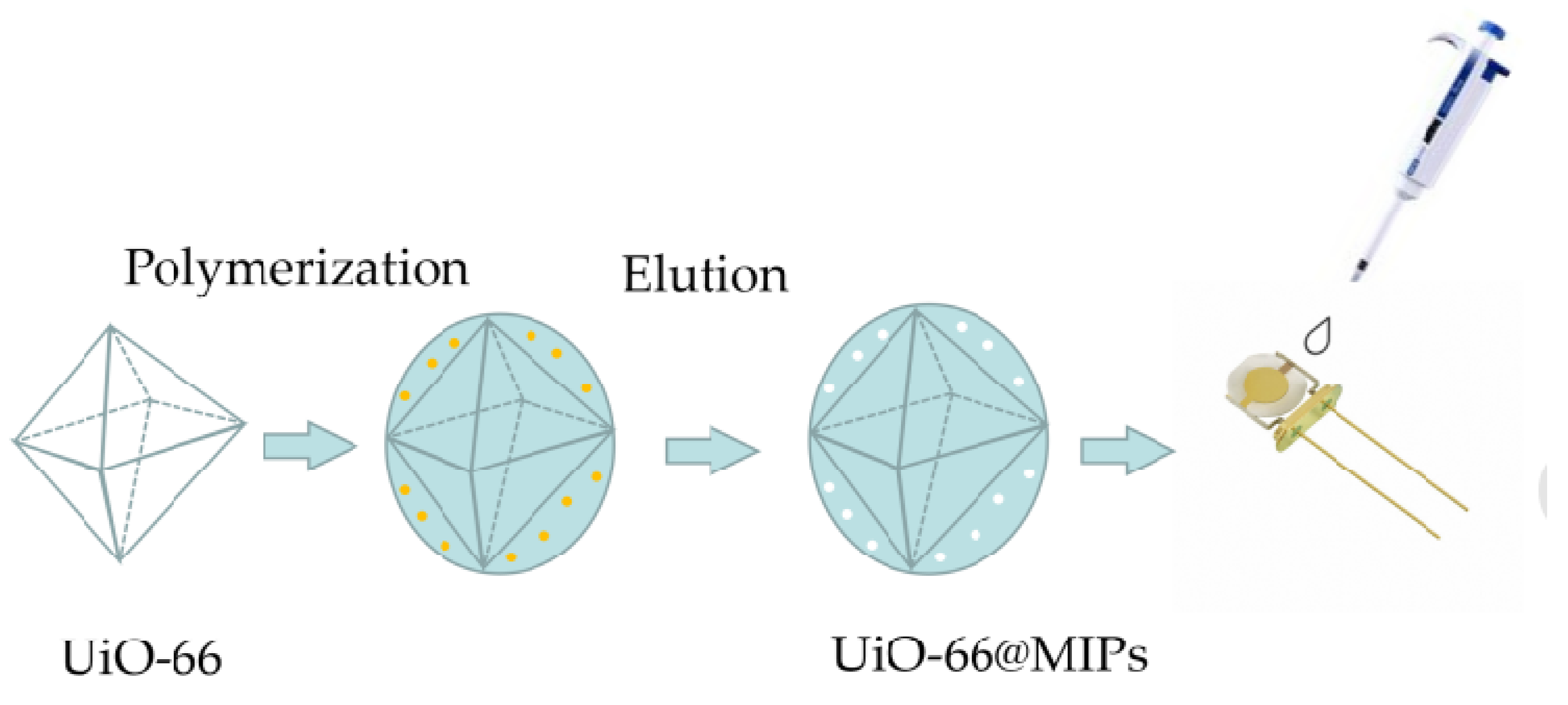
2.2. Synthesis of UiO-66 and UiO-66@MIPs/NIPs
2.3. Adsorption of Tyramine.
2.4. QCM Measurements
2.5. Sample Preparation
3. Results and Discussion
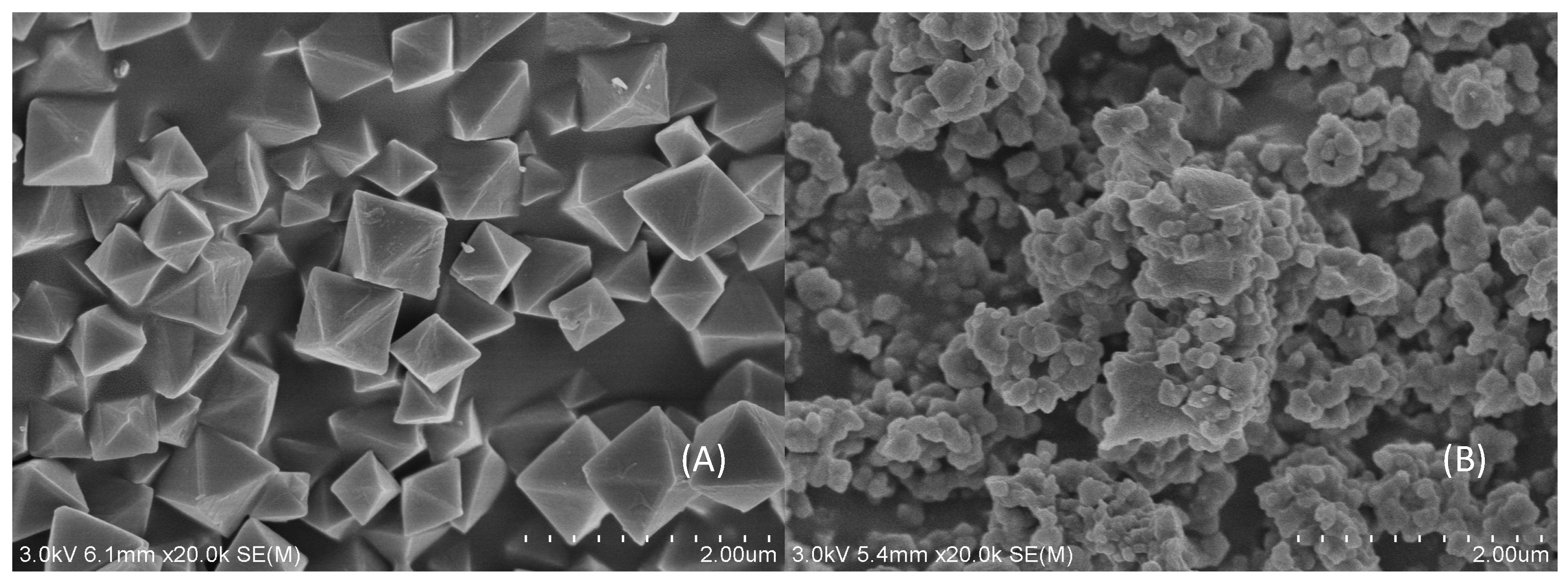
3.1. Characterization
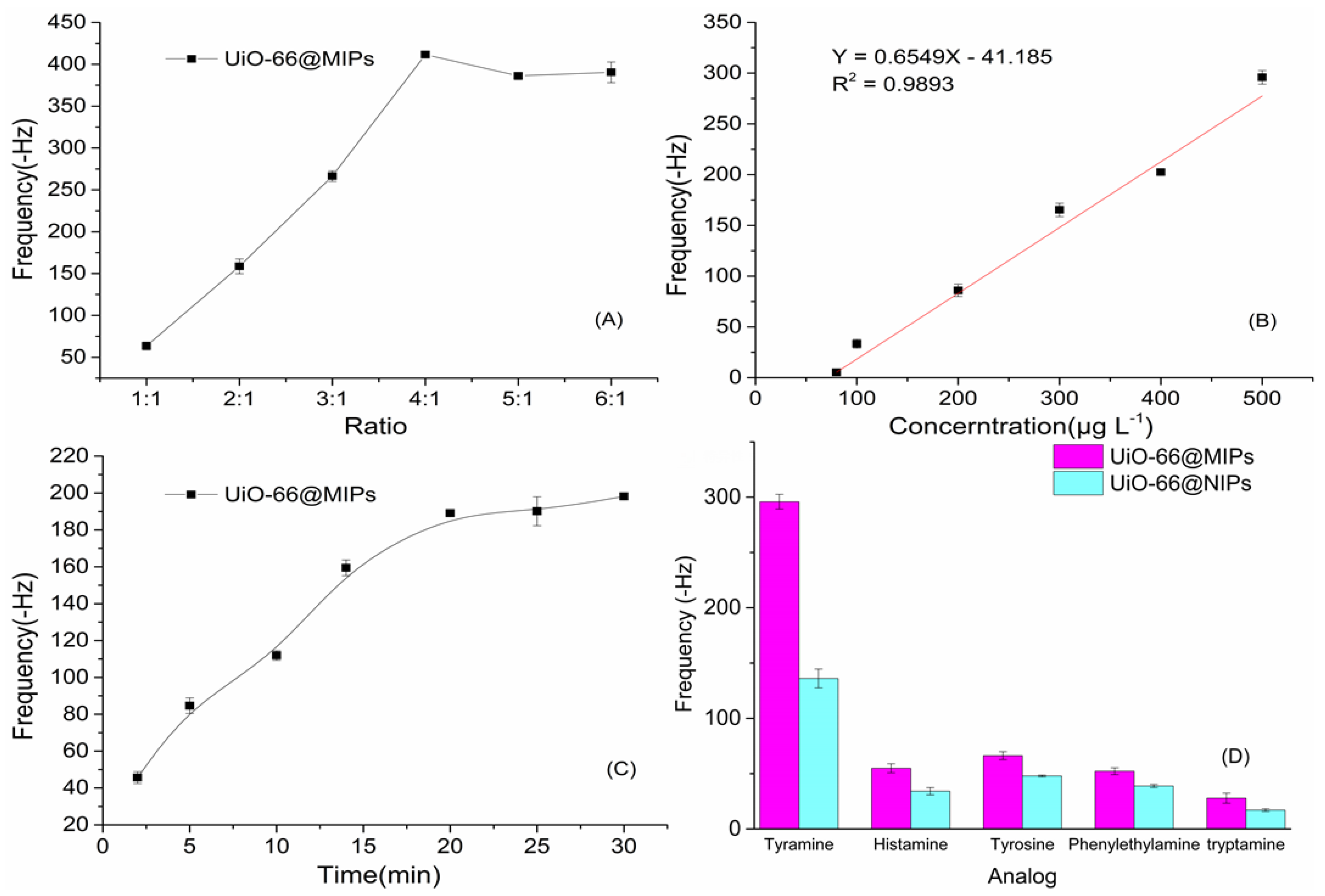
3.2. Adsorption of Tyramine-MIPs
3.3. The Performance of QCM Sensor
3.4. Evaluation of sample analysis and comparison of the methods
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mazzucco, E.; Gosetti, F.; Bobba, M.; Marengo, E.; Robotti, E.; Gennaro, M.C. High-performance liquid chromatography-ultraviolet detection method for the simultaneous determination of typical biogenic amines and precursor amino acids. Applications in food chemistry. J. Agric. Food. Chem. 2010, 58, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Antal, E.; Hendershot, P.; Batts, D.; Sheu, W.; Hopkins, N.; Donaldson, K. Linezolid, a novel oxazolidinone antibiotic: Assessment of monoamine oxidase inhibition using pressor response to oral tyramine. J. Clin. Pharmacol. 2001, 41, 552–562. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Özkan, A.; Atar, N.; Yola, M.L. Enhanced surface plasmon resonance (SPR) signals based on immobilization of core-shell nanoparticles incorporated boron nitride nanosheets: Development of molecularly imprinted SPR nanosensor for anticancer drug, etoposide. Biosens. Bioelectron. 2019, 130, 293–298. [Google Scholar] [CrossRef]
- Li, Y.; Hsieh, C.-H.; Lai, C.-W.; Chang, Y.-F.; Chan, H.-Y.; Tsai, C.-F.; Ho, J.-A.A.; Wu, L.-C. Tyramine detection using PEDOT:PSS/AuNPs/1-methyl-4-mercaptopyridine modified screen-printed carbon electrode with molecularly imprinted polymer solid phase extraction. Biosens. Bioelectron. 2017, 87, 142–149. [Google Scholar] [CrossRef]
- Bieck, P.; Antonin, K.-H. Tyramine potentiation during treatment with MAO inhibitors: Brofaromine and moclobemide vs irreversible inhibitors. J. Neural Transm. 1989, 28, 21–31. [Google Scholar]
- Huang, K.J.; Wei, C.Y.; Liu, W.L.; Xie, W.Z.; Zhang, J.F.; Wang, W. Ultrasound-assisted dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-fluorescence detection for sensitive determination of biogenic amines in rice wine samples. J. Chromatogr. A 2009, 1216, 6636–6641. [Google Scholar] [CrossRef]
- Lapainis, T.; Scanlan, C.; Rubakhin, S.S.; Sweedler, J.V. A multichannel native fluorescence detection system for capillary electrophoretic analysis of neurotransmitters in single neurons. Anal. Bioanal. Chem. 2007, 387, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Lee, J.-I.; Kim, J.-H.; Mah, J.-H.; Hwang, H.-J.; Kim, Y.-W. Comparison of ELISA and HPLC methods for the determination of biogenic amines in commercial Doenjang and Gochujang. Food Sci. Biotechnol. 2011, 20, 1747–1750. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Amperometric biosensor based on polypyrrole and tyrosinase for the detection of tyramine in food samples. Sens. Actuators B Chem. 2013, 178, 40–46. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.; Yu, C.; Tang, H.; Yu, H.; Li, X.; Zhang, X. Synthesis of surface molecularly imprinted silica micro-particles in aqueous solution and the usage for selective off-line solid-phase extraction of 2,4-dinitrophenol from water matrixes. Anal. Chim. Acta. 2008, 618, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Piletska, E.V.; Guerreiro, A.R.; Romero-Guerra, M.; Chianella, I.; Turner, A.P.F.; Piletsky, S.A. Design of molecular imprinted polymers compatible with aqueous environment. Anal. Chim. Acta 2008, 607, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.S.; Wu, M.S.; Zuo, H.G.; Jiang, C.; Jin, S.F.; Lu, Y.C.; Yang, H. Core-shell magnetic molecularly imprinted polymers as sorbent for sulfonylurea herbicide residues. J. Agric. Food Chem. 2015, 63, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, C.; Anfossi, L.; Giovannoli, C. Solid phase extraction of food contaminants using molecular imprinted polymers. Anal. Chim. Acta 2007, 591, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Allender, C.J.; Richardson, C.; Woodhouse, B.; Heard, C.M.; Brain, K.R. Pharmaceutical applications for molecularly imprinted polymers. Int. J. Pharm. 2000, 195, 39–43. [Google Scholar] [CrossRef]
- Medetalibeyoğlu, H.; Manap, S.; Yokuş, Ö.A.; Beytur, M.; Kardaş, F.; Akyıldırım, O.; Özkan, V.; Yüksek, H.; Yola, M.L.; Atar, N. Fabrication of Pt/Pd nanoparticles/polyoxometalate/ionic liquid nanohybrid for electrocatalytic oxidation of methanol. J. Electrochem. Soc. 2018, 165, F338–F341. [Google Scholar] [CrossRef]
- Kıran, T.R.; Atar, N.; Yola, M.L. A methyl parathion recognition method based on carbon nitride incorporated hexagonal boron nitride nanosheets composite including molecularly imprinted polymer. J. Electrochem. Soc. 2019, 166, H495–H501. [Google Scholar] [CrossRef]
- Yola, M.L.; Eren, T.; Atar, N. A molecular imprinted voltammetric sensor based on carbon nitride nanotubes: Application to determination of melamine. J. Electrochem. Soc. 2016, 163, B588–B593. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L. Core-shell nanoparticles/two-dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer for electrochemical sensing of cypermethrin. J. Electrochem. Soc. 2018, 165, H255–H262. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Gold nanoparticles/two-dimensional (2D) hexagonal boron nitride nanosheets including diethylstilbestrol imprinted polymer: Electrochemical detection in urine samples and validation. J. Electrochem. Soc. 2018, 165, H897–H902. [Google Scholar] [CrossRef]
- Yola, M.L.; Göde, C.; Atar, N. Molecular imprinting polymer with polyoxometalate/carbon nitride nanotubes for electrochemical recognition of bilirubin. Electrochim. Acta 2017, 246, 135–140. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Development of cardiac troponin-I biosensor based on boron nitride quantum dots including molecularly imprinted polymer. Biosens. Bioelectron. 2019, 126, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Atar, N. Development of molecular imprinted sensor including graphitic carbon nitride/N-doped carbon dots composite for novel recognition of epinephrine. Compos. Part B Eng. 2019, 175, 107113. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Quartz-crystal microbalance (QCM) for public health: An overview of its applications. Adv. Protein. Chem. Struct. Biol. 2015, 101, 149–211. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Han, K. Development of a real-time QCM bond-rupture system for POCT applications. IEEE Sens. J. 2016, 16, 8731–8735. [Google Scholar] [CrossRef]
- Li, W.; Wen, X.Y.; Li, S.M.; Wang, X.; Wang, J.Z.; Tang, H. Determination of DMMP using a polymer coated QCM sensor. Adv. Mater. Res. 2012, 542–543, 959–962. [Google Scholar] [CrossRef]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Olfactory receptor based piezoelectric biosensors for detection of alcohols related to food safety applications. Sens. Actuators B Chem. 2011, 155, 8–18. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Özgür, E.; Bereli, N.; Denizli, A. Microcontact imprinted quartz crystal microbalance nanosensor for protein C recognition. Colloids Surf. B Biointerfaces 2017, 151, 264–270. [Google Scholar] [CrossRef]
- Hussain, M.; Kotova, K.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles for formaldehyde sensing with QCM. Sensors 2016, 16, 1011. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Hu, C.-H.; Chou, T.-C. Determination of albumin concentration by MIP-QCM sensor. Biosens. Bioelectron. 2004, 20, 75–81. [Google Scholar] [CrossRef]
- Kayal, S.; Sun, B.; Chakraborty, A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks). Energy 2015, 91, 772–781. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D. Beyond equilibrium: Metal–organic frameworks for molecular sieving and kinetic gas separation. Cryst. Growth Des. 2017, 17, 2291–2308. [Google Scholar] [CrossRef]
- Yang, X.; Lv, J.; Yang, Z.; Yuan, R.; Chai, Y. A sensitive electrochemical aptasensor for thrombin detection based on electroactive co-based metal-organic frameworks with target-triggering NESA strategy. Anal. Chem. 2017, 89, 11636–11640. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Du, W.; Shi, D.; Ning, W.; Lu, X.; Hou, Z. Metal-organic framework derived Cu/ZnO catalysts for continuous hydrogenolysis of glycerol. Appl. Catal. B Environ. 2017, 203, 146–153. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Naimi-Jamal, M.R.; Panahi, L. Mechanochemical solvent-free in situ synthesis of drug-loaded {Cu2(1,4-bdc)2(dabco)}n MOFs for controlled drug delivery. J. Solid State Chem. 2018, 259, 35–42. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Polyzoidis, A.; Schwarzer, M.; Loebbecke, S. Stability of UiO-66 under acidic treatment: Opportunities and limitations for post-synthetic modifications. Microporous Mesoporous Mater. 2015, 208, 30–35. [Google Scholar] [CrossRef]
- Chavan, S.M.; Vitillo, J.; Uddin, M.J.; Bonino, F.; Lamberti, C.; Groppo, E.; Lillerud, K.; Bordiga, S. Functionalization of UiO-66 metal−organic framework and highly cross-linked polystyrene with Cr(CO)3: In situ formation, stability, and photoreactivity. Chem. Mater. 2010, 22, 4602–4611. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal–organic frameworks: From nano to single crystals. Chem. Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Lu, G.; Cui, C.; Zhang, W.; Liu, Y.; Huo, F. Synthesis and self-assembly of monodispersed metal-organic framework microcrystals. Chem. Asian J. 2013, 8, 69–72. [Google Scholar] [CrossRef]
- Arrua, R.; Peristyy, A.; Nesterenko, P.; Das, A.; D’Alessandro, D.; Hilder, E. UiO-66@SiO2 core-shell microparticles as stationary phases for the separation of small organic molecules. Analyst 2017, 142, 517–524. [Google Scholar] [CrossRef]
- Abid, H.; Ang, H.; Wang, S. Effects of ammonium hydroxide on the structure and gas adsorption of nanosized Zr-MOFs (UiO-66). Nanoscale 2012, 4, 3089–3094. [Google Scholar] [CrossRef]
- Shokouhfar, N.; Aboutorabi, L.; Morsali, A. Improving the capability of UiO-66 for Cr(VI) adsorption from aqueous solutions by introducing the isonicotinate N–Oxide as the functional group. Dalton Trans. 2018, 47, 14549–14555. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef]
- Tang, P.; Bao, T.; Chen, Z. Novel Zn-based MOFs stationary phase with large pores for capillary electrochromatography. Electrophoresis 2016, 37, 2181–2189. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.; Zhu, G.; Hungerford, J.; Bhattacharyya, S.; Lively, R.; Sholl, D.; Walton, K. Heat-treatment of defective UiO-66 from modulated synthesis: Adsorption and stability studies. J. Phys. Chem. C 2017, 121, 23471–23479. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, W.; Gao, Y.; Wu, J.; Tang, B. Facile synthesis and supercapacitive properties of Zr-metal organic frameworks (UiO-66). RSC Adv. 2015, 5, 17601–17605. [Google Scholar] [CrossRef]
- Chang, S.-F.; Ayres, J.W.; Sandine, W.E. Analysis of cheese for histamine, tyramine, tryptamine, histidine, tyrosine, and tryptophane1. J. Dairy Sci. 1985, 68, 2840–2846. [Google Scholar] [CrossRef]
- Jia, L.; Rong-Fa, G.; Xi-Ming, W.; Jian-Chu, C.; Ya-Qin, H.; Dong-Hong, L.; Xing-Qian, Y. Detection of ten biogenic amines in Chinese commercial soybean paste by HPLC. Int. J. Food Prop. 2018, 21, 1344–1350. [Google Scholar] [CrossRef]
- Antolini, F.; Franciosini, S.; Floridi, A.; Floridi, A. An ion pair HPLC method for the determination of histamine, tyramine, tryptamine, β-phenylethylamine and their amino acid precursors in cheeses for industrial purposes. Ital. J. Food Sci. 1999, 11, 335–346. [Google Scholar]
- Veciana-Nogués, M.T.; Mariné-Font, A.; Vidal-Carou, M.C. Changes in biogenic amines during the storage of Mediterranean anchovies immersed in oil. J. Agric. Food Chem. 1997, 45, 1385–1389. [Google Scholar] [CrossRef]
- Şenel, E.; Yildiz, F.; Yetişemiyen, A.; Durlu-Özkaya, F.; Oztekin, S.; Şanli, E. Evaluation of the biogenic amine content and some chemical and microbiological properties of urfa and van herby cheeses. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 2012, 18, 537–544. [Google Scholar] [CrossRef]
- Calbiani, F.; Careri, M.; Elviri, L.; Mangia, A.; Pistarà, L.; Zagnoni, I. Rapid assay for analyzing biogenic amines in cheese: Matrix solid-phase dispersion followed by liquid chromatography−electrospray−tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3779–3783. [Google Scholar] [CrossRef]
- Apetrei, I.; Apetrei, C. The biocomposite screen-printed biosensor based on immobilization of tyrosinase onto the carboxyl functionalised carbon nanotube for assaying tyramine in fish products. J. Food Eng. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Bóka, B.; Adányi, N.; Virág, D.; Sebela, M.; Attila, K. Spoilage detection with biogenic amine biosensors, comparison of different enzyme electrodes. Electroanalysis 2012, 24, 181–186. [Google Scholar] [CrossRef]
- Teepoo, S.; Promta, A.; Phapugrangkul, P. A Competitive colorimetric immunosensor for detection of tyramine in fish samples. Food Anal. Methods 2019, 12, 1886–1894. [Google Scholar] [CrossRef]


| Dosage (μg·L−1) | Measured Quantity (μg·L−1) | Rate of Recovery (%) | RSD (%) |
|---|---|---|---|
| 200 | 182.96 | 91.47 | 3.7 |
| 300 | 280.88 | 93.62 | 2.2 |
| 400 | 346.95 | 86.74 | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.-X.; Zhao, N.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Ultra-Stable UiO-66 Involved Molecularly Imprinted Polymers for Specific and Sensitive Determination of Tyramine Based on Quartz Crystal Microbalance Technology. Polymers 2020, 12, 281. https://doi.org/10.3390/polym12020281
Yao C-X, Zhao N, Liu J-M, Fang G-Z, Wang S. Ultra-Stable UiO-66 Involved Molecularly Imprinted Polymers for Specific and Sensitive Determination of Tyramine Based on Quartz Crystal Microbalance Technology. Polymers. 2020; 12(2):281. https://doi.org/10.3390/polym12020281
Chicago/Turabian StyleYao, Chi-Xuan, Ning Zhao, Jing-Min Liu, Guo-Zhen Fang, and Shuo Wang. 2020. "Ultra-Stable UiO-66 Involved Molecularly Imprinted Polymers for Specific and Sensitive Determination of Tyramine Based on Quartz Crystal Microbalance Technology" Polymers 12, no. 2: 281. https://doi.org/10.3390/polym12020281
APA StyleYao, C.-X., Zhao, N., Liu, J.-M., Fang, G.-Z., & Wang, S. (2020). Ultra-Stable UiO-66 Involved Molecularly Imprinted Polymers for Specific and Sensitive Determination of Tyramine Based on Quartz Crystal Microbalance Technology. Polymers, 12(2), 281. https://doi.org/10.3390/polym12020281





