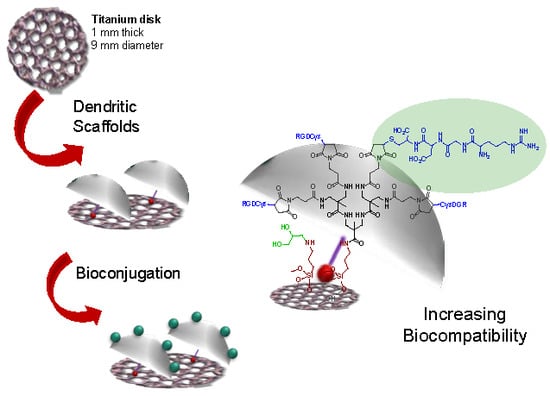
Dendritic Scaffold onto Titanium Implants. A Versatile Strategy Increasing Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. D-Printing of Titanium Disks
2.2. General Materials and Methods
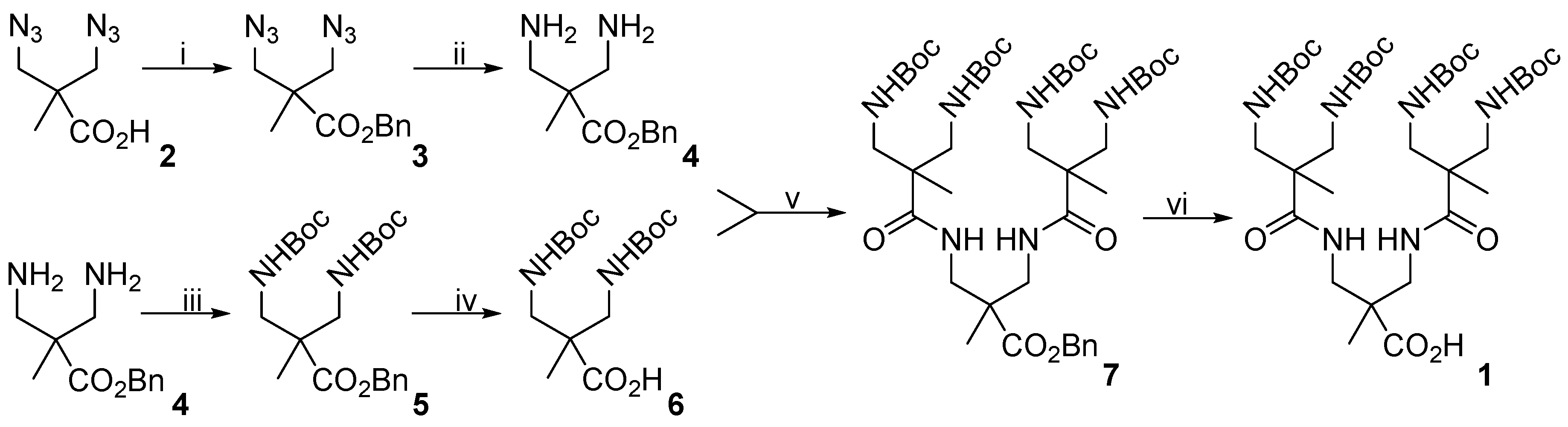
2.3. Synthesis of 3,3′-Diazidopivalic Acid (2)
2.4. Synthesis of Benzyl-3,3′-Diazidopivaloate (3)
2.5. Synthesis of Benzyl-3,3’-Diaminopivaloate (4)
2.6. Synthesis of Benzyl-3,3´-Bis(Tert-Butoxycarbonyl) Aminopivaloate (5)
2.7. Synthesis of 3,3´-Bis(Tert-Butoxycarbonyl) Aminopivalic Acid (6)
2.8. Synthesis of Compound 7
2.9. Synthesis of Compound 1
2.10. Preparation of Ti disk (VII)
2.10.1. Preparation of Ti Disk (I)
2.10.2. Preparation of Ti Disk (II)
2.10.3. Preparation of Ti Disk (III)
2.10.4. Preparation of Ti Disk (IV)
2.10.5. Preparation of Ti Disk (V)
2.10.6. Preparation of Ti Disk (VI)
2.10.7. Preparation of Ti Disk (VII)
2.11. Quantification of Free Primary Amino Groups Bonded to the Ti disk
2.12. Culture of Osteoblastic Cells on the Ti6Al4V ELI
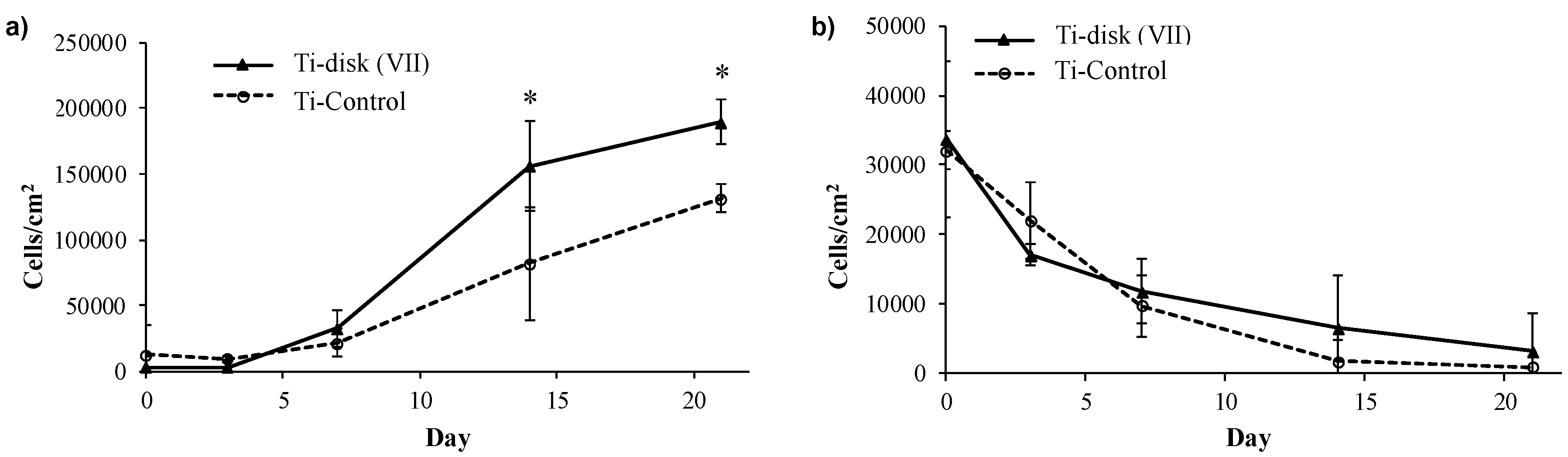
2.13. Cell Adhesion and Proliferation Essays
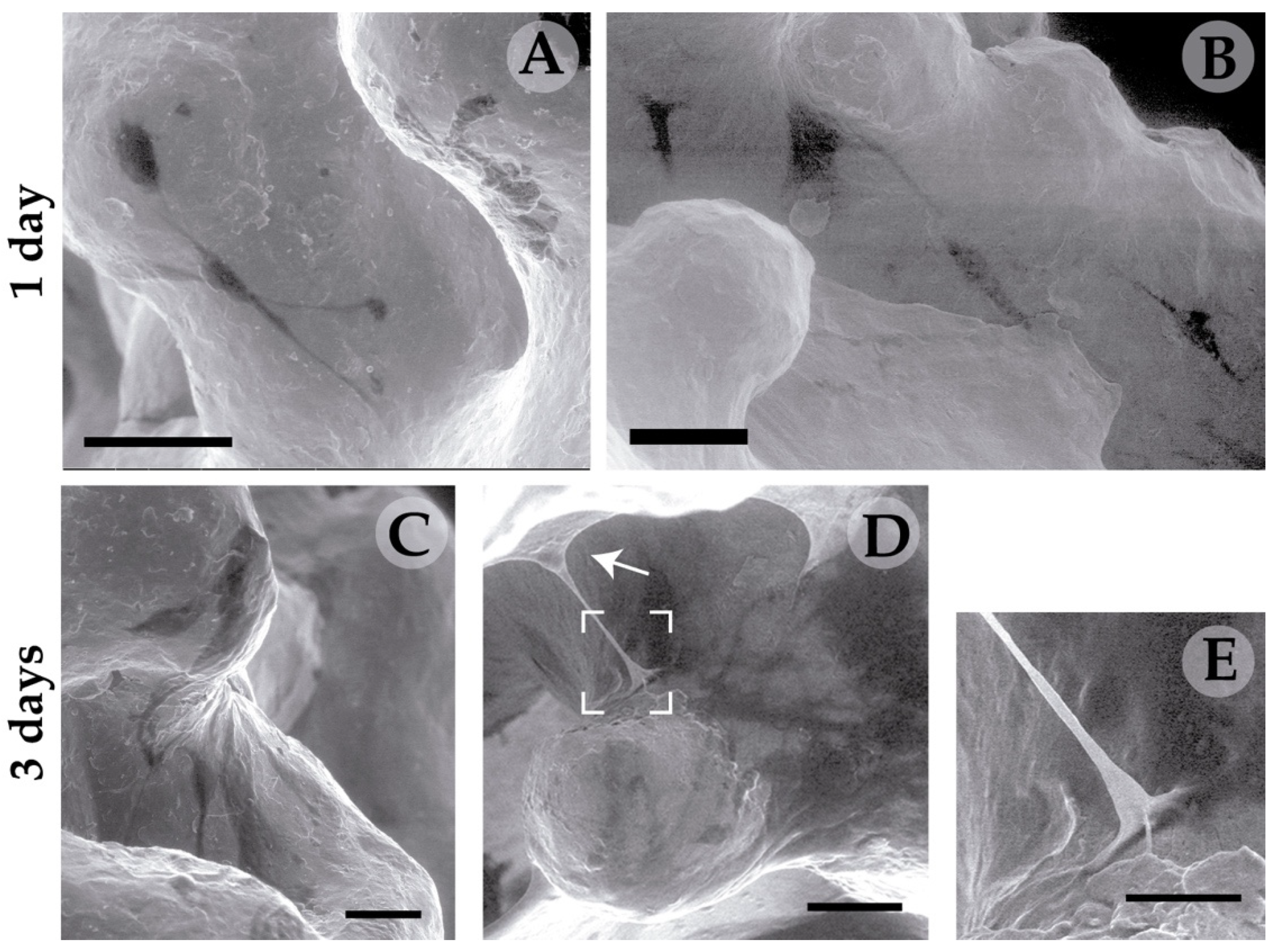
2.14. Environmental Scanning Electron Microscopy (ESEM)
3. Results
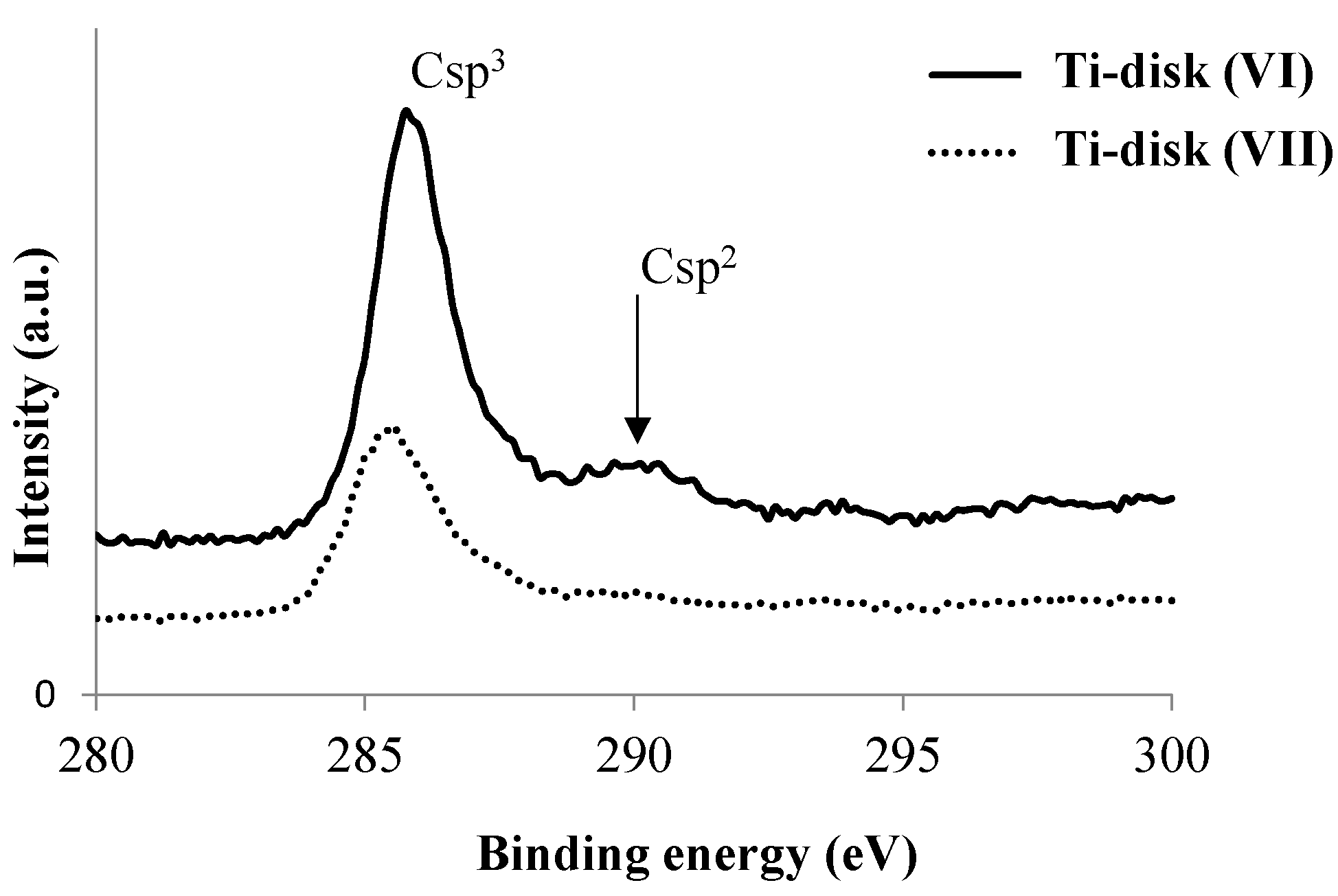
3.1. Modification of Ti Disk Surfaces
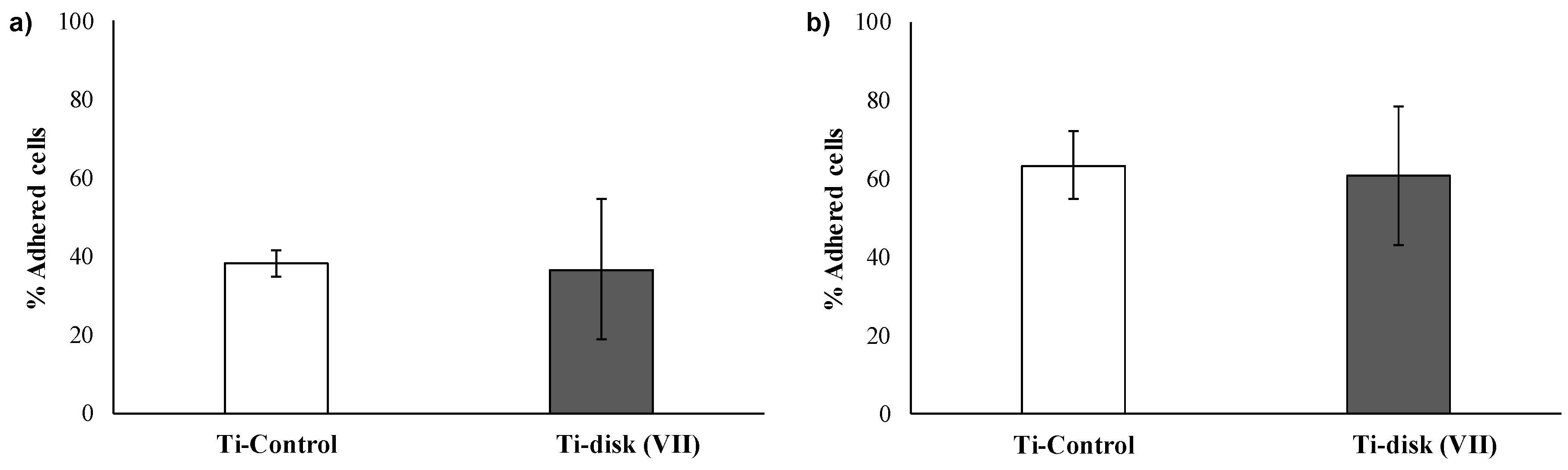
3.2. Essays of RGD-Functionalized Titanium Surfaces with Pre-Osteoblastic Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. Part A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Zhou, Y.; Takebe, J.; Guo, J.; Abron, A.; Holmén, A.; Ellingsen, J.E. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Abrahamsson, I.; Albouy, J.P.; Lindhe, J. Bone healing at implants with a fluoride-modified surface: An experimental study in dogs. Clin. Oral Implants Res. 2007, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, D.; Sladkova, M.; Palmer, M.; Xia, W.; Engqvist, H.; de Peppo, G.M. Stem cell-mediated functionalization of titanium implants. J. Mater. Sci. Mater. Med. 2017, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R.; Al, W.E.T. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Goriainov, V.; McEwan, J.K.; Oreffo, R.O.; Dunlop, D.G. Application of 3D-printed patient-specific skeletal implants augmented with autologous skeletal stem cells. Regen. Med. 2018, 13, 283–294. [Google Scholar] [CrossRef]
- Schwartz, Z.; Kieswetter, K.; Dean, D.D.; Boyan, B.D. Underlying mechanisms at the bone-surface interface during regeneration. J. Periodontal Res. 1997, 32, 166–171. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7, S93–S105. [Google Scholar] [CrossRef] [Green Version]
- Treccani, L.; Yvonne Klein, T.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: A versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Markusen, J.F.; Mason, C.; Hull, D.A.; Town, M.A.; Tabor, A.B.; Clements, M.; Boshoff, C.H.; Dunnill, A.P. Behavior of Adult Human Mesenchymal Stem Cells Entrapped in Alginate-GRGDY Beads. Tissue Eng. 2006, 12, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers. Discovery, Applications, and the Future; Cambridge University Press The Edinburgh Building: Cambridge, UK, 2012; ISBN 9781139048859. [Google Scholar]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Salgado, A.J.; Sousa, N.; Mano, J.F.; Reis, R.L. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—A review. Prog. Polym. Sci. 2010, 35, 1163–1194. [Google Scholar] [CrossRef] [Green Version]
- Vida, Y.; Collado, D.; Najera, F.; Claros, S.; Becerra, J.; Andrades, J.A.; Perez-Inestrosa, E. Dendrimer surface orientation of the RGD peptide affects mesenchymal stem cell adhesion. RSC Adv. 2016, 6, 49839–49844. [Google Scholar] [CrossRef]
- Lagunas, A.; Castaño, A.G.; Artés, J.M.; Vida, Y.; Collado, D.; Gorostiza, P.; Claros, S.; Andrades, J.A.; Samitier, J. Large-scale dendrimer-based uneven nanopatterns for the study of local arginine–glycine–aspartic acid ( RGD ) density effects on cell adhesion. Nano Res. 2014, 7, 399–409. [Google Scholar] [CrossRef]
- Lagunas, A.; Tsintzou, I.; Vida, Y.; Collado, D.; Pérez-inestrosa, E.; Pereira, C.R.; Magalhaes, J.; Andrades, J.A.; Samitier, J. Tailoring RGD local surface density at the nanoscale toward adult stem cell chondrogenic commitment. Nano Res. 2016, 10, 1959–1971. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Song, H.Y.; Ngai, M.H.; Song, Z.Y.; Macary, P.A.; Lear, M.J. Practical synthesis of maleimides and coumarin-linked probes for protein and antibody labelling via reduction of native disulfides. Org. Biomol. Chem. 2009, 7, 3400–3406. [Google Scholar] [CrossRef]
- Blanca, M.; Mayorga, C.; Perez, E.; Suau, R.; Juarez, C.; Vega, J.M.; Carmona, M.J.; Perez-Estrada, M.; Garcia, J. Determination of IgE antibodies to the benzyl penicilloyl determinant. J. Inmunol. Methods 1992, 153, 99–105. [Google Scholar] [CrossRef]
- Montañez, M.I.; Perez-Inestrosa, E.; Suau, R.; Mayorga, C.; Torres, M.J.; Blanca, M. Dendrimerized Cellulose as a Scaffold for Artificial Antigens with Applications in Drug Allergy Diagnosis. Biomacromolecules 2008, 9, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchez, A.J.; Mesa-Antunez, P.; Barbero, N.; Collado, D.; Vida, Y.; Najera, F.; Perez-Inestrosa, E. Synthesis of all-aliphatic polyamide dendrimers based on a 3,3′-diaminopivalic acid scaffold. Polym. Chem. 2015, 6, 3031–3038. [Google Scholar] [CrossRef]
- Hernández de Gatica, N.L.; Jones, G.L.; Gardella, J.A. Surface characterization of titanium alloys sterilized for biomedical applications. Appl. Surf. Sci. 1993, 68, 107–121. [Google Scholar] [CrossRef]
- Wälivaara, B.; Aronsson, B.-O.; Rodahl, M.; Lausmaa, J.; Tengvall, P. Titanium with different oxides: In vitro studies of protein adsorption and contact activation. Biomaterials 1994, 15, 827–834. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How To Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir 2012, 28, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocas, P.; Hoyos-Nogués, M.; Rocas, J.; Manero, J.M.; Gil, J.; Albericio, F.; Mas-Moruno, C. Installing Multifunctionality on Titanium with RGD-Decorated Polyurethane-Polyurea Roxithromycin Loaded Nanoparticles: Toward New Osseointegrative Therapies. Adv. Healthc. Mater. 2015, 4, 1956–1960. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef]
- Asenath Smith, E.; Chen, W. How To Prevent the Loss of Surface Functionality Derived from Aminosilanes. Langmuir 2008, 24, 12405–12409. [Google Scholar] [CrossRef] [Green Version]
- Vida, Y.; Montañez, M.I.; Collado, D.; Najera, F.; Ariza, A.; Blanca, M.; Torres, M.J.; Mayorga, C.; Perez-Inestrosa, E. Dendrimeric antigen–silica particle composites: An innovative approach for IgE quantification. J. Mater. Chem. B 2013, 1, 3044–3050. [Google Scholar] [CrossRef]
- Curtis, A.S.G.; Forrester, J.V. The Compettitive Effects of Serum Proteins on Cell Adhesion. J. Cell Sci. 1984, 71, 17–35. [Google Scholar]
- Ying, P.Q.; Jin, G.; Tao, Z.L. Effects of surface chemistry and protein competitive adsorption on cell adhesion. In Proceeding of the 2001 Conference Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; IEEE: Piscataway, NJ, USA, 2001; Volume 3, pp. 2957–2960. [Google Scholar]
- Verdanova, M.; Sauerova, P.; Hempel, U.; Kalbacova, M.H. Initial cell adhesion of three cell types in the presence and absence of serum proteins. Histochem. Cell Biol. 2017, 148, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Delfini, R.H. Dental Implant Complications. Semin. Ultrasound CT MRI 2015, 36, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ghosh (Pal), B. Metallic biomaterials for dental implant systems. In Fundamental Biomaterials: Metals; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Tedesco, J.; Lee, B.E.J.; Lin, A.Y.W.; Binkley, D.M.; Delaney, K.H.; Kwiecien, J.M.; Grandfield, K. Osseointegration of a 3D Printed Stemmed Titanium Dental Implant: A Pilot Study. Int. J. Dent. 2017, 2017, 5920714. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Zhou, Q.; Gong, Y.; Li, R.; Li, C.; Klämpfl, F.; Freund, S.; Wu, X.; Sun, Y.; et al. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implants: Fabrication, biocompatibility analysis and photoelastic study. Sci. Rep. 2017, 7, 45360. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Additively manufactured porous metallic biomaterials. J. Mater. Chem. B 2019, 7, 4088–4117. [Google Scholar] [CrossRef] [Green Version]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Tomisa, A.P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.K.; Saiz, E. Nanotechnology approaches to improve dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 25–44. [Google Scholar]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [Green Version]
- Perlin, L.; MacNeil, S.; Rimmer, S. Production and performance of biomaterials containing RGD peptides. Soft Matter 2008, 4, 2331–2349. [Google Scholar] [CrossRef]
- Wang, L.; Erasquin, U.J.; Zhao, M.; Ren, L.; Zhang, M.Y.; Cheng, G.J.; Wang, Y.; Cai, C. Stability, antimicrobial activity, and cytotoxicity of poly(amidoamine) dendrimers on titanium substrates. ACS Appl. Mater. Interfaces 2011, 3, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Charitidis, C.A.; Skarmoutsou, A.; Tsetsekou, A.; Brasinika, D.; Tsiourvas, D. Nanomechanical properties of hydroxyapatite (HAP) with DAB dendrimers (poly-propylene imine) coatings onto titanium surfaces. Mater. Sci. Eng. B 2013, 178, 391–399. [Google Scholar] [CrossRef]
- Stübinger, S.; Nuss, K.; Bürki, A.; Mosch, I.; le Sidler, M.; Meikle, S.T.; von Rechenberg, B.; Santin, M. Osseointegration of titanium implants functionalised with phosphoserine-tethered poly(epsilon-lysine) dendrons: A comparative study with traditional surface treatments in sheep. J. Mater. Sci. Mater. Med. 2015, 26, 87. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.B.; Skubitz, A.P.N.; Zhao, Q.; Yi, X.; Mickelson, D.J.; Klein, D.J.; Furcht, L.T. RGD-independent cell adhesion to the carboxy-terminal heparin-binding fragment of fibronectin involves heparin-dependent and -independent activities. J. Cell Biol. 1990, 110, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.H.; Moffatt, S.; Horton, M. Cell adhesion molecules in human osteoblasts: Structure and function. Histol. Histopathol. 2001, 16, 603–611. [Google Scholar]
- Verderio, E.A.M.; Telci, D.; Okoye, A.; Melino, G.; Griffin, M. A novel RGD-independent cel adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J. Biol. Chem. 2003, 278, 42604–42614. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef]
- Cowles, E.A.; Brailey, L.L.; Gronowicz, G.A. Integrin-mediated signaling regulates AP-1 transcription factors and proliferation in osteoblasts. J. Biomed. Mater. Res. 2000, 52, 725–737. [Google Scholar] [CrossRef]

| Ti Disk | II | III | IV | V | VI |
|---|---|---|---|---|---|
| Free –NH2a | + | − | − | + | − |
| Ti2p | O1s | Si2p | C1s | N1s | S2p | |
|---|---|---|---|---|---|---|
| Ti disk VI | 12.9 | 32.0 | 6.1 | 38.3 | 10.6 | - |
| Ti disk VII | 13.5 | 37.3 | 7.2 | 29.2 | 8.2 | 4.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, N.; González, A.; Monopoli, D.; Mentado, B.; Becerra, J.; Santos-Ruiz, L.; Vida, Y.; Perez-Inestrosa, E. Dendritic Scaffold onto Titanium Implants. A Versatile Strategy Increasing Biocompatibility. Polymers 2020, 12, 770. https://doi.org/10.3390/polym12040770
Molina N, González A, Monopoli D, Mentado B, Becerra J, Santos-Ruiz L, Vida Y, Perez-Inestrosa E. Dendritic Scaffold onto Titanium Implants. A Versatile Strategy Increasing Biocompatibility. Polymers. 2020; 12(4):770. https://doi.org/10.3390/polym12040770
Chicago/Turabian StyleMolina, Noemi, Ana González, Donato Monopoli, Belinda Mentado, José Becerra, Leonor Santos-Ruiz, Yolanda Vida, and Ezequiel Perez-Inestrosa. 2020. "Dendritic Scaffold onto Titanium Implants. A Versatile Strategy Increasing Biocompatibility" Polymers 12, no. 4: 770. https://doi.org/10.3390/polym12040770
APA StyleMolina, N., González, A., Monopoli, D., Mentado, B., Becerra, J., Santos-Ruiz, L., Vida, Y., & Perez-Inestrosa, E. (2020). Dendritic Scaffold onto Titanium Implants. A Versatile Strategy Increasing Biocompatibility. Polymers, 12(4), 770. https://doi.org/10.3390/polym12040770