The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of the Polymeric Monomer THAAB
2.3. Synthesis of SX-1
2.4. Methods
2.4.1. Characterization of SX-1
2.4.2. Inhibition Performance Evaluations
2.4.3. Inhibition Mechanism Study
3. Results and Discussion
3.1. IR Characterization of SX-1
3.1.1. Molecular Weight Measurement
3.1.2. TGA-DSC Measurement
3.2. Inhibition Property Evaluation
3.2.1. Hot-Rolling Recovery Tests
3.2.2. Linear Swelling Tests
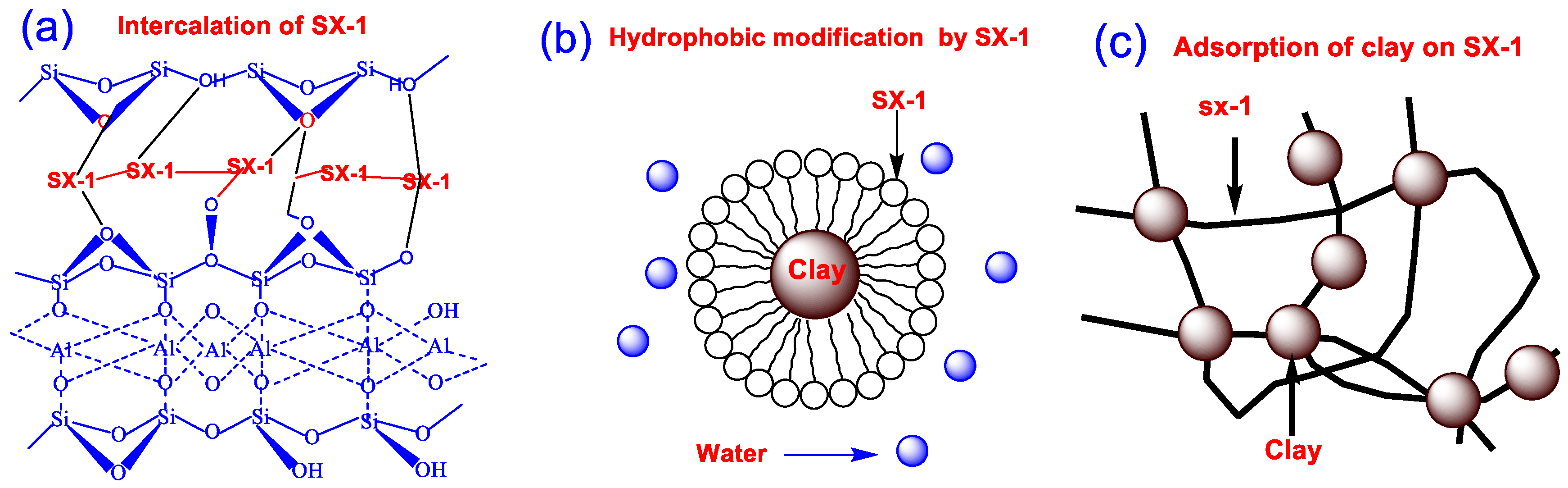
3.3. Inhibition Mechanism Analysis
3.3.1. FT-IR Analysis
3.3.2. Particle Distribution Tests
3.3.3. XRD Analysis
3.3.4. SEM Observations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lai, N.J.; Tang, L.; Jia, N.; Qiao, D.Y.; Chen, J.L.; Wang, Y.; Zhao, X.B. Feasibility study of applying modified nano-SiO2 hyperbranched copolymers for enhanced oil recovery in low-mid permeability reservoirs. Polymers 2019, 11, 1483. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Jo Jin, H.; Choi, M.J.; Jang, H.W.; Kim Ju, Y.; Hwang, W.R.; Kim, S.Y. Influence of MoS2 nanosheet size on performance of drilling mud. Polymers 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.J.; Wilson, L. Clay mineralogy and shale instability: An alternative conceptual analysis. Clay Miner. 2014, 49, 127–145. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.L.; Ratcliffe, I.; Greenwell, H.C.; Williams, P.A.; Cliffe, S.; Coveney, P.V. Clay swelling-A challenge in the oilfield. Earth-Sci. Rev. 2010, 98, 201–216. [Google Scholar] [CrossRef]
- Gholami, R.; Elochukwu, H.; Fakhari, N.; Sarmadivaleh, M. A review on borehole instability in active shale formations: Interactions, mechanisms and inhibitors. Earth-Sci. Rev. 2018, 177, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Wang, X.; Chen, G.; Zhang, J.; Slaný, M. Synthesis, property and mechanism analysis of a novel polyhydroxy organic amine shale hydration inhibitor. Minerals 2020, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.; Arfaj, M.K.; Saleh, T.A. Advanced developments in shale inhibitors for oil production with low environmental footprints: A review. Fuel 2019, 247, 237–249. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M. Polymeric and low molecular weight shale inhibitors: A review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Gou, S.H.; Yin, T.; Liu, K.; Guo, Q.P. Water-soluble complexes of an acrylamide copolymer and ionic liquids for inhibiting shale hydration. New J. Chem. 2015, 39, 2155–2161. [Google Scholar] [CrossRef]
- Liu, X.J.; Liu, K.; Gou, S.H.; Liang, L.X.; Luo, C.; Guo, Q.P. Water-soluble acrylamide sulfonate copolymer for inhibiting shale hydration. Ind. Eng. Chem. Res. 2014, 53, 2903–2910. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.X.; Han, Y.G.; Wang, S.Y.; Zhang, F.; Pan, W.; Lv, K.H. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.S.; Zhang, Y.J.; Zhong, H.Y.; Huang, W.A.; Tang, Z.C. Zwitterionic polymer P(AM-DMC-AMPS) as a low-molecular-weight encapsulator in deepwater drilling fluid. Appl. Sci. 2017, 7, 594. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zhu, Z.; Shi, W.; Hu, Y. Synthesis and application of a novel betaine-type copolymer as fluid loss additive for water-based drilling fluid. Colloid Polym. Sci. 2017, 295, 53–66. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Yi, Z.; Zhu, L.-P.; Xu, Y.-Y. Versatile antifouling polyethersulfone filtration membranes modified via surface grafting of zwitterionic polymers from a reactive amphiphilic copolymer additive. J. Colloid Interf. Sci. 2015, 448, 380–388. [Google Scholar] [CrossRef]
- Morimoto, N.; Wakamura, M.; Muramatsu, K.; Toita, S.; Nakayama, M.; Shoji, W.; Suzuki, M.; Winnik, F.M. Membrane Translocation and Organelle-Selective Delivery Steered by Polymeric Zwitterionic Nanospheres. Biomacromolecules 2016, 17, 1523–1535. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Lahmer, T.; Zhuang, X.; Nguyen-Thoi, T.; Rabczuk, T. A software framework for probabilistic sensitivity analysis for computationally expensive models. Adv. Eng. Softw. 2016, 100, 19–31. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Rafiee, R.; Zhuang, X.; Lahmer, T.; Rabczuk, T. Uncertainty quantification for multiscale modeling of polymer nanocomposites with correlated parameters. Compos. Part B Eng. 2015, 68, 446–464. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Silani, M.; Lahmer, T.; Zhuang, X.; Rabczuk, T. A unified framework for stochastic predictions of mechanical properties of polymeric nanocomposites. Comp. Mater. Sci. 2015, 96, 520–535. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Lahmer, T.; Zhang, Y.; Zhuang, X.; Rabczuk, T. Stochastic predictions of interfacial characteristic of polymeric nanocomposites (PNCs). Compos. Part. B Eng. 2014, 59, 80–95. [Google Scholar] [CrossRef]
- Pu, X.L.; Du, W.C.; Sun, J.S.; Luo, X.; Zhang, H.D. Synthesis and application of a novel polyhydroxy amine clay anti-swelling agent. Petrochem. Technol. 2016, 45, 595–600. [Google Scholar]
- Silva, F.; Siopa, F.; Figueiredo, B.; Gonçalves, A.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.; Afonso, C.; Ventura, S. Sustainable design for environment-friendly monoand dicationic cholinium-based ionic liquids. Ecotox. Environ. Safe 2014, 108, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Uranta, K.G.; Rezaei-Gomari, S.; Russell Paul-Hamad, F. Studying the effectiveness of polyacrylamide (PAM) application in hydrocarbon reservoirs at different operational conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.H.; Qiu, Y.L.; Guo, P.; Du, J.F.; Liu, H.; Hu, Y.S.; Zeng, F.H. Experimental Investigation of the Damage Mechanisms of Drilling Mud in Fractured Tight gas Reservoir. J. Energ. Resour-Asme. 2019, 141, 092907. [Google Scholar] [CrossRef]
- Caglar, B.; Topcu, C.; Coldur, F.; Sarp, G.; Caglar, S.; Tabak, A.; Sahin, E. Structural, thermal, morphological and surface charge properties of dodecyl trimethylammonium-smectite composites. J. Mol. Struct. 2016, 1105, 70–79. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the Clay Minerals Society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Madejová, J.; Sekeráková, Ľ.; Bizovská, V.; Slaný, M.; Jankovič, Ľ. Near-infrared spectroscopy as an effective tool for monitoring the conformation of alkylammonium surfactants in montmorillonite interlayers. Vib. Spectrosc. 2016, 84, 44–52. [Google Scholar] [CrossRef]
- Júnior, L.P.C.; Silva, D.B.R.; Aguiar, M.F.; Melo, C.P.; Alves, K.G.B. Preparation and characterization of polypyrrole/organophilic montmorillonite nanofibers obtained by electrospinning. J. Mol. Liq. 2019, 275, 452–462. [Google Scholar] [CrossRef]
- Adewole, J.K.; Najimu-Musa, O. A Study on the Effects of Date Pit-Based Additive on the Performance of Water-Based Drilling Fluid. J. Energ. Resour-Asme. 2019, 140, 052903. [Google Scholar] [CrossRef]
- Boek, E.S.; Coveney, P.V.; Skipper, N.T. Monte carlo molecular modeling studies of hydrated Li-, Na- and K-smectites: Understanding the role of potassium as a clay swelling inhibitor. J. Am. Chem. Soc. 1995, 117, 12608–12617. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.S.; Wang, M.L.; Huang, W.A.; Zhang, S.F. Performance Evaluation of a Highly Inhibitive Water-Based Drilling Fluid for Ultralow Temperature Wells. J. Energ. Resour-Asme. 2017, 140, 012906. [Google Scholar] [CrossRef]
- Du, W.C.; Pu, X.L.; Sun, J.S.; Luo, X.; Zhang, Y.N.; Li, L. Synthesis and evaluation of a novel monomeric amine as sodium montmorillonite swelling inhibitor. Adsorpt. Sci. Technol. 2018, 36, 655–668. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.; Montes, M.; Molina, R.; Moreno, S. Modified clays as catalysts for the catalytic oxidation of ethanol. Appl. Clay Sci. 2014, 95, 18–24. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.L.; Song, K.L.; French, A.D.; Mei, C.T.; Lei, T.Z. pH-responsive water-based drilling fluids containing bentonite and chitin nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 33783–33795. [Google Scholar] [CrossRef]
- Li, M.C.; Ren, S.X.; Zhang, X.Q.; Dong, L.L.; Lei, T.Z.; Lee, S.Y.; Wu, Q.L. Surface-chemistry-tuned cellulose nanocrystals in a bentonite suspension for water-based drilling fluids. ACS Appl. Nano Mater. 2018, 1, 7039–7051. [Google Scholar] [CrossRef]
- Suter, J.L.; Coveney, P.V.; Anderson, R.L.; Greenwell, H.C.; Cliffe, S. Rule based design of clay-swelling inhibitor. Energ. Environ. Sci. 2011, 4, 4572–4586. [Google Scholar] [CrossRef]











| Mineral Compositions | Kaolinite | Chlorite | Illite | Sodium Bentonite | Illite/Sodium Bentonite |
|---|---|---|---|---|---|
| Content/% | 0.0 | 26.3 | 65.1 | 8.6 | 10.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Slaný, M.; Wang, X.; Chen, G.; Zhang, J. The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability. Polymers 2020, 12, 708. https://doi.org/10.3390/polym12030708
Du W, Slaný M, Wang X, Chen G, Zhang J. The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability. Polymers. 2020; 12(3):708. https://doi.org/10.3390/polym12030708
Chicago/Turabian StyleDu, Weichao, Michal Slaný, Xiangyun Wang, Gang Chen, and Jie Zhang. 2020. "The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability" Polymers 12, no. 3: 708. https://doi.org/10.3390/polym12030708
APA StyleDu, W., Slaný, M., Wang, X., Chen, G., & Zhang, J. (2020). The Inhibition Property and Mechanism of a Novel Low Molecular Weight Zwitterionic Copolymer for Improving Wellbore Stability. Polymers, 12(3), 708. https://doi.org/10.3390/polym12030708







