Effect of Donor-Acceptor Concentration Ratios on Non-Radiative Energy Transfer in Zero-Dimensional Cs4PbBr6 Perovskite/MEH-PPV Nanocomposite Thin Films
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussion
3.1. Structural and Morphology of the Hybrid Thin Films
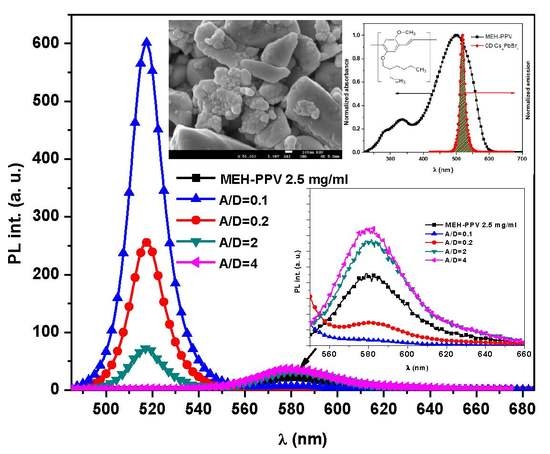
3.2. Absorption and Fluorescence Spectra of the Thin Films
3.3. Energy Transfer Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing optoelectronic properties of metal halide perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Li, G. Highly efficient perovskite nanocrystal light-emitting diodes enabled by a universal crosslinking method. Adv. Mater. 2016, 28, 3528–3534. [Google Scholar] [CrossRef]
- Li, X. A vacuum flash–assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Yakunin, S. Detection of X-ray photons by solution-processed lead halide perovskites. Nat. Photonics 2015, 9, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Choi, J. Organolead halide perovskites for low operating voltage multilevel resistive switching. Adv. Mater. 2016, 28, 6562–6567. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Im, S.H.; Park, N.-G. Organolead halide perovskite: New horizons in solar cell research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- Wei, H. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 2016, 10, 333. [Google Scholar] [CrossRef]
- Leijtens, T. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Murali, B. Surface restructuring of hybrid perovskite crystals. ACS Energy Lett. 2016, 1, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H. Enhanced environmental stability of planar heterojunction perovskite solar cells based on blade-coating. Adv. Energy Mater. 2015, 5, 1401229. [Google Scholar] [CrossRef]
- Chang, C.-Y. High-performance, air-stable, low-temperature processed semitransparent perovskite solar cells enabled by atomic layer deposition. Chem. Mater. 2015, 27, 5122–5130. [Google Scholar] [CrossRef]
- You, J. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Kulbak, M. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 2015, 7, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadhanala, A. Electroluminescence from Organometallic Lead Halide Perovskite-Conjugated Polymer Diodes. Adv. Electron. Mater. 2015, 1, 1500008. [Google Scholar] [CrossRef] [Green Version]
- Li, G. Efficient light-emitting diodes based on nanocrystalline perovskite in a dielectric polymer matrix. Nano Lett. 2015, 15, 2640–2644. [Google Scholar] [CrossRef] [Green Version]
- Al-Asbahi, B.A. Photophysical properties and energy transfer mechanism of PFO/Fluorol 7GA hybrid thin films. J. Lumin. 2013, 142, 57–65. [Google Scholar] [CrossRef]
- Sharma, N. Maleimide—Based donor-π-acceptor-π-donor derivative for efficient organic light-emitting diodes. Org. Electron. 2016, 38, 180–185. [Google Scholar] [CrossRef]
- Al-Asbahi, B. Influence of SiO2/TiO2 nanocomposite on the optoelectronic properties of PFO/MEH-PPV-based OLED devices. Polymers 2018, 10, 800. [Google Scholar] [CrossRef] [Green Version]
- Liu, B. High-performance doping-free hybrid white organic light-emitting diodes: The exploitation of ultra thin emitting nanolayers (<1 nm). Nano Energy 2016, 26, 26–36. [Google Scholar]
- Saidaminov, M.I. Pure Cs4PbBr6: Highly luminescent zero-dimensional perovskite solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Controlled synthesis of zero-dimensional phase-pure Cs4PbBr6 perovskites crystals with high photoluminescence quantum yield. J. Alloys Compd. 2019, 797, 1151–1156. [Google Scholar] [CrossRef]
- Zhang, Z. One-step on-chip synthesis of highly-luminescent Cs4PbBr6 microcrystal. Mater. Lett. 2018, 232, 118–121. [Google Scholar] [CrossRef]
- Jing, Q. A systematic study of the synthesis of cesium lead halide nanocrystals: Does Cs4PbBr6 or CsPbBr3 form? Nanoscale 2019, 11, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.; Baggot, J. Essentials of Molecular Photochemistry; Blackwell Scientific Publications: Oxford, UK, 1991; pp. 265–267. [Google Scholar]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef]
- Wu, P.; Brand, L. Resonance energy transfer: Methods and applications. Anal. Biochem. 1994, 218, 1–13. [Google Scholar] [CrossRef]
- Kong, F. Effect of distance between acceptor and donor on optical properties of composite semiconducting polymer films. J. Lumin. 2011, 131, 815–819. [Google Scholar] [CrossRef]
- Mallick, A. 7-Hydroxy-4-methyl-8-(4′-methylpiperazine-1′-yl) methyl coumarin: An efficient probe for fluorescence resonance energy transfer to a bioactive indoloquinolizine system. J. Lumin. 2006, 118, 165–172. [Google Scholar] [CrossRef]
- Mote, U. Fluorescence resonance energy transfer from tryptophan to folic acid in micellar media and deionised water. J. Photochem. Photobiol. B 2011, 103, 16–21. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: London, UK, 2009. [Google Scholar]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Al-Asbahi, B.A. Energy transfer mechanism and optoelectronic properties of (PFO/TiO2)/Fluorol 7GA nanocomposite thin films. Opt. Mater. 2017, 72, 644–649. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Long-range dipole–dipole energy transfer enhancement via addition of SiO2/TiO2 nanocomposite in PFO/MEH-PPV hybrid thin films. J. Appl. Polym. Sci. 2019, 136, 47845. [Google Scholar] [CrossRef]
- Heinrichova, P. Energy versus charge transfer in π-conjugated polymer: Fullerene blends. Chem. Phys. Lett. 2014, 592, 314–319. [Google Scholar] [CrossRef]
- Zhang, Y. Zero-Dimensional Cs4PbBr6 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2017, 8, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M. Improvement of solution based conjugate polymer organic light emitting diode by ZnO–graphene quantum dots. J. Mater. Sci.: Mater. Electron. 2015, 26, 3344–3351. [Google Scholar] [CrossRef]
- Virgili, T. Efficient energy transfer from blue to red in tetraphenylporphyrin-doped poly(9,9-dioctylfluorene) light-emitting diodes. Adv. Mater. 2000, 12, 58–62. [Google Scholar] [CrossRef]
- Förster, T. Fluoreszenz Organischer Verbindungen; Vandenhoeck and Ruprecht: Göttingen, Germany, 1951. [Google Scholar]











| A/D Ratio | ||||
|---|---|---|---|---|
| 0.0 | 0.450 | 0.104 | −0.201 | 5.30 |
| 0.1 | 0.113 | 0.667 | −2.057 | 1.33 |
| 0.2 | 0.0484 | 1.670 | −2.979 | 0.57 |
| 2.0 | 0.0139 | 6.013 | −4.261 | 0.164 |
| 4.0 | 0.0037 | 22.64 | −5.586 | 0.044 |
| A/D Ratio | J(λ) × 1016 (M−1 cm−1 nm4) | R0 (Å) | RDA (Å) | TDR (ns−1) | |
|---|---|---|---|---|---|
| 0.1 | 4.97 | 74.87 | 62.45 | 0.56 | 0.75 |
| 0.2 | 7.66 | 80.47 | 56.55 | 1.57 | 1.76 |
| 2.0 | 7.49 | 80.17 | 45.15 | 5.91 | 6.10 |
| 4.0 | 4.88 | 74.65 | 33.64 | 22.55 | 22.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asbahi, B.A.; Qaid, S.M.H.; Aldwayyan, A.S. Effect of Donor-Acceptor Concentration Ratios on Non-Radiative Energy Transfer in Zero-Dimensional Cs4PbBr6 Perovskite/MEH-PPV Nanocomposite Thin Films. Polymers 2020, 12, 444. https://doi.org/10.3390/polym12020444
Al-Asbahi BA, Qaid SMH, Aldwayyan AS. Effect of Donor-Acceptor Concentration Ratios on Non-Radiative Energy Transfer in Zero-Dimensional Cs4PbBr6 Perovskite/MEH-PPV Nanocomposite Thin Films. Polymers. 2020; 12(2):444. https://doi.org/10.3390/polym12020444
Chicago/Turabian StyleAl-Asbahi, Bandar Ali, Saif M. H. Qaid, and Abdullah S. Aldwayyan. 2020. "Effect of Donor-Acceptor Concentration Ratios on Non-Radiative Energy Transfer in Zero-Dimensional Cs4PbBr6 Perovskite/MEH-PPV Nanocomposite Thin Films" Polymers 12, no. 2: 444. https://doi.org/10.3390/polym12020444