Highly Dual Antifouling and Antibacterial Ultrafiltration Membranes Modified with Silane Coupling Agent and Capsaicin-Mimic Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Filtration Experiments
2.5. Antifouling Property Evaluation
2.6. Antibacterial Activity Evaluation
3. Results
3.1. Chemical Structures and Compositions of the Membrane Surfaces
3.2. Morphologies of the Membranes
3.3. Surface Wettability of the Membranes
3.4. Mechanical Properties of the Membranes
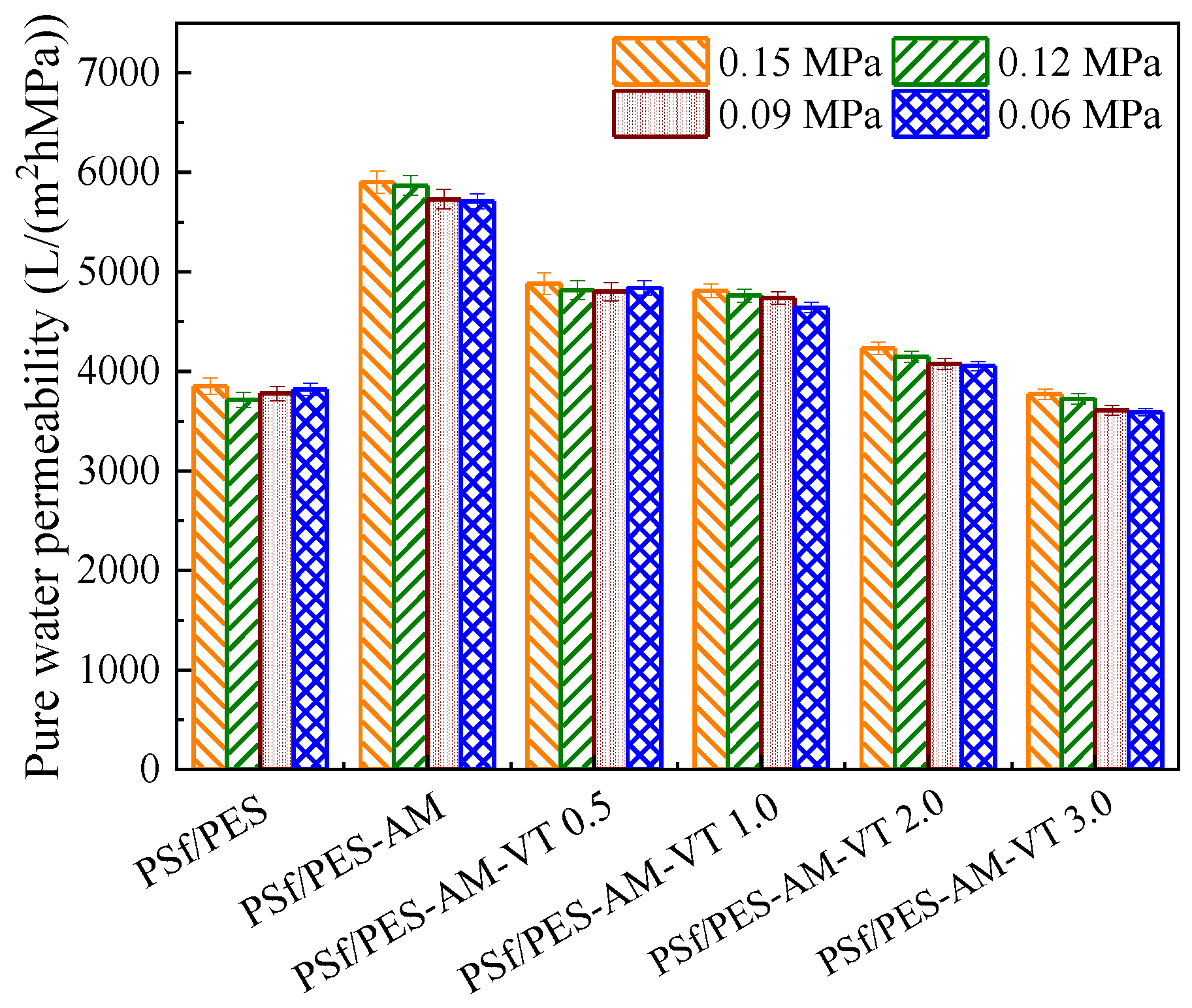
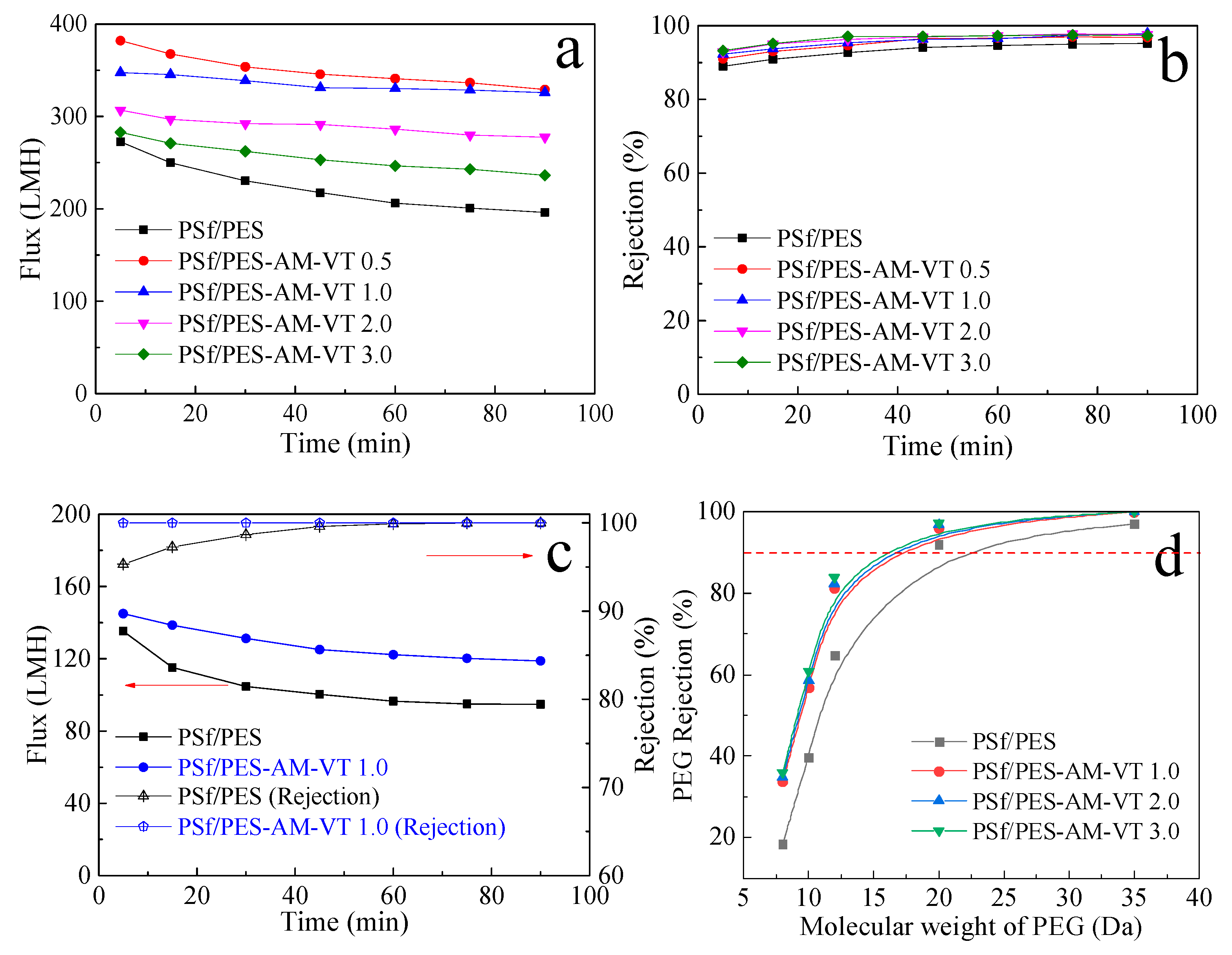
3.5. Separation Performance of the Membranes
3.6. Antibacterial Activity and Antibacterial Adhesion Ability of the Membranes
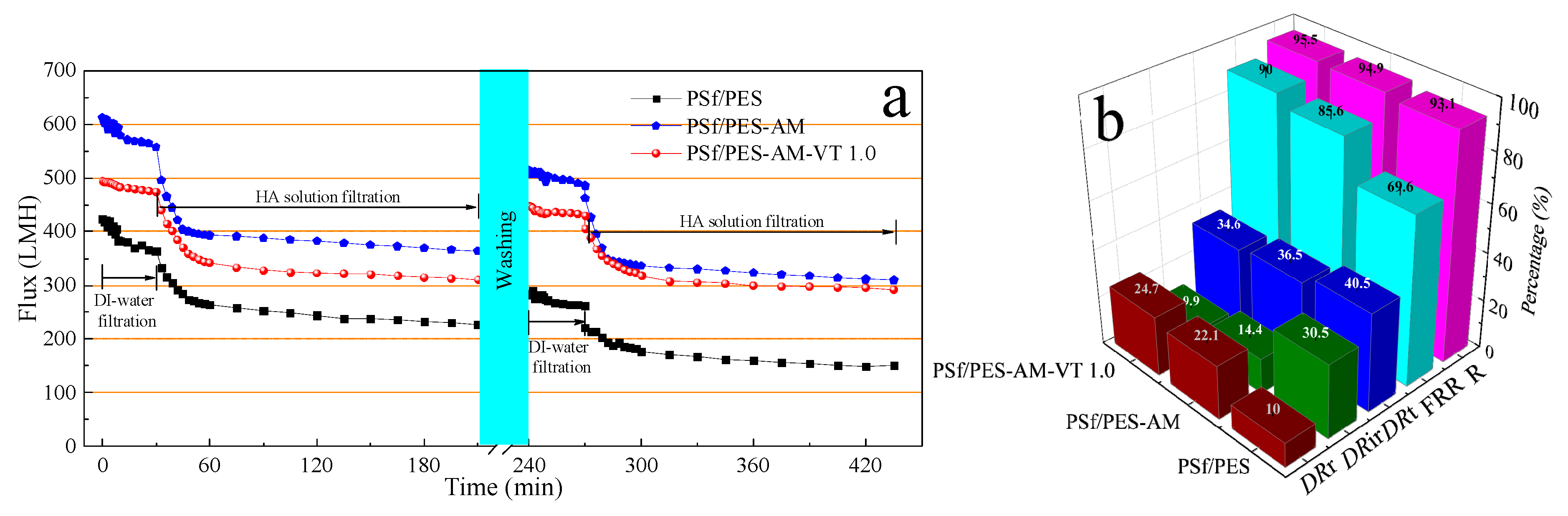
3.7. Antifouling Performances of the Membranes
3.8. Comparison with other UF Membranes Reported in Recent Years
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alsohaimi, I.H.; Kumar, M.; Algamdi, M.S.; Khan, M.A.; Nolan, K.; Lawler, J. Antifouling hybrid ultrafiltration membranes with high selectivity fabricated from polysulfone and sulfonic acid functionalized TiO2 nanotubes. Chem. Eng. J. 2017, 316, 573–583. [Google Scholar] [CrossRef]
- Mokhtari, S.; Rahimpour, A.; Shamsabadi, A.A.; Habibzadeh, S.; Soroush, M. Enhancing performance and surface antifouling properties of polysulfone ultrafiltration membranes with salicylate-alumoxane nanoparticles. Appl. Surf. Sci. 2017, 393, 93–102. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Wang, K.; Li, K.; Xu, L.; Wang, J. Fabrication of a novel conductive ultrafiltration membrane and its application for electrochemical removal of hexavalent chromium. J. Membr. Sci. 2019, 584, 191–201. [Google Scholar] [CrossRef]
- Yang, T.; Xiong, H.; Liu, F.; Yang, Q.; Xu, B.; Zhan, C. Effect of UV/TiO2 pretreatment on fouling alleviation and mechanisms of fouling development in a cross-flow filtration process using a ceramic UF membrane. Chem. Eng. J. 2019, 358, 1583–1593. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.; Souaya, E.; Badawy, M.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Pranantyo, D.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M.; Rittschof, D. Layer-by-layer click deposition of functional polymer coatings for combating marine biofouling. Biomacromolecules 2012, 13, 2769–2780. [Google Scholar] [CrossRef]
- Jhaveri, J.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Li, Z.; Hu, W.; Zhao, Y.; Ren, L.; Yuan, X. Integrated antibacterial and antifouling surfaces via cross-linking chitosan-g-eugenol/zwitterionic copolymer on electrospun membranes. Colloids Surf. B Biointerface 2018, 169, 151–159. [Google Scholar] [CrossRef]
- Chen, Z.; Du, X.; Liu, Y.; Ju, Y.; Song, S.; Dong, L. A high-efficiency ultrafiltration nanofibrous membrane with remarkable antifouling and antibacterial ability. J. Mater. Chem. A 2018, 6, 15191–15199. [Google Scholar] [CrossRef]
- Gao, K.; Su, Y.; Zhou, L.; He, M.; Zhang, R.; Liu, Y.; Jiang, Z. Creation of active-passive integrated mechanisms on membrane surfaces for superior antifouling and antibacterial properties. J. Membr. Sci. 2018, 548, 621–631. [Google Scholar] [CrossRef]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y.; Narain, R. Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J. Membr. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; Isloor, A.M.; Asiri, A.M.; Ismail, A.F.; Kumar, R.; Ahamed, M.I. Performance intensification of the polysulfone ultrafiltration membrane by blending with copolymer encompassing novel derivative of poly (styrene-co-maleic anhydride) for heavy metal removal from wastewater. Chem. Eng. J. 2018, 353, 425–435. [Google Scholar] [CrossRef]
- Qayum, A.; Wei, J.; Li, Q.; Chen, D.; Jiao, X.; Xia, Y. Efficient decontamination of multi-component wastewater by hydrophilic electrospun PAN/AgBr/Ag fibrous membrane. Chem. Eng. J. 2019, 361, 1255–1263. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, L.; Zhao, Y.; Zhu, B.; Xu, Y. Anti-fouling and anti-bacterial polyethersulfone membranes quaternized from the additive of poly (2-dimethylamino ethyl methacrylate) grafted SiO2 nanoparticles. J. Mater. Chem. A 2014, 2, 15566–15574. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Surface coating of UF membranes to improve antifouling properties: A comparison study between cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs). Chemosphere 2019, 217, 76–84. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, C.; Wang, Z.; Xu, Y.; Zhao, W.; Sun, S.; Zhao, C. Co-deposition towards mussel-inspired antifouling and antibacterial membranes by using zwitterionic polymers and silver nanoparticles. J. Mater. Chem. B 2017, 5, 7186–7193. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Huang, J.; Mao, L.; Chen, J.; Li, M. Preparation and characterization of antifouling and antibacterial polysulfone ultrafiltration membranes incorporated with a silver-polydopamine nanohybrid. J. Appl. Polym. Sci. 2018, 135, 46430. [Google Scholar] [CrossRef]
- Zhu, L.; Song, H.; Wang, J.; Xue, L. Polysulfone hemodiafiltration membranes with enhanced anti-fouling and hemocompatibility modified by poly(vinyl pyrrolidone) via in situ cross-linked polymerization. Mater. Sci. Eng. C 2017, 74, 159–166. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Tang, Y.; Hou, J.; Yu, L.; Gao, C. A novel long-lasting antifouling membrane modified with bifunctional capsaicin-mimic moieties via in situ polymerization for efficient water purification. J. Mater. Chem. A 2016, 4, 10352–10362. [Google Scholar] [CrossRef]
- Watts, L.J. Anti-Fouling Coating Composition Containing Capsaicin. U.S. Patent 5,397,385, 14 March 1995. [Google Scholar]
- Bullat, M.D.; Vasishtha, N. Biorepellent Matrix Coating. U.S. Patent 5,925,370, 20 July 1999. [Google Scholar]
- Clark, R.; Lee, S. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Zhou, Y.; Guan, X.; Zhu, W.; Liu, Z.; Wang, X.; Yu, H.; Wang, H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur. J. Clin. Microbiol. 2014, 33, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, H.; Wang, J.; Huang, X.; Gao, C. Surface-modified PSf UF membrane by UV-assisted graft polymerization of capsaicin derivative moiety for fouling and bacterial resistance. J. Membr. Sci. 2013, 445, 146–155. [Google Scholar]
- Xu, J.; Feng, X.; Hou, J.; Wang, X.; Shan, B.; Yu, L.; Gao, C. Preparation and characterization of a novel polysulfone UF membrane using a copolymer with capsaicin-mimic moieties for improved anti-fouling properties. J. Membr. Sci. 2013, 446, 171–180. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, C.; Jiang, X.; Li, X.; Yu, L. High hydrophilic antifouling membrane modified with capsaicin-mimic moieties via microwave assistance (MWA) for efficient water purification. Chem. Eng. J. 2018, 338, 688–699. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Costerton, J.; Stewart, P.; Greenberg, E. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Laghaei, M.; Sadeghi, M.; Ghalei, B.; Shahrooz, M. The role of compatibility between polymeric matrix and silane coupling agents on the performance of mixed matrix membranes: Polyethersulfone/MCM-41. J. Membr. Sci. 2016, 513, 20–32. [Google Scholar] [CrossRef]
- Sun, J.; Cao, Z.; Wu, L. Polyvinylidene fluoride/silane-treated hydroxyapatite mixed matrix membrane for enzyme capturing. Colloids Surf. B Biointerface 2015, 126, 265–272. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Yang, H. TEOS/silane coupling agent composed double layers structure: A novel super-hydrophilic coating with controllable water contact angle value. Appl. Energy 2017, 185, 2209–2216. [Google Scholar]
- Su, X.; Shi, B. Effect of silane coupling agents with different non-hydrolytic groups on tensile modulus of composite PDMS crosslinked membranes. React. Funct. Polym. 2016, 98, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Liu, M.; Zhao, F.; Wu, Z. In-situ modification of PVDF membrane during phase-inversion process using carbon nanosphere sol as coagulation bath for enhancing anti-fouling ability. J. Membr. Sci. 2017, 526, 272–280. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, J.; Xu, X.; Zhang, G.; Yang, Y.; Yang, F. Highly antifouling and antibacterial performance of poly (vinylidene fluoride) ultrafiltration membranes blending with copper oxide and graphene oxide nanofillers for effective wastewater treatment. J. Colloid Interface Sci. 2017, 505, 341–351. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, G.; Zhang, S.; Du, Y.; Lu, Y.; Na, R.; Mu, Y.; Zhang, Y. Preparation of organic–inorganic hybrid membranes with superior antifouling property by incorporating polymer-modified multiwall carbon nanotubes. RSC Adv. 2017, 7, 30564–30572. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; McGlade, D.; Ulbricht, M.; Lawler, J. Quaternized polysulfone and graphene oxide nanosheet derived low fouling novel positively charged hybrid ultrafiltration membranes for protein separation. RSC Adv. 2015, 5, 51208–51219. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Jiang, J.; Zhang, Q.; Gao, F.; Zhan, X.; Chen, F. Ultralow oil-fouling heterogeneous poly (ether sulfone) ultrafiltration membrane via blending with novel amphiphilic fluorinated gradient copolymers. Langmuir 2016, 32, 1380–1388. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Yu, L.; Wang, J. PANI-PMMA as cathodic electrode material and its application in cathodic polarization antifouling. Electrochem. Commun. 2017, 84, 57–60. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Yu, L.; Wang, J.; Zheng, T. The feasibility and application of PPy in cathodic polarization antifouling. Colloids Surf. B Biointerface 2018, 164, 247–254. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, M.S.; Choi, Y.; Lee, K.B.; Kim, J.H. Synthesis of PVA-g-POEM graft copolymers and their use in highly permeable thin film composite membranes. Chem. Eng. J. 2018, 346, 739–747. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Hu, H.; Liu, W. Surface modification of self-healing poly (urea-formaldehyde) microcapsules using silane-coupling agent. Appl. Surf. Sci. 2008, 255, 1894–1900. [Google Scholar] [CrossRef]
- Xing, J.; Wang, H.; Cheng, X.; Tang, X.; Luo, X.; Wang, J.; Wang, T.; Li, G.; Liang, H. Application of low-dosage UV/chlorine pre-oxidation for mitigating ultrafiltration (UF) membrane fouling in natural surface water treatment. Chem. Eng. J. 2018, 344, 62–70. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Duan, L.; Wang, Y.; Zhang, Y.; Liu, J. Graphene immobilized enzyme/polyethersulfone mixed matrix membrane: Enhanced antibacterial, permeable and mechanical properties. Appl. Surf. Sci. 2015, 355, 436–445. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Zhao, X.; He, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Antifouling, high-flux nanofiltration membranes enabled by dual functional polydopamine. ACS Appl. Mater. Interfaces 2014, 6, 5548–5557. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Q.; Liang, H.; Gu, L.; Xu, Z. Preparation and characterization of cellulose triacetate membranes via thermally induced phase separation. J. Appl. Polym. Sci. 2017, 134, 6. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; Xia, S.; Liu, J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-fouling and anti-bacterial modification of poly (vinylidene fluoride) membrane by blending with the capsaicin-based copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Goel, V.; Uttam, K.M. Surface modification of polysulfone ultrafiltration membrane by in-situ ferric chloride based redox polymerization of aniline-surface characteristics and flux analyses. Korean J. Chem. Eng. 2019, 36, 573–583. [Google Scholar] [CrossRef]
- Najjar, A.; Sabri, S.; Al-Gaashani, R.; Kochkodan, V.; Atieh, M.A. Enhanced fouling resistance and antibacterial properties of novel graphene oxide-Arabic gum polyethersulfone membranes. Appl. Sci. 2019, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Tafreshi, J.; Fashandi, H. Environmentally Friendly modification of polysulfone ultrafiltration membrane using organic plant-derived nanoparticles prepared from basil seed gum (BSG) and Ar/O2 low-pressure plasma. J. Environ. Chem. Eng. 2019, 7, 103245. [Google Scholar] [CrossRef]
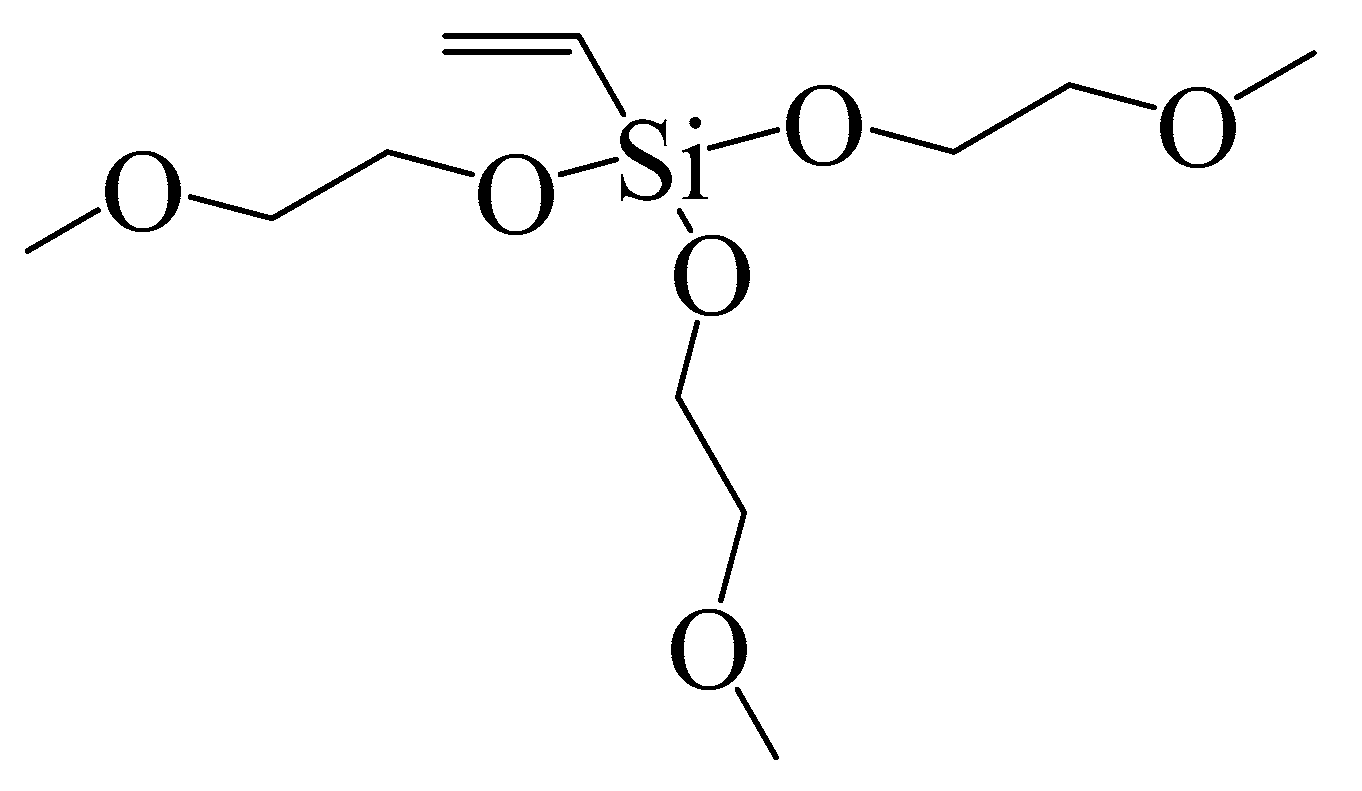
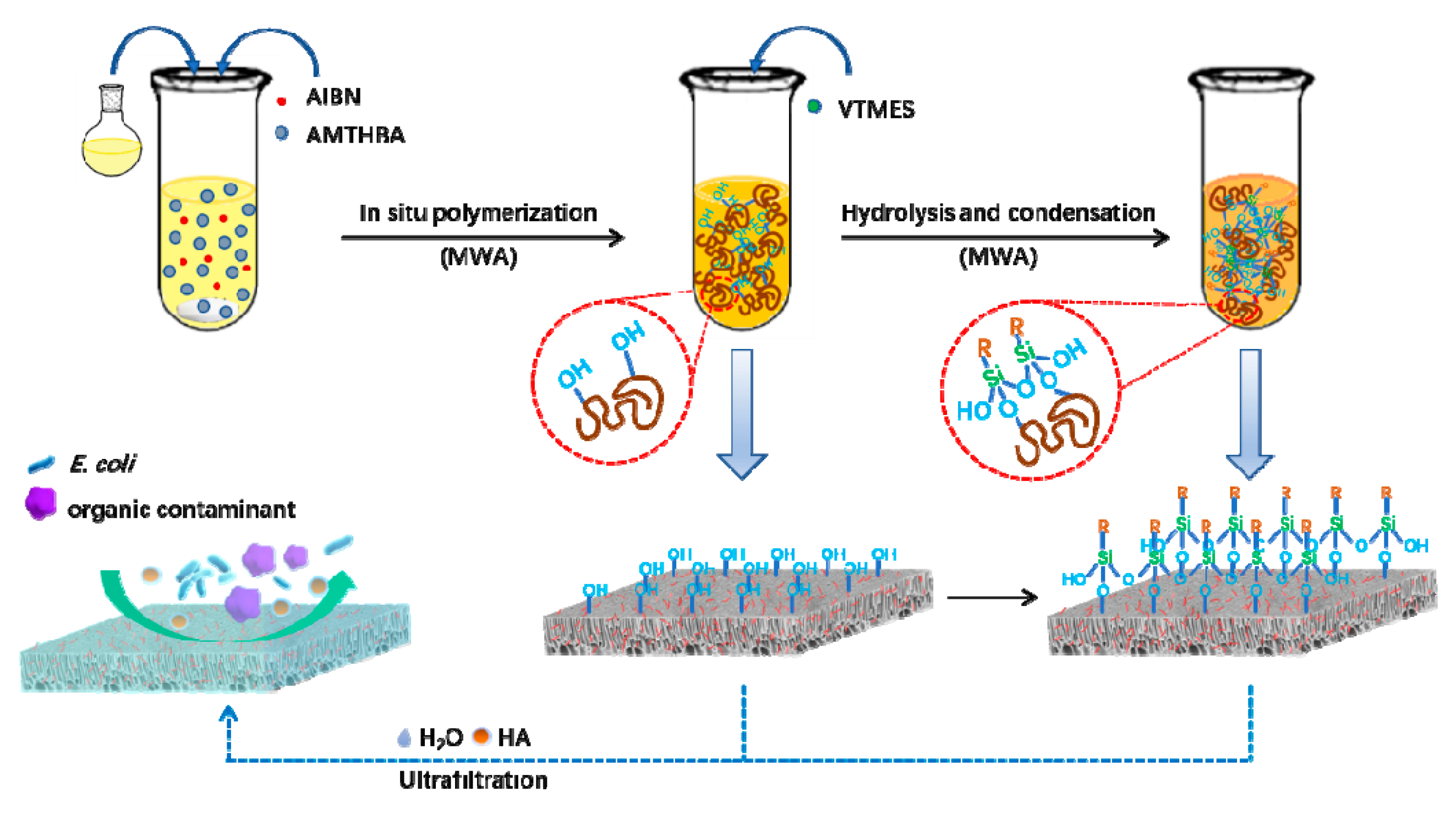




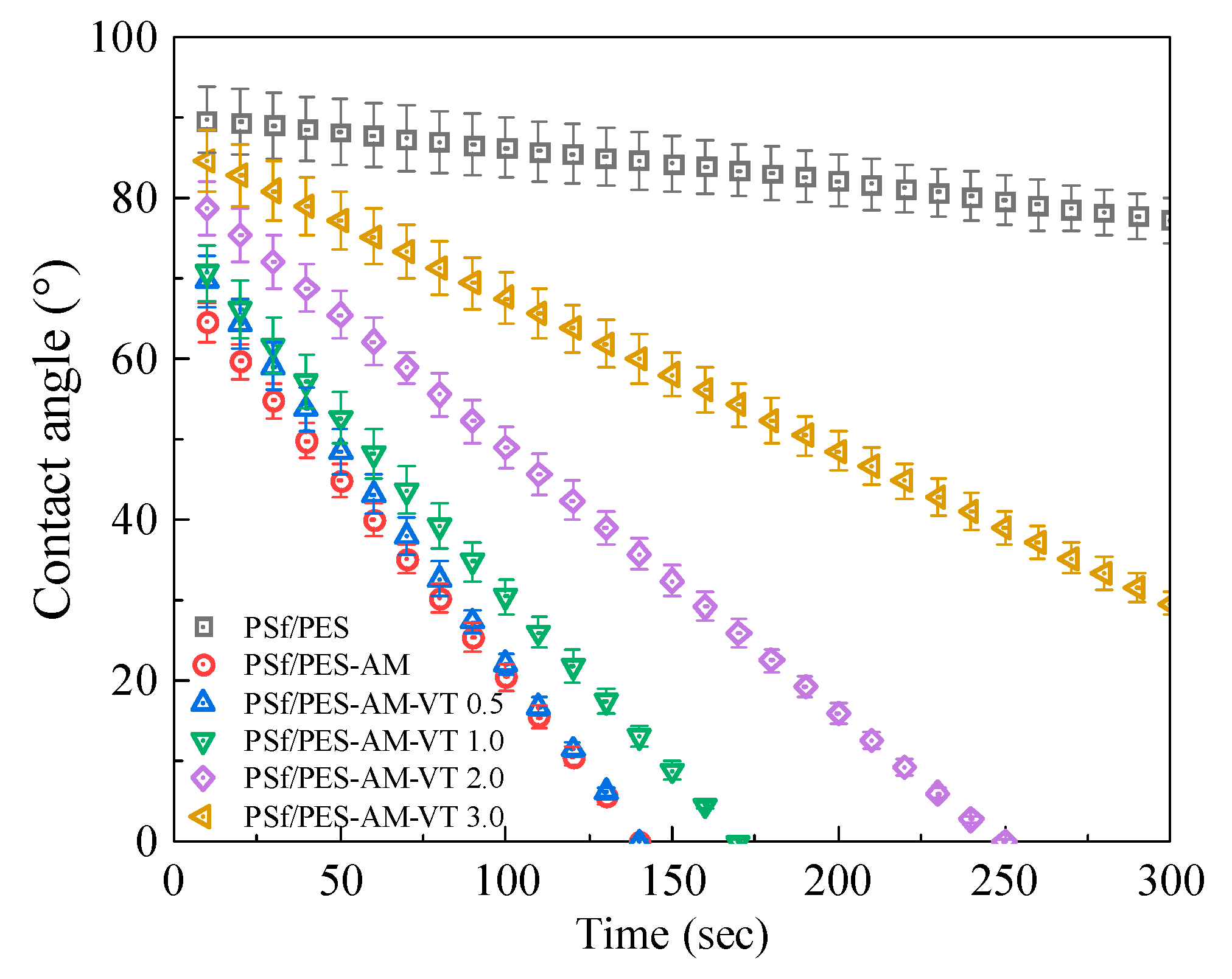
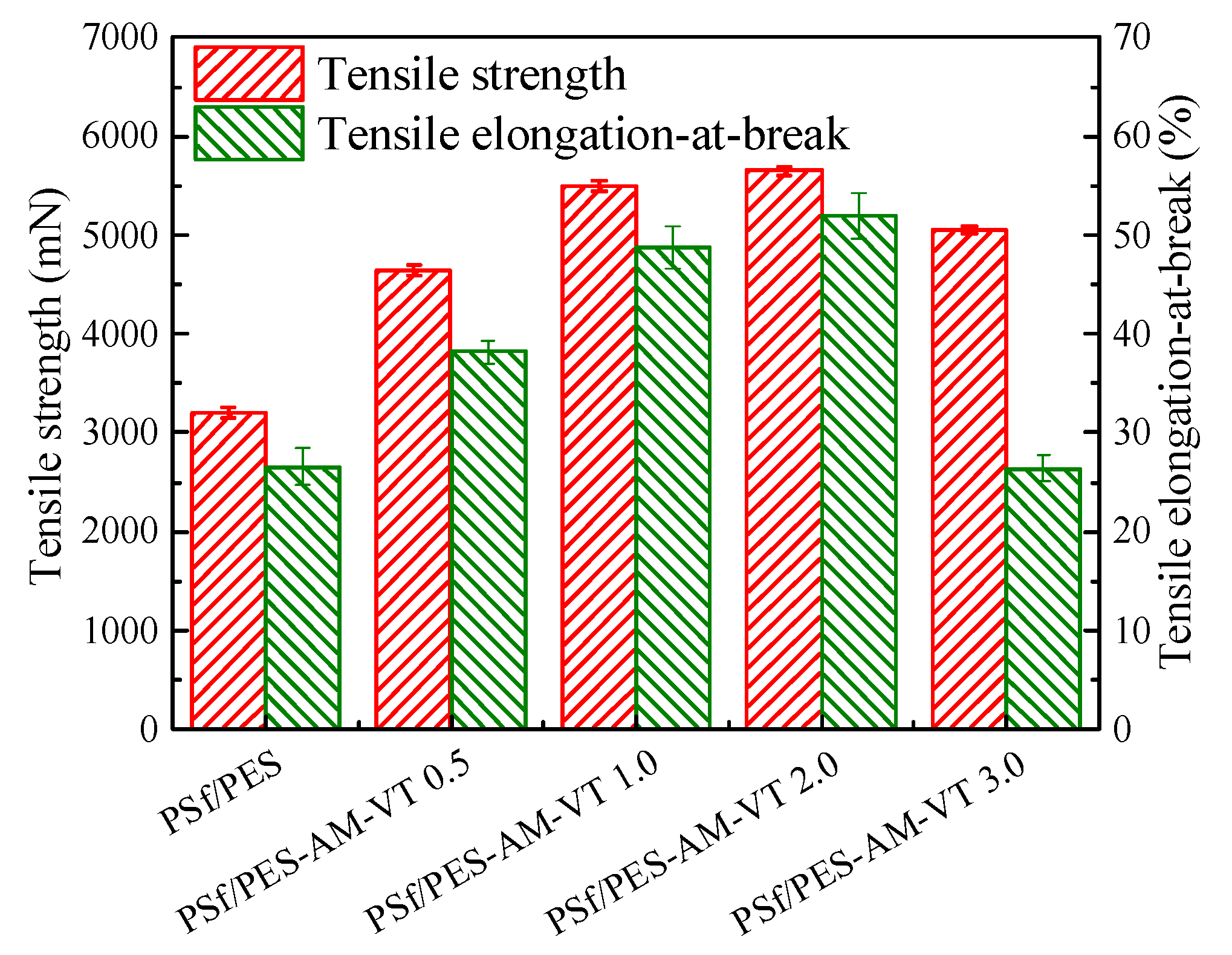


| Membrane | PSf (wt %) | PES (wt %) | DMAc (wt %) | PEG-400 (wt %) | H2O (wt %) | AMTHBA (wt %) | AIBN (wt %) | VTMES (wt %) |
|---|---|---|---|---|---|---|---|---|
| PSf/PES | 12.8 | 3.2 | 75.6 | 8 | 0.4 | 0 | 0 | 0 |
| PSf/PES-AM | 12.8 | 3.2 | 73.4 | 8 | 0.4 | 2 | 0.2 | 0 |
| PSf/PES-AM-VT 0.5 | 12.8 | 3.2 | 72.9 | 8 | 0.4 | 2 | 0.2 | 0.5 |
| PSf/PES-AM-VT 1.0 | 12.8 | 3.2 | 72.4 | 8 | 0.4 | 2 | 0.2 | 1 |
| PSf/PES-AM-VT 2.0 | 12.8 | 3.2 | 71.4 | 8 | 0.4 | 2 | 0.2 | 2 |
| PSf/PES-AM-VT 3.0 | 12.8 | 3.2 | 70.4 | 8 | 0.4 | 2 | 0.2 | 3 |
| Matrix Membrane | Monomer | Pure Water Flux (L·m−2·h−1) | BSA Rejection (%) | FRR (%) | Eb (%) | Year of Publication and Reference |
|---|---|---|---|---|---|---|
| PSf/PES | AMTHBA/VTMES | 473.8 | ~100 | 90 | 92.3 | This work |
| PSf/PES | AMTHBA | 586.8 | ~100 | 85.6 | -- | 2018 [28] |
| PVDF | PMMA-PACMO-capsaicin | 225.5 | 64.6 | ~90 | 88.5 | 2019 [49] |
| PSf | Aniline | 396.1 | ~98 | 66.5 | -- | 2019 [50] |
| PES | Graphene oxide/arabic gum | ~520 | ~79 | ~75 | -- | 2019 [51] |
| PSf | Basil seed gum (BSG) | 155.3 | ~84 | ~100 | -- | 2019 [52] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Tang, Y.; Jiang, X.; Yu, L.; Wang, C. Highly Dual Antifouling and Antibacterial Ultrafiltration Membranes Modified with Silane Coupling Agent and Capsaicin-Mimic Moieties. Polymers 2020, 12, 412. https://doi.org/10.3390/polym12020412
Zhang L, Tang Y, Jiang X, Yu L, Wang C. Highly Dual Antifouling and Antibacterial Ultrafiltration Membranes Modified with Silane Coupling Agent and Capsaicin-Mimic Moieties. Polymers. 2020; 12(2):412. https://doi.org/10.3390/polym12020412
Chicago/Turabian StyleZhang, Lili, Yuanyuan Tang, Xiaohui Jiang, Liangmin Yu, and Changyun Wang. 2020. "Highly Dual Antifouling and Antibacterial Ultrafiltration Membranes Modified with Silane Coupling Agent and Capsaicin-Mimic Moieties" Polymers 12, no. 2: 412. https://doi.org/10.3390/polym12020412





