Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design, Synthesis, NMR, and Photophysical Characterization
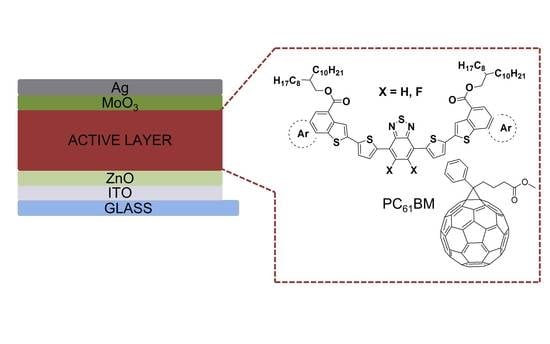
2.2. BHJ OPV Devices
3. Materials and Methods
3.1. General Experimental
3.2. General Procedure for the Stille Protocol for the Synthesis of the Small Oligomers
Selected Example for the Synthesis of Compound 7
3.3. Fabrication and Characterization of Photovoltaic Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Shi, W.; Song, J.; Jang, H.-J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and Biomolecule Sensing with Organic Field-Effect Transistors. Chem. Rev. 2019, 119, 3–35. [Google Scholar] [CrossRef]
- Torsi, L.; Magliulo, M.; Manolia, K.; Palazzo, G. Organic field-effect transistor sensors: a tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Usta, H.; Facchetti, A.; Marks, T.J. n-Channel Semiconductor Materials Design for Organic Complementary Circuits. Acc. Chem. Res. 2011, 44, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Wong, K.T. Perspective on Host Materials for Thermally Activated Delayed Fluorescence Organic Light Emitting Diodes. Adv. Opt. Mater. 2019, 7, 1800565. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Y.; Mao, Z.; Xie, Z.L.; Zhang, Y.; Liu, S.W.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef]
- Ahmadi, M.; Wu, T.; Hu, B. A Review on Organic–Inorganic Halide Perovskite Photodetectors: Device Engineering and Fundamental Physics. Adv. Mater. 2017, 29, 1605242. [Google Scholar] [CrossRef]
- Heeger, A.J. 25th anniversary article: Bulk heterojunction solar cells: understanding the mechanism of operation. Adv. Mater. 2014, 26, 10–28. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated polymer-based chemical sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, H.; Ding, L.; Wang, J. Thermostable single-junction organic solar cells with a power conversion efficiency of 14.62%. Sci. Bull. 2018, 63, 340–342. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of Light-Emitting Conjugated Polymers for Applications in Electroluminescent Devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Oligomer Molecules for Efficient Organic Photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, J.E.; Henson, Z.B.; Welch, G.C.; Bazan, G.C. Design and Synthesis of Molecular Donors for Solution-Processed High-Efficiency Organic Solar Cells. Acc. Chem. Res. 2014, 47, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Zhan, X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Po, R.; Bianchi, G.; Carbonera, C.; Pellegrino, A. “All That Glisters Is Not Gold”: An Analysis of the Synthetic Complexity of Efficient Polymer Donors for Polymer Solar Cells. Macromolecules 2015, 48, 453–461. [Google Scholar] [CrossRef]
- Po, R.; Bernardi, A.; Calabrese, A.; Carbonera, C.; Corso, G.; Pellegrino, A. From lab to fab: how must the polymer solar cell materials design change?—An industrial perspective. Energy Environ. Sci. 2014, 7, 925–943. [Google Scholar] [CrossRef]
- Nitti, A.; Po, R.; Bianchi, G.; Pasini, D. Direct Arylation Strategies in the Synthesis of π-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2017, 22, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobalasingham, N.S.; Thompson, B.C. Direct arylation polymerization: A guide to optimal conditions for effective conjugated polymers. Progr. Polym. Sci. 2018, 83, 135–201. [Google Scholar] [CrossRef]
- Matsidik, R.; Komber, H.; Luzio, A.; Caironi, M.; Sommer, M. Defect-free naphthalene diimide bithiophene copolymers with controlled molar mass and high performance via direct arylation polycon-densation. J. Am. Chem. Soc. 2015, 137, 6705–6711. [Google Scholar] [CrossRef] [PubMed]
- Bura, T.; Morin, P.-O.; Leclerc, M. En route to defect-free polythiophene derivatives by direct heteroarylation polymerization. Macromolecules 2015, 48, 5614–5620. [Google Scholar] [CrossRef]
- Okamoto, J.; Zhang, J.; Housekeeper, J.B.; Marder, S.R.; Luscombe, C.K. C–H arylation reaction: atom efficient and greener syntheses of π-conjugated small molecules and macromolecules for organic electronic materials. Macromolecules 2013, 46, 8059–8078. [Google Scholar] [CrossRef]
- Nitti, A.; Debattista, F.; Abbondanza, L.; Bianchi, G.; Po, R.; Pasini, D. Donor–acceptor conjugated copolymers incorporating tetrafluorobenzene as the π-electron deficient unit’. J. Polym. Sci. Polym. Chem. 2017, 55, 1601–1610. [Google Scholar] [CrossRef]
- Yu, J.; Yan, H.; Zhu, C. Synthesis of Multiply Substituted Polycyclic Aromatic Hydrocarbons by Iridium-Catalyzed Annulation of Ring-Fused Benzocyclobutenol with Alkyne through C−C Bond Cleavage. Angew. Chem. Int. Ed. 2016, 55, 1143–1146. [Google Scholar] [CrossRef]
- Koga, Y.; Kaneda, T.; Saito, Y.; Murakami, K.; Itami, K. Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization. Science 2018, 359, 435–439. [Google Scholar] [CrossRef] [Green Version]
- VanVeller, B.; Robinson, D.; Swager, T.M. Triptycene Diols: A Strategy for Planar π-Systems Demonstrated by the Catalytic Conversion of a PPE into a PPV. Angew. Chem. Int. Ed. 2012, 51, 1182–1186. [Google Scholar] [CrossRef]
- Nitti, A.; Bianchi, G.; Po, R.; Swager, T.M.; Pasini, D. Domino Direct Arylation and Cross-Aldol for Rapid Construction of Extended Polycyclic π-Scaffolds. J. Am. Chem. Soc. 2017, 139, 8788–8791. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Signorile, M.; Boiocchi, M.; Bianchi, G.; Po, R.; Pasini, D. Conjugated Thiophene-Fused Isatin Dyes through Intramolecular Direct Arylation. J. Org. Chem. 2016, 81, 11035–11042. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Osw, P.; Calcagno, G.; Botta, C.; Etkind, S.E.; Bianchi, G.; Po, R.; Swager, T.M.; Pasini, D. Regiodirected Annulations through One-Pot Direct Arylation-Cross Aldol Condensation for the Rapid Synthesis of π-Extended Oligomers. Submitted.
- Nitti, A.; Osw, P.; Abdullah, M.N.; Galbiati, A.; Pasini, D. Scalable Synthesis of Naphthothiophene-based D-π-D Extended Oligomers through Cascade Direct Arylation Processes. Synlett 2018, 29, 2577–2581. [Google Scholar]
- Yao, S.; Kim, B.; Yue, X.; Colon Gomez, M.Y.; Bondar, M.V.; Belfield, K.D. Synthesis of Near-Infrared Fluorescent Two-Photon-Absorbing Fluorenyl Benzothiadiazole and Benzoselenadiazole Derivatives. ACS Omega 2016, 1, 1149–1156. [Google Scholar] [CrossRef]
- Ellinger, S.; Graham, K.R.; Shi, P.; Farley, R.T.; Steckler, T.T.; Brookins, R.N.; Taranekar, P.; Mei, J.; Padilha, L.A.; Ensley, T.R.; et al. Donor–Acceptor–Donor-based π-Conjugated Oligomers for Nonlinear Optics and Near-IR Emission. Chem. Mater. 2011, 23, 3805–3817. [Google Scholar] [CrossRef]
- Jung, J.W.; Liu, F.; Russell, T.P.; Jo, W.H. Fluorination on BT consistent: Medium Bandgap Conjugated Polymer for High Per-formance Polymer Solar Cells Exceeding 9% Power Conversion Efficiency. Adv. Mater. 2015, 27, 7462–7468. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- He, Y.; Li, Y. Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 2011, 13, 1970–1983. [Google Scholar] [CrossRef]
- Brabec, C.J.; Winder, C.; Sariciftci, N.S.; Hummelen, J.C.; Dhanabalan, A.; van Hal, P.A.; Janssen, R.A.J. A low-bandgap semiconducting polymer for photovoltaic devices and infrared emitting diodes. Adv. Funct. Mater. 2002, 12, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Thompson, B.C.; Fréchet, J.M.J. Polymer–Fullerene Composite Solar Cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design Rules for Donors in Bulk-Heterojunction Solar Cells—Towards 10 % Energy-Conversion Efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Xin, Y.; Zeng, G.; Zhang, J.; Zhao, X.; Yang, X. A new copolymer based on a D-π-A or D-A-π repeat unit for polymer solar cells employing non-halogenated solvents. J. Mater. Chem. A 2018, 6, 9561–9568. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Dong, J.; Yang, M.; Liu, M.; Zhang, Y.; Wen, J.; Ma, H.; Gao, X.; Chen, W.; et al. Chlorinated Wide-Bandgap Donor Polymer Enabling Annealing Free Nonfullerene Solar Cells with the Efficiency of 11.5%. J. Phys. Chem. Lett. 2018, 9, 6955–6962. [Google Scholar] [CrossRef]
- Fox, D.; Metrangolo, P.; Pasini, D.; Pilati, T.; Resnati, G.; Terraneo, G. Site-selective supramolecular synthesis of halogen-bonded cocrystals incorporating the photoactive azo group. CrystEngComm 2008, 10, 1132–1136. [Google Scholar] [CrossRef]
- Agnes, M.; Nitti, A.; Vander Griend, D.A.; Dondi, D.; Merli, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Commun. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Qiao, W.; Gu, W.; Zhu, X.; Wang, C.; Wang, Z.Y. Broadband polymer photodetectors with a good trade-off between broad response and high detectivity by using combined electron-deficient moieties. J. Mater. Chem. C 2020. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]





| Compound | max abs (nm) | (cm−1·mol−1·L) | Egopt (eV) | em (nm) | (ns) | max abs (nm) 2 |
|---|---|---|---|---|---|---|
| 7 | 516 | 1.31·104 | 2.06 | 627 | 4.2 | 610 (broad) |
| 8 | 502 | 1.61·104 | 2.13 | 610 | 3.3 | 615 (broad) |
| 9 | 515 | 1.40·104 | 2.05 | 622 | 3.4 | 618 (broad) |
| 10 | 505 | 1.66·104 | 2.11 | 607 | 2.5 | 628 |
| Compound | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|
| 7 | 0.74 | 0.55 | 0.34 | 0.14 |
| 9 | 0.81 | 0.89 | 0.52 | 0.37 |
| 10 | 0.51 | 0.78 | 0.44 | 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osw, P.; Nitti, A.; Abdullah, M.N.; Etkind, S.I.; Mwaura, J.; Galbiati, A.; Pasini, D. Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells. Polymers 2020, 12, 720. https://doi.org/10.3390/polym12030720
Osw P, Nitti A, Abdullah MN, Etkind SI, Mwaura J, Galbiati A, Pasini D. Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells. Polymers. 2020; 12(3):720. https://doi.org/10.3390/polym12030720
Chicago/Turabian StyleOsw, Peshawa, Andrea Nitti, Media N. Abdullah, Samuel I. Etkind, Jeremiah Mwaura, Alessandro Galbiati, and Dario Pasini. 2020. "Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells" Polymers 12, no. 3: 720. https://doi.org/10.3390/polym12030720
APA StyleOsw, P., Nitti, A., Abdullah, M. N., Etkind, S. I., Mwaura, J., Galbiati, A., & Pasini, D. (2020). Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells. Polymers, 12(3), 720. https://doi.org/10.3390/polym12030720