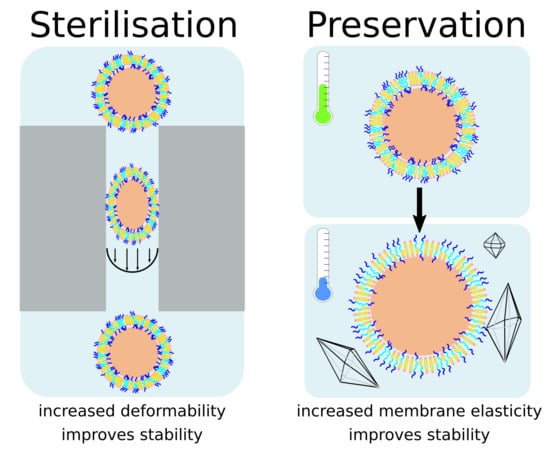
Hybrid Vesicle Stability under Sterilisation and Preservation Processes Used in the Manufacture of Medicinal Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vesicle Preparation
2.3. CF Release Assay
2.4. Dynamic Light Scattering (DLS)
2.5. Cryo-Electron Microscopy (cryo-EM)
3. Results
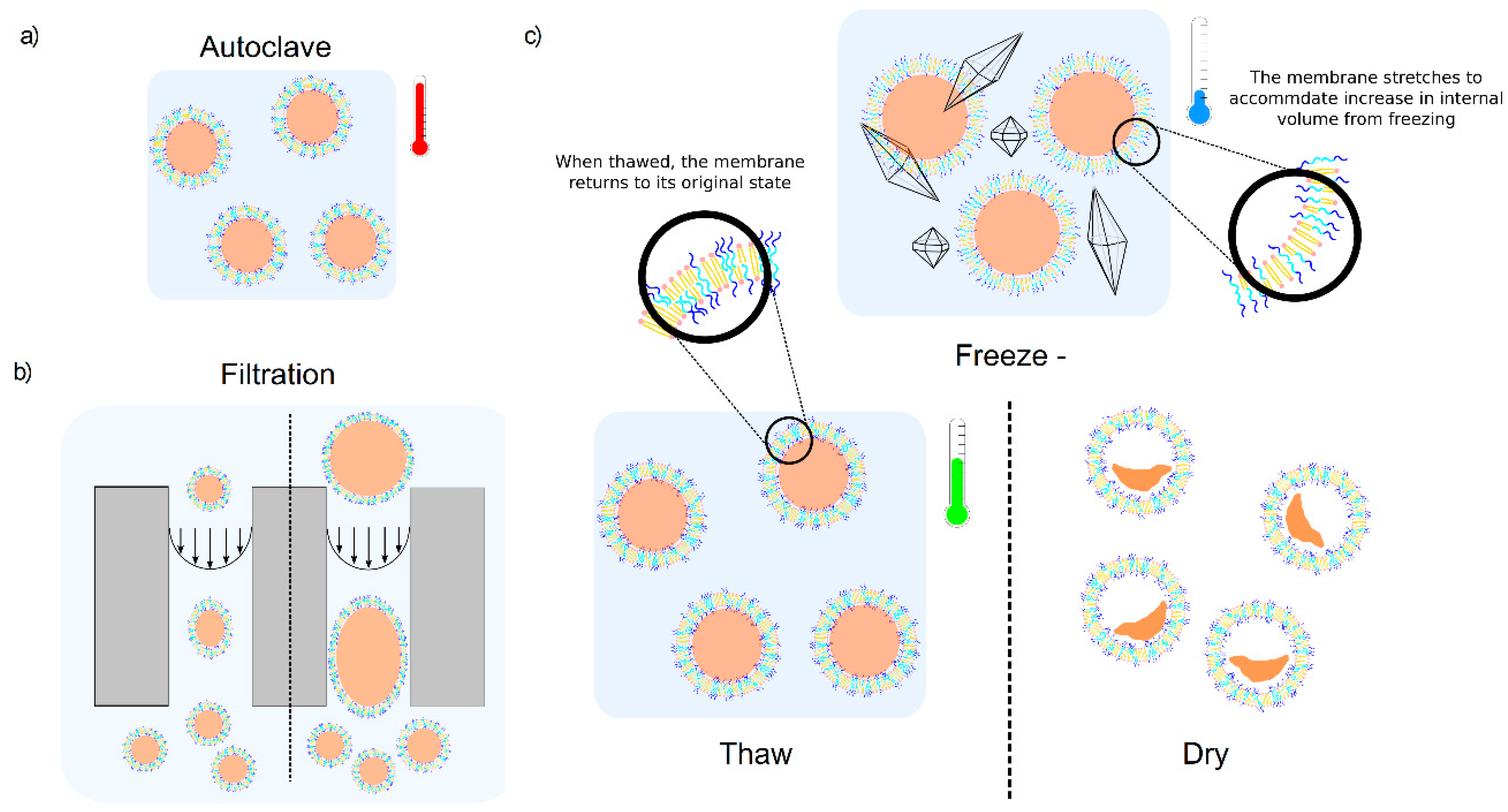
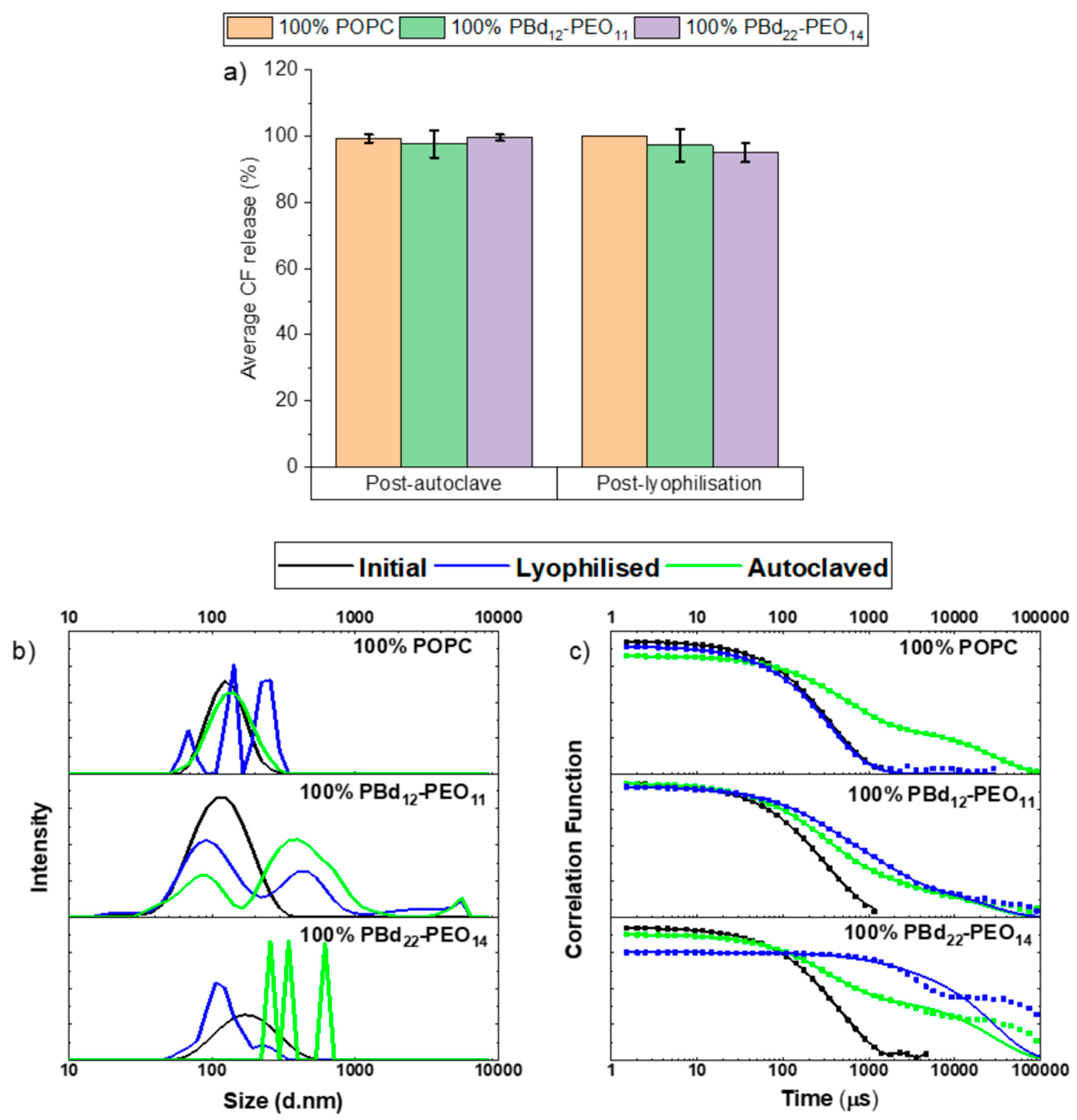
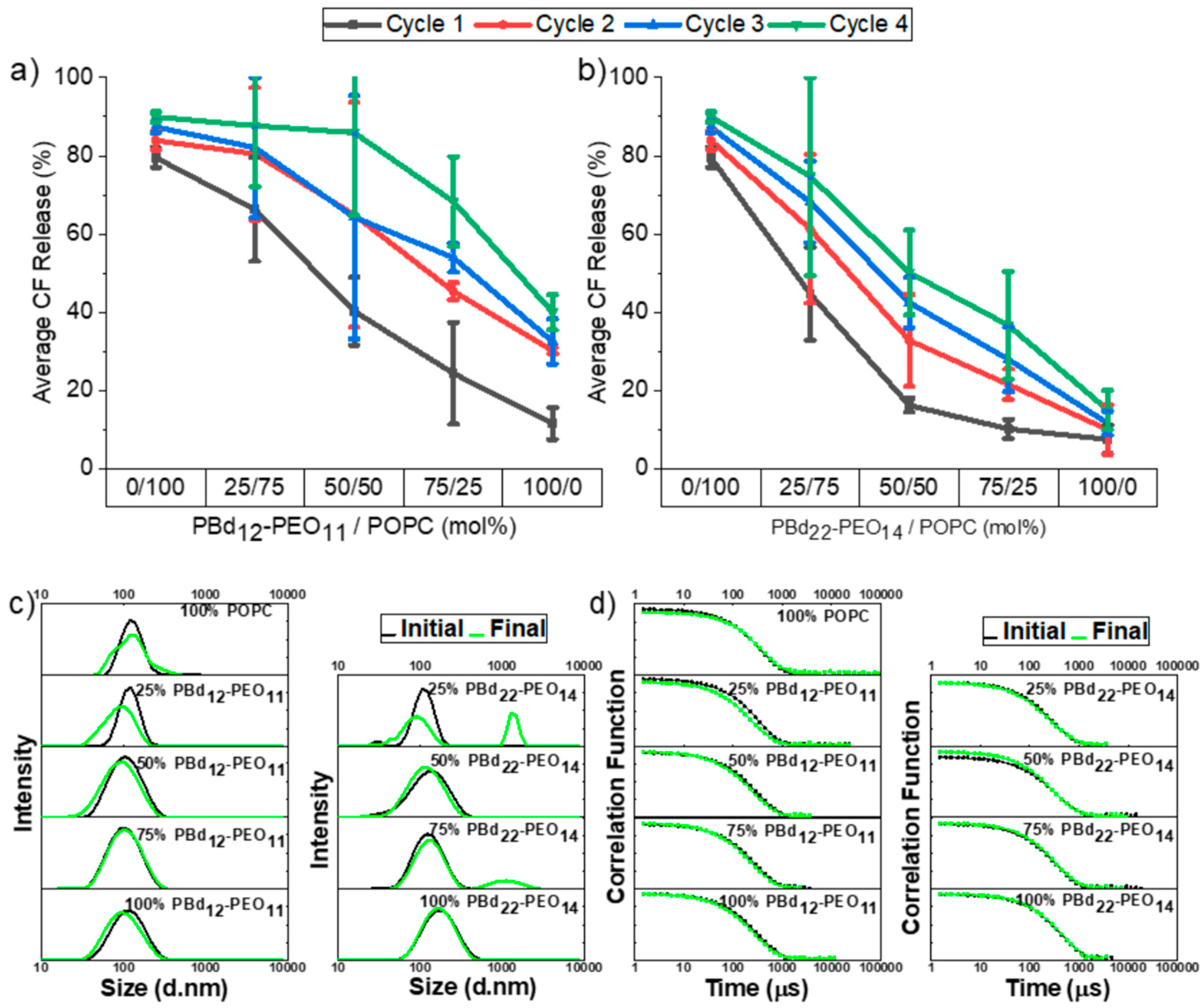
3.1. Autoclaving and Lyophilisation
3.2. Filtration
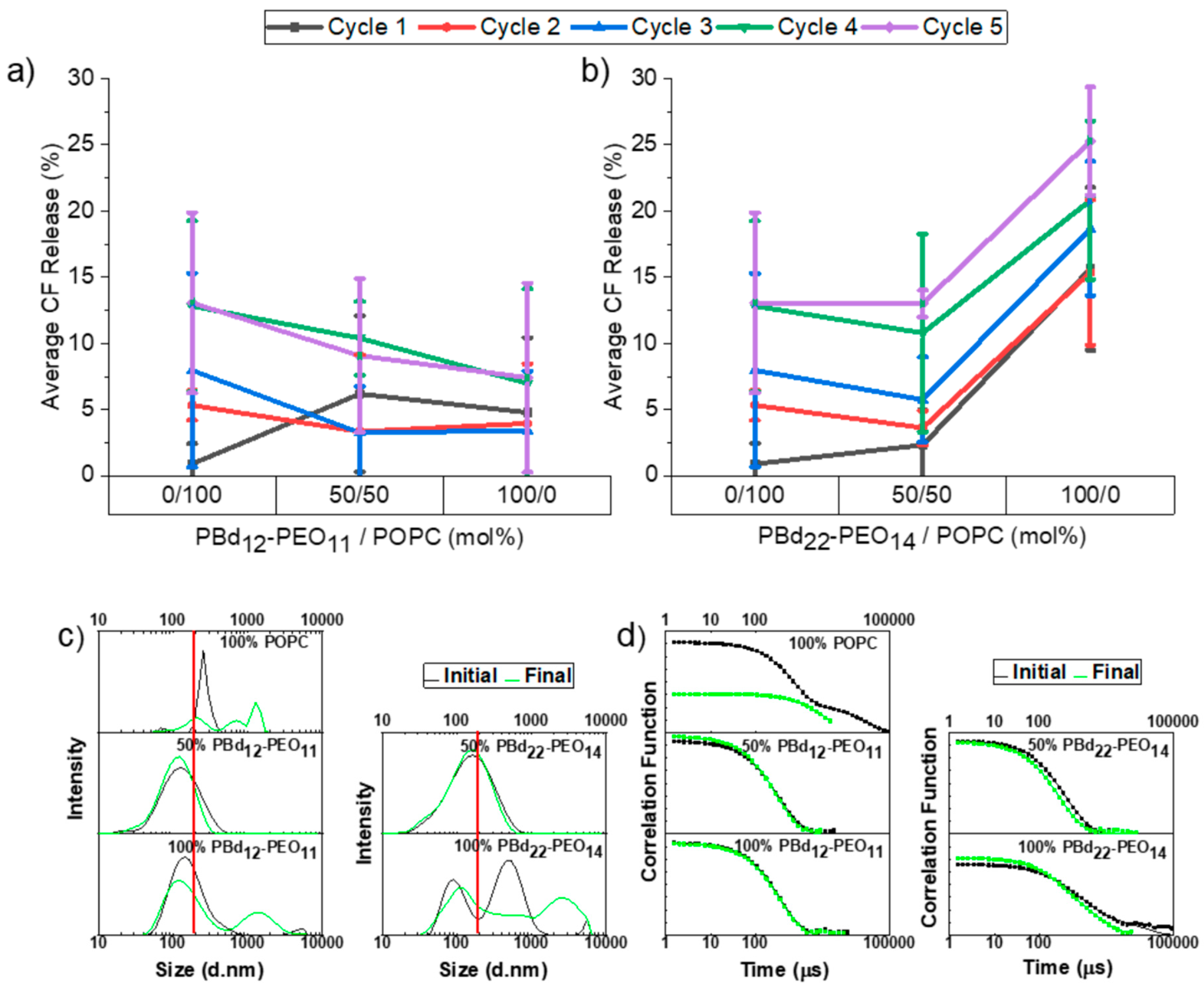
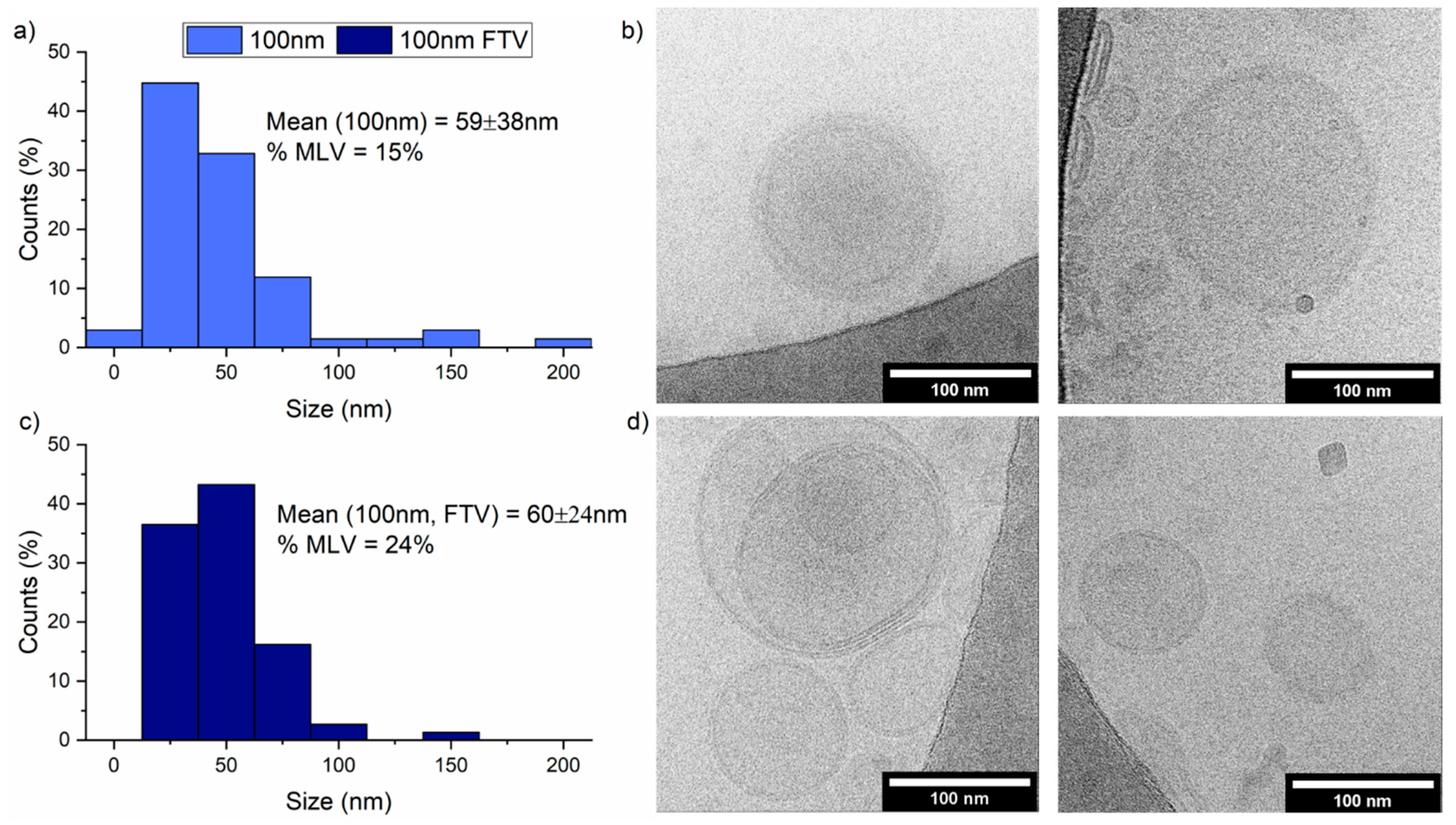
3.3. Freeze-Thaw-Vortex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chemin, M.; Brun, P.M.; Lecommandoux, S.; Sandre, O.; Le Meins, J.F. Hybrid polymer/lipid vesicles: Fine control of the lipid and polymer distribution in the binary membrane. Soft Matter 2012, 8, 2867–2874. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Santore, M.M. Hybrid copolymer-phospholipid vesicles: Phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control. Soft Matter 2015, 11, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.T.; Fernandes, F.; Ibarboure, E.; Ferji, K.; Prieto, M.; Sandre, O.; Le Meins, J.F. Modulation of phase separation at the micron scale and nanoscale in giant polymer/lipid hybrid unilamellar vesicles (GHUVs). Soft Matter 2017, 13, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, I.M.; Paxton, W.F. Salt, Shake, Fuse-Giant Hybrid Polymer/Lipid Vesicles through Mechanically Activated Fusion. Angew. Chem. Int. Ed. 2014, 53, 3372–3376. [Google Scholar] [CrossRef]
- Hu, S.W.; Huang, C.Y.; Tsao, H.K.; Sheng, Y.J. Hybrid membranes of lipids and diblock copolymers: From homogeneity to rafts to phase separation. Phys. Rev. E 2019, 99, 012403. [Google Scholar] [CrossRef]
- Lim, S.K.; de Hoog, H.P.; Parikh, A.N.; Nallani, M.; Liedberg, B. Hybrid, Nanoscale Phospholipid/Block Copolymer Vesicles. Polymers 2013, 5, 1102–1114. [Google Scholar] [CrossRef] [Green Version]
- Magnani, C.; Montis, C.; Mangiapia, G.; Mingotaud, A.F.; Mingotaud, C.; Roux, C.; Joseph, P.; Berti, D.; Lonettia, B. Hybrid vesicles from lipids and block copolymers: Phase behavior from the micro- to the nano-scale. Colloids Surf. B Biointerfaces 2018, 168, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Vanderlick, T.K.; Beales, P.A. Formation and dissolution of phospholipid domains with varying textures in hybrid lipo-polymersomes. Soft Matter 2012, 8, 7982–7988. [Google Scholar] [CrossRef]
- Schulz, M.; Binder, W.H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef]
- Schulz, M.; Glatte, D.; Meister, A.; Scholtysek, P.; Kerth, A.; Blume, A.; Bacia, K.; Binder, W.H. Hybrid lipid/polymer giant unilamellar vesicles: Effects of incorporated biocompatible PIB-PEO block copolymers on vesicle properties. Soft Matter 2011, 7, 8100–8110. [Google Scholar] [CrossRef]
- Abraham, T.; Mao, M.; Tan, C.E.M. Engineering approaches of smart, bio-inspired vesicles for biomedical applications. Phys. Biol. 2018, 15, 061001. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Bello, G.; Virk, M.; Kurzhals, S.; Scheberl, A.; Gal, N.; Matysik, A.; Kraut, R.; Reimhult, E. Magneto-Thermal Release from Nanoscale Unilamellar Hybrid Vesicles. Chemnanomat 2016, 2, 1111–1120. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Elias, D.R.; Kamat, N.P.; Johnston, E.D.; Poloukhtine, A.; Popik, V.; Hammer, D.A.; Tsourkas, A. Improved Tumor Targeting of Polymer-Based Nanovesicles Using Polymer-Lipid Blends. Bioconj. Chem. 2011, 22, 2021–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer—Lipid vesicles as potential drug delivery systems. J. Colloid Interface Sci. 2020, 562, 418–428. [Google Scholar] [CrossRef]
- Mohammadi, M.; Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Hybrid Vesicular Drug Delivery Systems for Cancer Therapeutics. Adv. Funct. Mater. 2018, 28, 1802136. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.Y.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [Green Version]
- Panneerselvam, K.; Lynge, M.E.; Riber, C.F.; Mena-Hernando, S.; Smith, A.A.A.; Goldie, K.N.; Zelikin, A.N.; Stadler, B. Phospholipid-polymer amphiphile hybrid assemblies and their interaction with macrophages. Biomicrofluidics 2015, 9, 052610. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.S.; Kumbhar, D.D.; Manwar, J.V.; Jadhao, R.G.; Bakal, R.L.; Wakode, S. Ultrasound-Assisted Facile Synthesis of Nanostructured Hybrid Vesicle for the Nasal Delivery of Indomethacin: Response Surface Optimization, Microstructure, Stability. AAPS PharmSciTech 2019, 20, 97. [Google Scholar] [CrossRef]
- Pippa, N.; Kaditi, E.; Pispas, S.; Demetzos, C. PEO-b-PCL-DPPC chimeric nanocarriers: Self-assembly aspects in aqueous and biological media and drug incorporation. Soft Matter 2013, 9, 4073–4082. [Google Scholar] [CrossRef]
- Pippa, N.; Merkouraki, M.; Pispas, S.; Demetzos, C. DPPC: MPOx chimeric advanced Drug Delivery nano Systems (chi-aDDnSs): Physicochemical and structural characterization, stability and drug release studies. Int. J. Pharm. 2013, 450, 1–10. [Google Scholar] [CrossRef]
- Robertson, J.D.; Yealland, G.; Avila-Olias, M.; Chierico, L.; Bandmann, O.; Renshaw, S.A.; Battaglia, G. pH-Sensitive Tubular Polymersomes: Formation and Applications in Cellular Delivery. ACS Nano 2014, 8, 4650–4661. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beales, P.A.; Khan, S.; Muench, S.P.; Jeuken, L.J.C. Durable vesicles for reconstitution of membrane proteins in biotechnology. Biochem. Soc. Trans. 2017, 45, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Li, M.Q.; Muench, S.P.; Jeuken, L.J.C.; Beales, P.A. Durable proteo-hybrid vesicles for the extended functional lifetime of membrane proteins in bionanotechnology. Chem. Commun. 2016, 52, 11020–11023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paxton, W.F.; McAninch, P.T.; Achyuthan, K.E.; Shin, S.H.R.; Monteith, H.L. Monitoring and modulating ion traffic in hybrid lipid/polymer vesicles. Colloids Surf. B Biointerfaces 2017, 159, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, R.; Khan, S.; Moscrop, E.; Rappolt, M.; Muench, S.P.; Jeuken, L.J.C.; Beales, P.A. A reconstitution method for integral membrane proteins in hybrid lipid-polymer vesicles for enhanced functional durability. Methods 2018, 147, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.L.; Boyd, M.A.; Kamat, N.P. Diblock copolymers enhance folding of a mechanosensitive membrane protein during cell-free expression. Proc. Natl. Acad. Sci. USA 2019, 116, 4031–4036. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.M.; Bermudez, H.; Discher, B.M.; Sheehan, M.A.; Won, Y.-Y.; Bates, F.S.; Discher, D.E. Preparation, stability, and in vitro performance of vesicles made with diblock copolymer. Biotechnol. Bioeng. 2001, 73, 135–145. [Google Scholar] [CrossRef]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant Phospholipid/Block Copolymer Hybrid Vesicles: Mixing Behavior and Domain Formation. Langmuir 2011, 27, 1–6. [Google Scholar] [CrossRef]
- Garni, M.; Wehr, R.; Avsar, S.Y.; John, C.; Palivan, C.; Meier, W. Polymer membranes as templates for bio-applications ranging from artificial cells to active surfaces. Eur. Polym. J. 2019, 112, 346–364. [Google Scholar] [CrossRef]
- Dao, T.P.T.; Brulet, A.; Fernandes, F.; Er-Rafik, M.; Ferji, K.; Schweins, R.; Chapel, J.P.; Schmutz, F.M.; Prieto, M.; Sandre, O.; et al. Mixing Block Copolymers with Phospholipids at the Nanoscale: From Hybrid Polymer/Lipid Wormlike Micelles to Vesicles Presenting Lipid Nanodomains. Langmuir 2017, 33, 1705–1715. [Google Scholar] [CrossRef] [Green Version]
- Stoenescu, R.; Graff, A.; Meier, W. Asymmetric ABC-triblock copolymer membranes induce a directed insertion of membrane proteins. Macromol. Biosci. 2004, 4, 930–935. [Google Scholar] [CrossRef]
- Otrin, L.; Marusic, N.; Bednarz, C.; Vidakovic-Koch, T.; Lieberwirt, I.; Landfester, K.; Sundmacher, K. Toward Artificial Mitochondrion: Mimicking Oxidative Phosphorylation in Polymer and Hybrid Membranes. Nano Lett. 2017, 17, 6816–6821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.Z.; O’Reilly, R.K. Advances and challenges in smart and functional polymer vesicles. Soft Matter 2009, 5, 3544–3561. [Google Scholar] [CrossRef]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Armenante, P.M.; Kirpekar, A.K. Sterilization in the Pharmaceutical and Biotechnology Industry. In Handbook of Downstream Processing; Springer: Dordrecht, The Netherlands, 1997; pp. 261–308. [Google Scholar]
- World Health Organisation. Methods of Sterilisation. In The International Pharmacopoeia; World Health Organization: Geneva, Switzerland, 2019; pp. 1–3. [Google Scholar]
- Zuidam, N.J.; Lee, S.S.L.; Crommelin, D.J.A. Sterilization of Liposomes by Heat-Treatment. Pharm. Res. 1993, 10, 1591–1596. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Cuhadar, S.; Koseoglu, M.; Atay, A.; Dirican, A. The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem. Med. 2013, 23, 70–77. [Google Scholar] [CrossRef]
- Sykes, C. Time- and Temperature-Controlled Transport: Supply Chain Challenges and Solutions. Pharm. Ther. 2018, 43, 154. [Google Scholar]
- de Castro, M.D.; Garcia, J.L. Analytical freeze-drying. Tech. Instrum. Anal. Chem. 2002, 24, 11–41. [Google Scholar]
- Khan, I.; Elhissi, A.; Shah, M.; Alhnan, M.; Ahmed, W. Liposome-Based Carrier Systems and Devices Used for Pulmonary Drug Delivery. In Biomaterials and Medical Tribology; Woodhead Publishing: Cambridge, UK, 2013; pp. 395–443. [Google Scholar]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Engbers, G.; Feijen, J. Biodegradable polymersomes as a basis for artificial cells: Encapsulation, release and targeting. J. Control. Release 2005, 101, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Goldbach, P.; Brochart, H.; Wehrlé, P.; Stamm, A. Sterile filtration of liposomes: Retention of encapsulated carboxyfluorescein. Int. J. Pharm. 1995, 117, 225–230. [Google Scholar] [CrossRef]
- Drulyte, I.; Johnson, R.M.; Hesketh, E.L.; Hurdiss, D.L.; Scarff, C.A.; Porav, S.A.; Ranson, N.A.; Muench, S.P.; Thompson, R.F. Approaches to altering particle distributions in cryo-electron microscopy sample preparation. Acta Crystallogr. Sect. D Struct. Biol. 2018, 74, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.L.S.; Borgnia, M.J.; Bartesaghi, A.; Tran, E.E.H.; Earl, L.A.; Schauder, D.M.; Lengyel, J.; Pierson, J.; Patwardhan, A.; Subramaniam, S. Cryo-electron microscopy—A primer for the non-microscopist. FEBS J. 2013, 280, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.J.; Cheng, A.; Noble, A.J.; Eng, E.T.; Kim, L.Y.; Carragher, B.; Potter, C.S. Routine determination of ice thickness for cryo-EM grids. J. Struct. Biol. 2018, 204, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sejwal, K.; Chami, M.; Baumgartner, P.; Kowal, J.; Mller, S.A.; Stahlberg, H. Proteoliposomes—A system to study membrane proteins under buffer gradients by cryo-EM. Nanotechnol. Rev. 2017, 6, 57–74. [Google Scholar] [CrossRef]
- Karlsson, G. Thickness measurements of lacey carbon films. J. Microsc. Oxf. 2001, 203, 326–328. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, H.; Carlsson, A.; Yachi, K.; Hirota, S. Possibility of heat sterilization of liposomes. Chem. Pharm. Bull. 1991, 39, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Mann, A.; Kiefer, M.; Leuenberger, H. Thermal sterilization of heat-sensitive products using high-temperature short-time sterilization. J. Pharm. Sci. 2001, 90, 275–287. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seneviratne, R.; Jeuken, L.J.C.; Rappolt, M.; Beales, P.A. Hybrid Vesicle Stability under Sterilisation and Preservation Processes Used in the Manufacture of Medicinal Formulations. Polymers 2020, 12, 914. https://doi.org/10.3390/polym12040914
Seneviratne R, Jeuken LJC, Rappolt M, Beales PA. Hybrid Vesicle Stability under Sterilisation and Preservation Processes Used in the Manufacture of Medicinal Formulations. Polymers. 2020; 12(4):914. https://doi.org/10.3390/polym12040914
Chicago/Turabian StyleSeneviratne, Rashmi, Lars J. C. Jeuken, Michael Rappolt, and Paul A. Beales. 2020. "Hybrid Vesicle Stability under Sterilisation and Preservation Processes Used in the Manufacture of Medicinal Formulations" Polymers 12, no. 4: 914. https://doi.org/10.3390/polym12040914
APA StyleSeneviratne, R., Jeuken, L. J. C., Rappolt, M., & Beales, P. A. (2020). Hybrid Vesicle Stability under Sterilisation and Preservation Processes Used in the Manufacture of Medicinal Formulations. Polymers, 12(4), 914. https://doi.org/10.3390/polym12040914