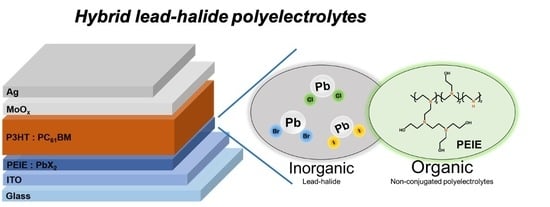
Hybrid Lead-Halide Polyelectrolytes as Interfacial Electron Extraction Layers in Inverted Organic Solar Cells
Abstract
:1. Introduction
2. Experimental
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedley, G.J.; Ruseckas, A.; Samuel, I.D.W. Light harvesting for organic photovoltaics. Chem. Rev. 2017, 117, 796–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Ye, L.; Hou, J. Breaking the 10% Efficiency Barrier in Organic Photovoltaics: Morphology and Device Optimization of Well-Known PBDTTT Polymers. Adv. Energy Mater. 2016, 6, 1502529. [Google Scholar] [CrossRef]
- Walker, B.; Choi, H.; Kim, J.Y. Interfacial engineering for highly efficient organic solar cells. Curr. Appl. Phys. 2017, 17, 370–391. [Google Scholar] [CrossRef]
- Ryno, S.M.; Ravva, M.K.; Chen, X.; Li, H.; Brédas, J.L. Molecular Understanding of Fullerene—Electron Donor Interactions in Organic Solar Cells. Adv. Energy Mater. 2017, 7, 1601370. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk-heterojunction solar cells—Towards 10 % energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT: PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Mai, C.K.; Collins, S.D.; Nguyen, T.Q.; Bazan, G.C.; Heeger, A.J. Conductive conjugated polyelectrolyte as hole-transporting layer for organic bulk heterojunction solar cells. Adv. Mater. 2014, 26, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wen, T.C.; Lee, T.H.; Guo, T.F.; Huang, J.C.A.; Lin, Y.C.; Hsu, Y.J. An inverted polymer photovoltaic cell with increased air stability obtained by employing novel hole/electron collecting layers. J. Mater. Chem. 2009, 19, 1643–1647. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.M.; Yang, G.; Huang, C.H.; Hou, J.; Wu, Y.; Li, G.; Hsu, C.S.; Yang, Y. Vertical phase separation in poly(3-hexylthiophene): Fullerene derivative blends and its advantage for inverted structure solar cells. Adv. Funct. Mater. 2009, 19, 1227–1234. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Li, X.; Min, C.; Jiu, T.; Zhu, Y.; Dai, N.; Fang, J. Solution-processed hybrid cathode interlayer for inverted organic solar cells. ACS Appl. Mater. Interfaces 2013, 5, 10428–10432. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Park, J.S.; Jeong, E.; Kim, G.H.; Lee, B.R.; Kim, S.O.; Song, M.H.; Woo, H.Y.; Kim, J.Y. Combination of titanium oxide and a conjugated polyelectrolyte for high-performance inverted-type organic optoelectronic devices. Adv. Mater. 2011, 23, 2759–2763. [Google Scholar] [CrossRef]
- Waldauf, C.; Morana, M.; Denk, P.; Schilinsky, P.; Coakley, K.; Choulis, S.A.; Brabec, C.J. Highly efficient inverted organic photovoltaics using solution based titanium oxide as electron selective contact. Appl. Phys. Lett. 2006, 89, 233517. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, C.; Zhu, C.; Zhang, J. Development of inverted organic solar cells with TiO2 interface layer by using low-temperature atomic layer deposition. ACS Appl. Mater. Interfaces 2013, 5, 713–718. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Liao, H.H.; Chen, L.M.; Xu, Z.; Li, G.; Yang, Y. Highly efficient inverted polymer solar cell by low temperature annealing of Cs2 C O3 interlayer. Appl. Phys. Lett. 2008, 92, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zhang, K.; Zhong, C.; Huang, F.; Cao, Y. Recent advances in water/alcohol-soluble π-conjugated materials: New materials and growing applications in solar cells. Chem. Soc. Rev. 2013, 42, 9071–9104. [Google Scholar] [CrossRef]
- Walker, B.; Tamayo, A.; Yang, J.; Brzezinski, J.Z.; Nguyen, T.Q. Solution-processed small molecule-based blue light-emitting diodes using conjugated polyelectrolytes as electron injection layers. Appl. Phys. Lett. 2008, 93, 91–94. [Google Scholar] [CrossRef]
- Lee, W.; Seo, J.H.; Woo, H.Y. Conjugated polyelectrolytes: A new class of semiconducting material for organic electronic devices. Polymer 2013, 54, 5104–5121. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A universal method to produce low-work function electrodes for organic electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Cha, M.J.; Yoon, Y.J.; Cho, S.; Kim, J.Y.; Seo, J.H.; Walker, B. Improved Performance in n-Type Organic Field-Effect Transistors via Polyelectrolyte-Mediated Interfacial Doping. Adv. Electron. Mater. 2017, 3, 1700184. [Google Scholar] [CrossRef]
- Seo, J.H.; Gutacker, A.; Sun, Y.; Wu, H.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A.J.; Bazan, G.C. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc. 2011, 133, 8416–8419. [Google Scholar] [CrossRef]
- Yip, H.L.; Jen, A.K.Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Jung, H.S.; Nguyen, T.Q. Electronic properties of conjugated polyelectrolyte thin films. J. Am. Chem. Soc. 2008, 130, 10042–10043. [Google Scholar]
- Kang, H.; Hong, S.; Lee, J.; Lee, K. Electrostatically self-assembled nonconjugated polyelectrolytes as an ideal interfacial layer for inverted polymer solar cells. Adv. Mater. 2012, 24, 3005–3009. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Uddin, A. Organic-inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Herz, L.M. Charge-Carrier Mobilities in Metal Halide Perovskites: Fundamental Mechanisms and Limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Saliba, M.; Correa-Baena, J.P.; Grätzel, M.; Hagfeldt, A.; Abate, A. Perovskite Solar Cells: From the Atomic Level to Film Quality and Device Performance. Angew. Chem. Int. Ed. 2018, 57, 2554–2569. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, D.H.; Gupta, V.; Zhang, J.; Chand, S.; Bazan, G.C.; Heeger, A.J. Efficient solution-processed small-molecule solar cells with inverted structure. Adv. Mater. 2013, 25, 2397–2402. [Google Scholar] [CrossRef]
- Seo, J.H.; Yang, R.; Brzezinski, J.Z.; Walker, B.; Bazan, G.C.; Nguyen, T.Q. Electronic properties at gold/conjugated-polyelectrolyte interfaces. Adv. Mater. 2009, 21, 1006–1011. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, Y.J.; Cha, M.J.; Yi, Y.; Song, A.; Chung, K.B.; Seo, J.H.; Walker, B. Effect of counter-ions on the properties and performance of non-conjugated polyelectrolyte interlayers in solar cell and transistor devices. RSC Adv. 2019, 9, 20670–20676. [Google Scholar] [CrossRef] [Green Version]




| ETLs | JSC (mA/cm2) | VOC (V) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| Average | Best | ||||
| without | 5.674 | 0.150 | 29.40 | 0.250 | 0.300 |
| PEIE | 8.865 | 0.544 | 59.98 | 2.687 | 2.894 |
| PEIE:PbCl2 | |||||
| 99:1 | 8.755 | 0.590 | 56.47 | 2.784 | 2.917 |
| 98:2 | 8.508 | 0.596 | 60.53 | 2.589 | 3.069 |
| 97:3 | 7.690 | 0.622 | 65.65 | 2.871 | 3.140 |
| 96:4 | 6.990 | 0.605 | 64.19 | 2.709 | 2.715 |
| PEIE:PbBr2 | |||||
| 99:1 | 8.278 | 0.624 | 69.06 | 2.991 | 3.567 |
| 98:2 | 8.337 | 0.608 | 63.26 | 2.922 | 3.207 |
| 97:3 | 9.159 | 0.593 | 61.40 | 3.097 | 3.335 |
| 96:4 | 7.991 | 0.587 | 61.07 | 2.263 | 2.865 |
| PEIE:PbI2 | |||||
| 99:1 | 8.499 | 0.602 | 64.70 | 3.052 | 3.310 |
| 98:2 | 8.916 | 0.596 | 59.21 | 2.821 | 3.146 |
| 97:3 | 8.135 | 0.599 | 66.63 | 2.839 | 3.247 |
| 96:4 | 7.378 | 0.545 | 64.16 | 1.854 | 2.579 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Park, Y.J.; Seo, J.H.; Walker, B. Hybrid Lead-Halide Polyelectrolytes as Interfacial Electron Extraction Layers in Inverted Organic Solar Cells. Polymers 2020, 12, 743. https://doi.org/10.3390/polym12040743
Lee JH, Park YJ, Seo JH, Walker B. Hybrid Lead-Halide Polyelectrolytes as Interfacial Electron Extraction Layers in Inverted Organic Solar Cells. Polymers. 2020; 12(4):743. https://doi.org/10.3390/polym12040743
Chicago/Turabian StyleLee, Jin Hee, Yu Jung Park, Jung Hwa Seo, and Bright Walker. 2020. "Hybrid Lead-Halide Polyelectrolytes as Interfacial Electron Extraction Layers in Inverted Organic Solar Cells" Polymers 12, no. 4: 743. https://doi.org/10.3390/polym12040743